Background: We understand that most diseases which are accompanied with aging must relate to oxidative stress, i.e. oxidative damage of lipid lamellar bimolecular membranes of mitochondria (mt). So-called cancer and tumor are groups of such diseases and are diagnosed on the basis of hard spot touch and cell-deformed invasion image obtained by optical microscope, but molecular-level definition of cancer and tumor cells is unclear. The hard spot and/or the cell invasion will be symptoms relating to metabolic dysfunctions of cells, and hydrogen peroxide-derived hydroxyl radical must induce oxidative degradation of lipid lamellar membranes of mt in the cells. Our goal is to gain insights into reactivity of hydrated HOOH and hydroxyl radicals which produce and exist inevitably in mt, and instability of the mt’s lipid bimolecular membrane. The result allows us to propose candidates for anticancer medicines which may have antioxidative effects on sustainable mt’s membranes.
Materials and methods: Quantum chemistry molecular modeling, i.e., density functional theory-based molecular modeling (DFT/MM) can be regarded as theory-based “experiments”. DFT/MM can be carried out very quickly using high-end supercomputer-like personal computers. The molecular unit consisting bimolecular membranes is glycerin triester of lauric acid [Gly(n-C11H23COO)3]. DFT/MM are applicable to molecular aggregates which are induced by van der Waals (vdW) force (i.e. hydrogen bonding and Coulomb interactions). DFT/MM for structuring of aggregates predicts heat of formation, dipole moment for understanding solubility in water, and volume of CPK model of the aggregates as quantitative structure activity for considering penetration of lipid bimolecular membranes of cells, and the energy structures of aggregates, i.e. surface molecular orbital electron energy structures (almost degenerate highest molecular orbitals (HOMO(n), n=0~12) and the lowest unoccupied molecular orbital (LUMO) configurations. Their orbital potentials of EHOMO(n), (n), n=0~6, and ELUMO(0), density distribution configuration of surface electron, radical density (spin density) configuration of radical species are evaluated as theory-based “experimental” data for verification and prediction of interacting molecules and radicals. As reported in the preceding papers, we performed DFT/MM using the B3LYP exchange correlation functional and the 6–31G(d) basis set with Spartan’18 (Wavefunction, Inc. Irvine, CA). Prior to the DFT calculation, for electronic properties, the geometries of the molecular aggregates were determined by the molecular mechanics simulation employing the Merck Molecular Force Field (MMFF). Details on the result obtained with DFT/MM are described in Figures and Tables of supplementary material.
Results: DFT/MM using glycerin triester of lauric acid [Gly(n-C11H23COO)3] as a lipid model molecule verifies that (1) cooperative van der Waals (vdW) force makes bimolecular lipid-like tetramer, [Gly(n- C11H23COO)3]4. (2) The lipid-like tetramer undergoes oxidative degradation by vdW aggregation with hydrogen peroxide and degradation reaction with hydroxyl radical. (3) Hydrated HOOH reacts with superoxide radical anion(O2.-) which always exists in functioning mt, giving hydroxyl radical. (4) Oxidative degradation starts from ester sites of mt’s lipid bilayer membranes, leading to degrative swelling of the mt’s membranes. (5) The oxidative degradation causes swelling and/or invasion of the cells containing such degraded mt’s membranes. Well-known anticancer medicines, 5-Fluorouracil, Cisplatin, Oxaliplatin, Vitamin C and thyroid hormone, thyroxine (T4) are verified to suppress oxidation power of HOOH and HO.. DFT/MM verifies that the swelling and/or invasion tissues filled with the swollen and dysfunctional mt is an authentic model of cancer.
density functional theory (DFT), 5-fluorouracil, cisplatin, sorafenib, vitamin C
Mitochondria (mt) are bean-shaped organelles found in every cell of the body (cell size: 6~25mm, cell numbers: 4~6×1013), being composed by cytoplasmic membranes and lamella. Size and length (diameter: 0.5µm, length: ~10mm, numbers: 4~6×1016) are comparable to those of bacteria with different shapes. The mt’s important role is to carry out the way a cell gets energy, i.e. chemical energy for production of ATP for peptide source of cells, and electromagnetic energy (EME) from electron transmitter-like mt in brain and nerve cells. We predict that the highest energetic molecule for ATP and EME in mt is hydrated superoxide anion radical of O2.-.
Recent studies on mt revealed that active cells with the most mt are heart (each cell is known to contain ~2000 pieces of mt), brain, stomach, intestines, liver, pancreas, kidney and muscle, and that most illness must come from dysfunctions of mt-filled cells, i.e. decline of mt as bioenergy source of cells [1-4]. In fact, cancer and tumor often occur on tissues of mt-filled cells.
Japan National Cancer Center Research Institute (NCCRI) reported that cancer is a disease associated with aging and environmental risk factors, and that most cancers develop with genomic instability and a number of mutations after middle age [5,6]. We understand, however, that while the genomic instability occurs in mt, oxidative degradation starts on mt’s lipid bimolecular membranes. It is proposed that the lipid bilayer membranes accumulate hydrogen peroxide (HOOH) with age, and hydroxyl radical (HO.) will form in situ from HOOH and O2.-. Particularly, HO. will cause dysfunction of mt as bioenergy source of cells.
DFT/MM validates: 1) Equilibrium geometry of hydrated HOOH, HOOH(H2O)2 and their energy structures, 2) The in-situ formation of hydrated hydroxyl radical and its destructive reactivity, 3) Energy structures of lipid bimolecular membranes and stability, 4) Oxidative degradation of lipid membranes, and 5) Antioxidative effects of anticancer medicines.
Verification of equilibrium geometry and energy structure of HOOH(H2O)2
In the preceding papers, DFT/MM verified that superoxide radical anion (O2.-) is produced from D-glucose and triplet oxygen 3O2, and O2.- works as energy source for mt metabolism. However, O2.- undergoes a side reaction, giving hydrogen peroxide (HOOH) [1,2]. We now verify on the basis of DFT/MM (Figure 1) that bicyclic hydrated HOOH, and monocyclic hydrated HOOH will form in water phase of mt with heat of formation of respective ΔE=-23.8 and ΔE=-25.9 kcal/mol. In both cases, the lowest electron accepting molecular orbital configuration (LUMO configuration; mesh presentation) locates on the HOOH site, verifying that preferential reductive electron transfer is likely to occur to HOOH, generating HO. (Figure 2). In addition, we find that HOOH(H2O)2 may aggregate with D-glucose and with O2.- (Figure 1). Their electron energy potential verifies that reductive electron transfer will result in giving HO. preferentially and instantly.
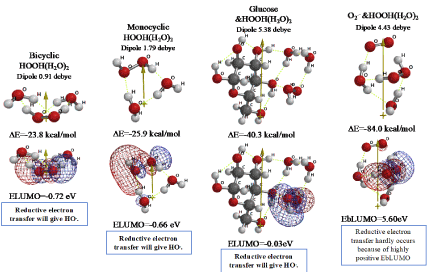
Figure 1. Verification of equilibrium geometries of HOOH-aggregated species and their energy structures
Verification of in-situ formation of hydrated HO. and destructive reactivity
Figure 2 shows that monocyclic hydrated hydrogen peroxide, HOOH(H2O)2 undergoes one electron transfer reduction from superoxide radical anion(O2.-), and removable of hydroxide anion (HO-) leads to exothermic formation of hydrated hydroxyl radical [HO.(H2O)2]. Formation of intermediary radical anion, [HOOH(H2O)2].-] validates the bond fission between O-O bond of HOOH by one-electron transfer reduction. In general, aging of the creature accompanies lack of aerobic exercise, and the lack of aerobic exercise accelerates a side reaction with O2.-, i.e. in-situ production of harmful HO. from O2.- and HOOH.
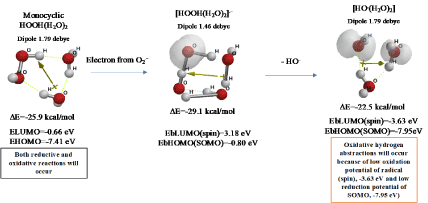
Figure 2. Verification of hydroxyl radical (HO.) formation from HOOH and O2.-
DFT/MM also verifies that radical (spin) density of [HO.(H2O)2] locates on both of HO. and H2O, indicating delocalization of spin. However, electron accepting potential, i.e. spin oxidation potential EbLUMO (spin) is verified to be low -3.63 eV, and electron donating potential, i.e. SOMO potential EbHOMO(SOMO) to be also low -7.95 eV. In addition, electron accepting potential of bare HO. is verified to be -4.16 eV. Hydrated HO. will be in equilibrium with the bare HO., keeping high reactivity of oxidative hydrogen abstraction. The hydrogen abstractions are often observed as intermediates in DFT/MM-based equilibrium geometry determination.
Verification of energy structures of lipid bimolecular membranes and stability
In order to demonstrate energy structures of lipid lamellar membranes, self-aggregation of glycerin triester of lauric acid, Gly(n-C11H23COO)3 is examined. The self-aggregation is molecular-modeled by single point energy (SPE) determination for molecular mechanics-based structure (Figure S1, Table S1). As the energy structures, heat of formation for self-organization and distribution of HOMO’s distribution for SPE-based determined aggregate structures are shown. The SPE-based aggregates have compact structures when compared to equilibrium geometry (EQG)-based determined structures, implying that the SPE-determined structures are appropriate for lipid stacked state bimolecular membranes. Previously, we reported a similar verification for the three-dimension structure of crystalline benzene.
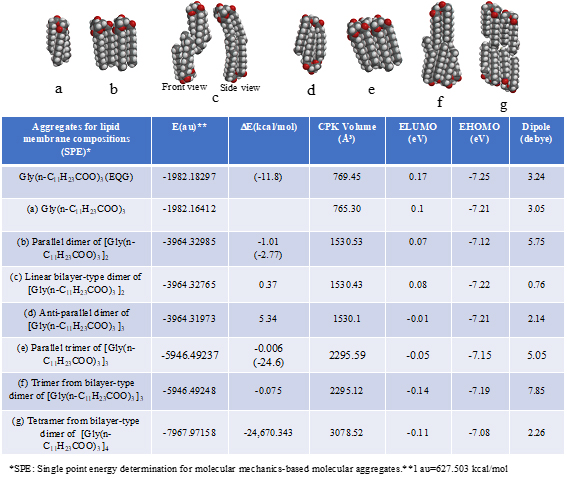
Table S1. Verification and prediction for van der Waals aggregates (SPE) of glycerin tri-ester of lauric acid [Gly(n-C11H23COO)3] for construction of lipid lamellar bimolecular membrane model. The DE in parentheses indicates heat of formation for the equilibrium geometry (EQG).
Scheme S1. Prediction for equilibrium structures of glycerin triester of lauric acid as a packing unit of lamellar-type lipid molecular membranes
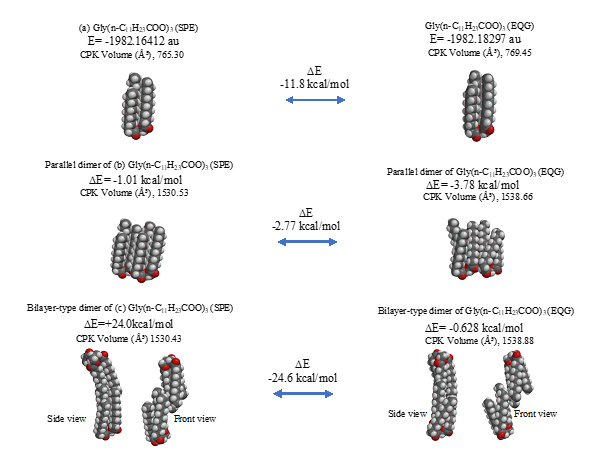
The parallel dimer of (b)[Gly(n-C11H23COO)3]2, is verified to form with heat of formation of ΔE=-1.01 kcal/mol (Table S1), and the bilayer-type dimer of (c)[Gly(n-C11H23COO)3]2, with ΔE=+0.37 (A in Figure 3). Interestingly, the tetramer from the bilayer-type dimer, i.e., the lamellar-type tetramer of (g)[Gly(n-C11H23COO)3]4 is verified to form with heat of formation of ΔE=-24,670 kcal/mol (B in Figure 3). On the other hand, the heats of formation of DE=-1.01 kcal/mol (SPE) and ΔE=-2.77 kcal/mol (EQG) are verified for the parallel dimer, and ΔE=-0.006 kcal/mol (SPE) and ΔE=-24.6 kcal/mol (EQG) for the parallel trimer combined (see Table S1). Given the heat of formation of ΔE=+0.37 kcal/mol (SPE) and ΔE=-0.628 kcal/mol (EQG) for the bilayer-type dimer, remarkable and cooperative vdW-force stabilization must play a decisive role in construction of the lamellar-type tetramer of [Gly (n-C11H23COO)3]4. In other words, lipid lamellar membranes are stabilized by taking both parallel and bilayer type aggregation of lipid triglyceride unit.
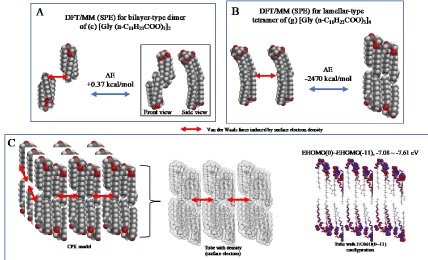
Figure 3. Lamellar-type lipid membrane model predicted by vdW force-induced aggregates composed of DFT/MM(SPE)-based lamellar-type tetramer of (g) [Gly(n C11H23COO)3]4 (SPE)
Taking into account the cooperative vdW force in the lamellar-type tetramer, a three-dimension membrane model is speculated by arranging nine units of the lamellar-type tetramer. The vdW forces via surface electron density and HOMO distribution are also depicted to predict the stability and reactivity of lipid lamellar membranes (C in Figure 3).
The surface energy structure, distribution of configurations of HOMO(0)~(11) is worth noting for structural stability. The HOMO distribution predicts that alkyl chain sites are stable enough for oxidation but surface ester sites are not, predicting that lipid bilayer membranes aggregate with hydrated HOOH and HO. especially at polar ester sites of lamellar membranes. In other words, the lipid bilayer membranes are fairly stable in biological redox reaction systems like cells and mt. The surface ester site undergoes firstly oxidative degradation, and the swollen lamellar membranes will have space at alkyl chain sites, undergo the attack by hydroxyl radical, and the lamellar membrane structures will be destroyed oxidatively.
Verification of oxidative degradation of lipid membranes
Energy structures of glycerin triester (a)Gly(n-C11H23COO)3 (SPE), the parallel trimer (e) [Gly(n-C11H23COO)3]3 (SPE), and the bilayer-type tetramer (g)[Gly(n-C11H23COO)3]4(SPE) are shown in Figure S2, S3, and Figure 4. The HOMO configurations distribute at ester sites and alkyl chain sites, indicating that HOOH and HO. react not only at ester sites but also at alkyl chain sites of the triglyceride and their aggregates. Aggregation of hydrated HOOH, HOOH(H2O)2(cyclic) at alkyl chain sites and at ester sites are molecular modeled (EQG) for heat of formation (ΔE), configurations and energy potential of HOMO and LUMO and volume of the CPK model (Figure 4, Table S2). As for the aggregation at alkyl chain sites, HOOH molecule still have potential to be reduced to HO. by attack of O2.- since the LUMO configuration locates also at the HOOH molecule on the alkyl chain site.
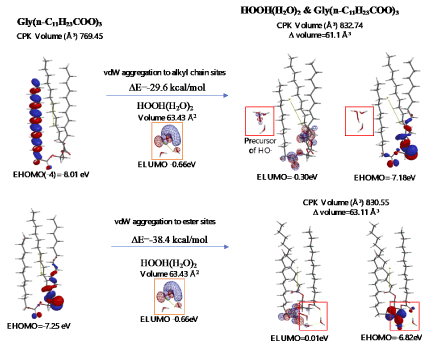
Figure 4. Equilibrium geometry and energy structure of van der Waals aggregate of HOOH(H2O)2 on lipid membrane unit model of Gly(n-C11H23COO)3
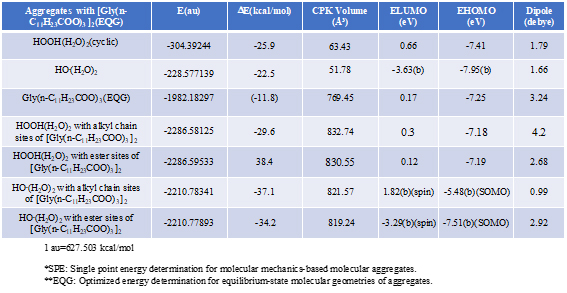
Table S2. Verification and prediction of degradative aggregation of [Gly(n-C11H23COO)3] with respective HOOH(H2O)2 and HO.(H2O)
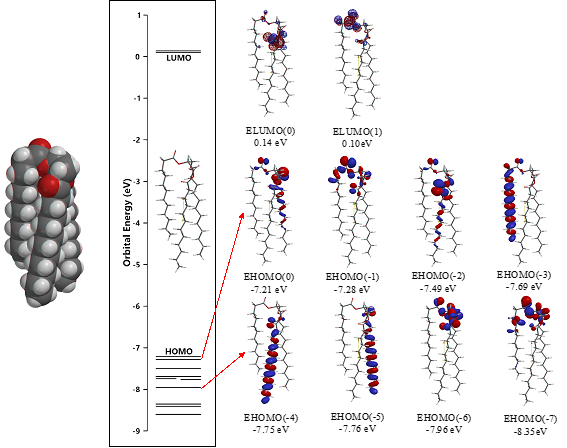
Scheme S2. DFT/MM-verified surface electron energy structure of glycerin tri-ester of lauric acid, Gly(n-C11H23COO)3 (SPE)
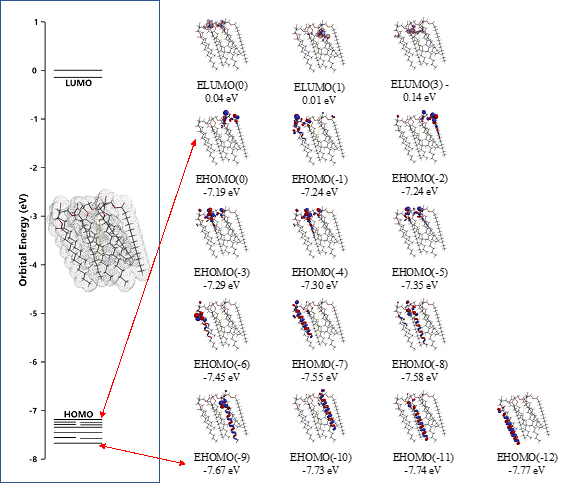
Scheme S3. DFT/MM-verified surface electron energy structure of lipid membrane model unit, parallel trimer of glycerin tri-ester of lauric acid, [Gly(n-C11H23COO)3]3 (SPE)
The heat of formation, (ΔE=-30.3 kcal/mol, ΔE=-38.4 kcal/mol) and the energy structure change verify that the aggregation occurs exothermically and HOOH molecules are more stabilized by vdW force at the ester sites. Interestingly, LUMO is verified not to locate at the HOOH molecule which is aggregated on ester sites, indicating that HOOH molecules can be accumulated at ester sites of lamellar membranes.
As for the aggregation of HO.(H2O)2 with Gly(n-C11H23COO)3 (Figure 5), hydrogen abstraction reaction occurs at alkyl chain sites. It is worth noting that the ester-site aggregation with HO.(H2O)2 accompanies large conformation change. Larger exothermic heat of formation (ΔE=-37.1 kcal/mol, ΔE=-34.2 kcal/mol) and 3-dimension molecular change validate vivid oxidative degradation of lipid molecules, especially when HOOH molecules on ester sites are converted into HO. in biological redox systems, i.e. in mt. Oxidative morphological change accelerates oxidation of alkyl chain sites of collapsing membranes.
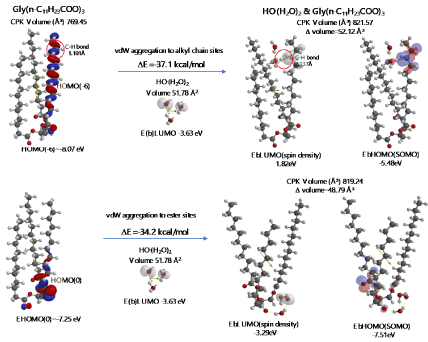
Figure 5. Equilibrium geometry and energy structure of van der Waals aggregate of HO.(H2O)2 on lipid membrane model of Gly(n-C11H23COO)3
Verification of antioxidative effects of anticancer medicines
Cancer and tumor cells induce dysfunction of metabolism in organs of body, and any dysfunction of cells results from initial degradation of powerhouse of cells, i.e. oxidative damage of lipid lamellar membranes of mt. From perspective of chemotherapy of cancer and tumor, oxidative collapse of lipid lamellar membranes will be suppressed by anticancer medicines if their molecular size is small enough to reach vicinity of mt through lipid lamellar membranes of cells. DFT/MM of the smallest anticancer medicine, Flurouracil (5-Fluorouracil) [8] is evaluated to clarify difference in antioxidative effect from that of uracil in the nucleic acid of RNA [6].
Both 5-Fluorouracil and uracil have three different ring structures due to the tautomerism; 1. amid, hydroxyl, 2. amid, amid, 3. hydroxyl, hydroxyl in the pyrimidine ring structure. Three equilibrium geometry structures and energy structures of the aggregates with HOOH(H2O)2, and with HO.(H2O)2 validate the vdW aggregation (Figure 6 and Figure S4).
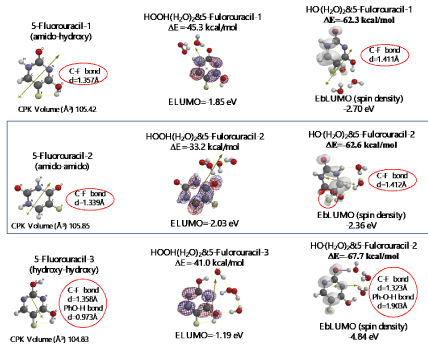
Figure 6. Equilibrium geometry and energy structures of van der Waals aggregation of 5-Fuluoruracil with HOOH(H2O)2 and HO.(H 2O) 2
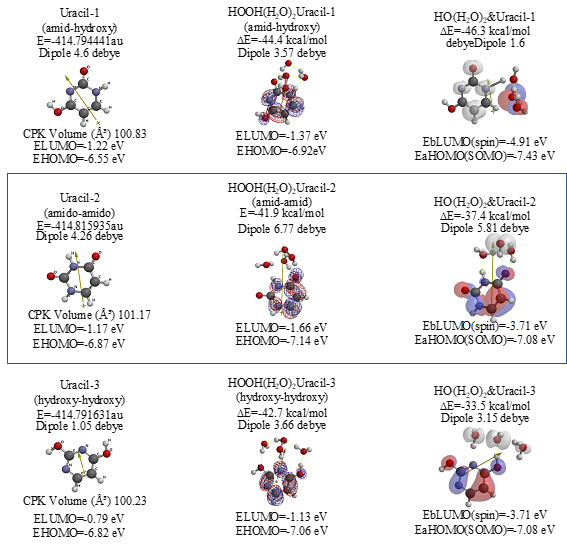
Scheme S4. Equilibrium geometry and energy structures of vdW aggregates of Uracil with HOOH(H2O)2 and HO.(H 2 O) 2; equilibrium geometry and energy structures
Interestingly, each of the aggregations with HO.(H2O)2 provides transition states to oxidation products, which is formed by C-F or O-H bond fission (Figure S5, S6, S7). It is noteworthy that although spin density potential (EbLUMO) of each intermediate ranges from -4.84 ~ -2.36 eV, the spin density is all delocalized on pyrimidine rings, not on HO.. The large negative heat of formation of ΔE = -62 ~ -68 kcal/mol also verifies antioxidative effect of 5-Fluorouracil.
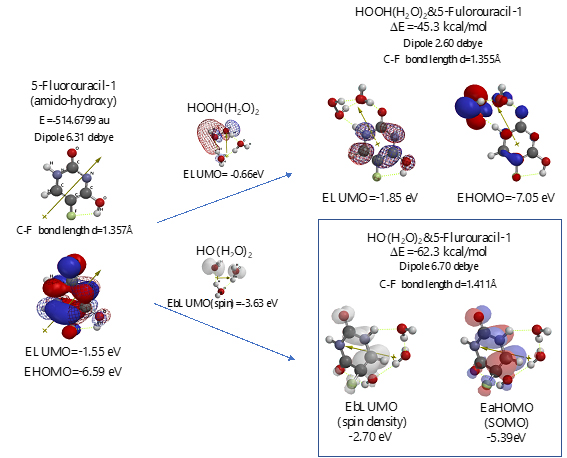
Scheme S5. Verification of 5-Fluorouracil-1 (amid-hydroxyl) as an anticancer medicine
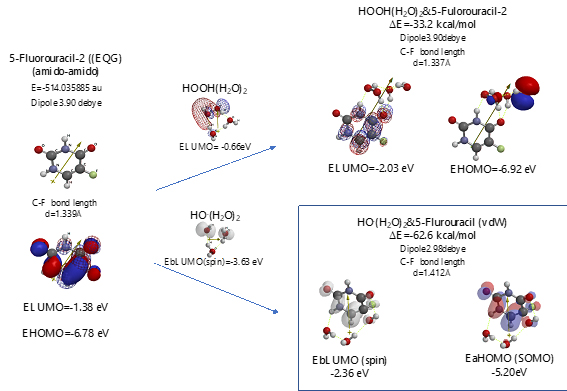
Scheme S6. Verification of 5-Fluorouracil-2 (amid & amid) as an anticancer medicine
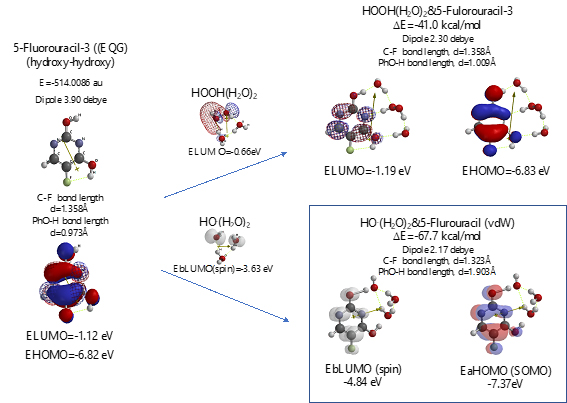
Scheme S7. Verification for 5-Fluorouracil-3 (hydroxyl-hydroxyl) as an anticancer medicine
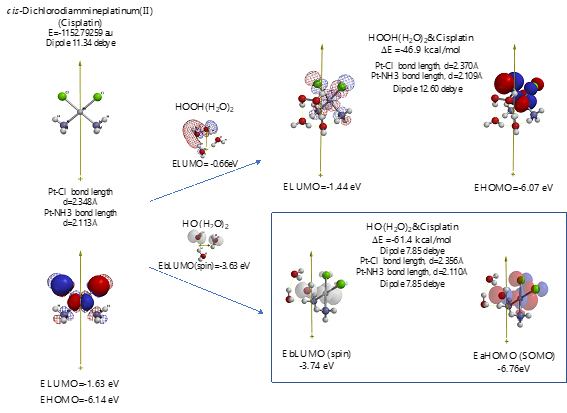
Scheme S8. Verification of Cisplatin as an anticancer medicine
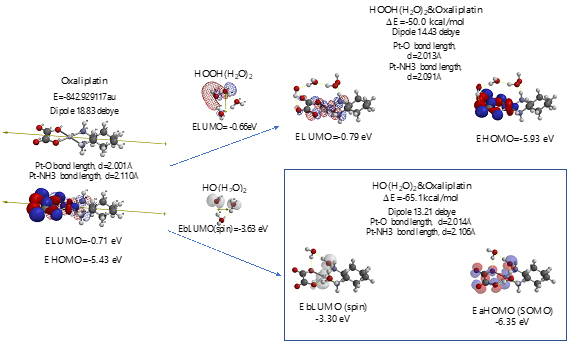
Scheme S9. Verification of Oxaliplatin as an anticancer medicine
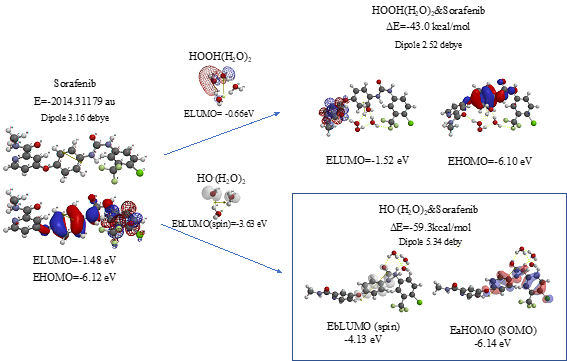
Scheme S10. Verification of Sorafenib as an anticancer medicine
The same verification is true for uracil isomers (Figure S4). It is worth noting, however, that difference in antioxidative effects comes from different exothermic heat of formation. 5-Fluorouracil reacts more exothermically with HOOH and HO. than uracil. DFT/MM results verify that 5-Fluorouracil is able to eliminate HOOH and HO. from mt vicinity, i.e. 5-Fluorouracil is more effective antioxidant than uracil (Table 1).
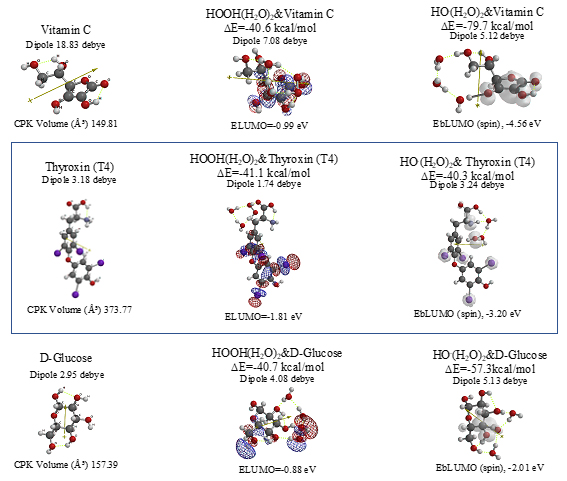
Table 1. Comparison for antioxidant effects of antitumor medicines and nutrients in view of heat of formation of aggregation with HOOH(H2O)2 and HO.(H2O)2
Antitumor medicines and netrients for mitochondria targeting chemotherapy |
CPK volume
(ų) |
Heat of formation for van der Waals aggregation with HOOH (H2O)2
ΔE (kcal/mol) |
Heat of formation (ΔE) for van der Waals aggregation with
HO. (H2O)2
ΔE (kcal/mol) |
Total of heat of formation (ΔE)
as a measure of antioxidant effect
ΔE (kcal/mol) |
5-Fluorouracil (amid, hydroxy)
Uracil-1(amid hydroxy) |
105.42
100.83 |
-45.3
-44.5 |
-55.7
-46.3 |
-101
-90.7 |
5-Fluorouracil (amid, amid)
Uracil-2 (amid amid) |
105.85
101.17 |
-33.2
-41.9 |
-62.6
-37.4 |
-95.8
-79.7 |
5-Fluorouracil (hydroxy hydroxy)
Uracil-3 (hydroxy hydroxy) |
104.83
100.23 |
-41.0
-42.7 |
-67.7
-33.5 |
-109
-76.3 |
Cisplatin |
96.02 |
-46.9 |
-61.4 |
-108 |
Oxaliplatin |
205.37 |
-50.0 |
-65.1 |
-115 |
Sorafenib |
411.92 |
-43.0 |
-59.3 |
-102 |
Ascorbic acid (Vitamin C) |
149.81 |
-37.3 |
-77.8 |
-115 |
Thyroxine (T4) |
373.77 |
-41.1 |
-40.3 |
-81.4 |
Gly(n-C11H23COO)3 (EQG) at alkyl sites |
769.45 |
-29.6* |
-37.1 |
-66.7 |
Gly(n-C11H23COO)3 (EQG) at ester sites |
769.45 |
-38.4 |
-34.2 |
-72.6 |
D-Glucose |
157.39 |
-40.7* |
-57.3 |
-98.1 |
As shown in Figure 7 and Table 1, platinum complexes, Cisplatin and Oxaliplatin [8], and chlorine atom-containing Sorafenib all aggregate with HOOH(H2O)2, and react with HO.(H2O)2. They all proceed highly exothermic, thus implying effective antioxidative effects. It is noteworthy that platinum metal has an interesting capability of decreasing oxidative reactivity of HO. . Both aggregates with HO.(H2O)2 have spin density which is delocalized onto the platinum (II) atom with spin density potential, EbLUMO(spin) = -3.74 and -3.30 eV, being much higher than that of bare HO. (EbLUMO=-4.18 eV).
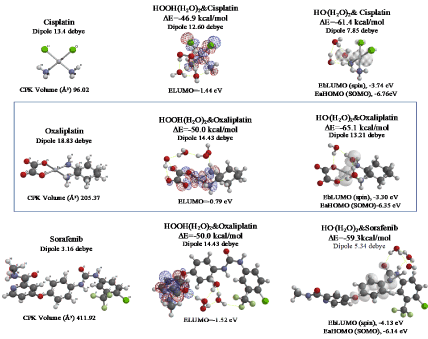
Figure 7. Equilibrium geometry and energy structures of vdW aggregates of cisplatin, oxaliplatin and Sorafenib with HOOH(H2O)2 and HO.(H 2O) 2
Aggregation of Sorafenib with HOOH(H2O)2 and its degradative reaction with HO.(H2O)2 proceeds exothermically, and thus results in large heat of formation. Effective delocalization of spin density with spin potential (EbLUMO(spin)=-4.13 eV) verifies antioxidative effects as an anticancer medicine. The heat of formation of the aggregation and reactions of Sorafenib is summarized together with those of Vitamin C, and thyroxine (T4) of thyroid hormone in Table 1 and Figure S11. To our knowledge, Vitamin C, and thyroxine (T4) are also effective for cancer therapy [9].
Scheme S11. Equilibrium geometry and energy structures of vdW aggregates of cisplatin, oxaliplatin and Sorafenib with HOOH(H2O)2 and HO.(H 2O) 2
As for mt-targeted cancer therapy, DFT/MM verifies importance of protection of oxidative degradation of lipid bimolecular lamellar membranes of mt. As a measure of effectiveness of anticancer medicines, we propose DFT/MM-based heat of formation for aggregations with HOOH(H2O)2 and with HO。(H2O)2. Table 1 summarizes them with total heat of formation and CPK volume of medicines. Effective anticancer medicines must have excellent antioxidative effects on lipid bilayer membranes of mt and the size must be smaller than the lipid size. Otherwise the anticancer medicines cannot go through lipid lamellar membranes of cells and mt. Based on the perspective on cancer and tumor syndrome and pharmacological antioxidative effects, we propose that so-called cancer tissues, i.e. swelling and/or invasion tissues must be filled with mt where lipid bimolecular membranes are oxidatively damaged by HOOH-derived hydroxyl radical (HO.). Further DFT/MM is in progress to validate and predict side-effect free mt-targeted therapy.
- Shozo Yanagida, Kenji Osabe, Takeharu Nagai, Nobuyuki Murakami (2019) Quantum chemistry molecular modeling for longevity: Importance of antioxidative effects in mitochondria as battery of cells. Integr Mol Med 6: 1-10.
- Shozo Yanagida, Akio Kaname, Nobuyuki Murakami (2019) Quantum chemistry-based verification of antioxidative action of iodide in mitochondria. Integr Mol Med 6: 1-6.
- sciencepointmnj.wordpress.com/2016/16/science-point-biology-cell-mitochondria-by-manoj-k-sonkaria/
- https://www.ncc.go.jp/en/ri/division/carcinogenesis_and_prevention/environmental_carcinogenesis/index.html
- Valavanidis A, Vlachogianni T, Fiotakis C (2009) There is experimental evidence that oxidative damage permanently occurs to DNA. J Environ Sci Health C 27: 120-139.
- Shozo Yanagida, Susumu Yanagisawa, Masatoshi Yanagida, Hiroshi Segawa (2018) Validity of density-functional-theory-based molecular modeling for UV/visible spectroscopy and rationale of panchromatic PbI64-(MeNH3+)4-structured molecular solar cells. Jpn J Appl Phys 57: 1602.
- Information from Wikipedia
- Kidani Y, Inagaki K, Iigo M, Hoshi A, Kuretani K (1978) Antitumor activity of -diaminocyclohexaneplatinum complexes against Sarcoma-180 ascites form”. J Med Chem 21: 1315-1318.
- https://lpi.oregonstate.edu/mic/minerals/iodine




















