Background: Primitive neuroectodermal tumors frequently express NeuGlycolylGM3 gangliosides (NeuGcGM3). Racotumomab-alum is a therapeutic vaccine able to induce specific antibody response against NeuGcGM3. The aim of this study was to evaluate safety and immunogenicity of this vaccine in pediatric patients.
Methods: A phase I, single arm, multicenter clinical trial was conducted in four pediatric hospitals in Cuba. Patients with NeuGlycolylGM3 positive neuroblastoma, Ewing’s sarcoma and nefroblastoma were included. Racotumomab-alum vaccine was administered intradermal 0.5 mg, every two weeks during induction phase (5 doses) and monthly reimmunizations after that period. Main objectives of the study were to evaluate safety and immunogenicity. Adverse events were registered in every patient visit and classified according CTCAE version Immunological response was evaluated with antibody titers against racotumomab and NeuGcGM3 before entering the study and every month.
Results: Ten patients were included between November 2016 and May 2018. Most common vaccine-related adverse event were erythema (9.7%), local rash (6.4%) and nasal discharge (6.4%). All of them were graded as mild according CTCAE. No serious adverse event or death were related to vaccine administration. All vaccinated patients developed antibody response against racotumomab, titers ranged from 1:400 to 1:102400. Antibody response against NeuGcGm3 was low, with maximum titers of 1:100 for IgM and 1:50 for IgG.
Conclusions: Racotumomab-alum vaccine is safe and immunogenic in neuroblastoma, Ewing’s sarcoma and nefroblastoma. Further clinical research is needed in order to evaluate clinical efficacy.
Primitive neuroectodermal tumors are a group of malignant neoplasms composed by small round blue cells derived from neural crest. They can appear in any group of age, and there are no differences on incidence according to sex. Early diagnose and properly treatment is related with better overall survival and quality of life [1].
Gangliosides are a family of complex glycosphingolipids involved in key cellular functions. They are considered attractive targets for cancer immunotherapy due to their higher presence in tumoral tissues compared with normal cells. There has been reported ganglioside expression in neuroectodermal tumors, supporting the rational of ganglioside-directed immunotherapy approaches [2-5].
Racotumomab-alum is an anti–idiotype vaccine composed by a murine monoclonal antibody and alum as adjuvant. This vaccine is able to induce an immune response against NeuGcGM3, a tumor associated antigen [6]. Racotumomab-alum vacine has demonstrated anti-tumoral effect and immunogenicity across different tumor types: small-cell lung cancer (SCLC), non-small cell lung cancer (NSCLC), breast cancer, melanoma [7-11]. In NSCLC population there are two clinical trials demonstrating efficacy and safety as switch maintenance therapy. A randomized, placebo-controlled phase II/III trial published by Alfonso et al reported a clinical benefit in terms of overall survival and progression-free survival in 176 advanced non-small cell lung cancer patients [12]. A non-inferiority Phase III clinical trial compared racotumomab versus docetaxel in terms of overall survival in 238 patients no-progressing after first-line oncospecific therapy. Median overall survival in racotumomab group was non-inferior than docetaxel group [13]. In pediatric population, there are reported cases with clinical benefit treated with this vaccine in NGcGM3 expressing tumors [14]. There is a Phase I clinical trial reporting safety and immunogenicity in refractory neuroectodermal tumors treated with racotumomab-alum vaccine [15].
Study design
This was a phase I, single arm, and multicenter clinical trial conducted in four Cuban pediatric hospitals recruiting patients with neurobastoma, nefroblastoma or Ewing’s sarcoma without other treatment option. Ten patients with NeuGcGM3 positive expression in tumoral samples were included. All patients received racotumomab-alum vaccine, 0.5 mg per dose intradermally bi-weekly during induction phase (five doses), followed by monthly reimmunizations until complete one year of treatment. Discontinuation criteria included voluntary withdrawal, worsening of performance status, death, and serious adverse event related to the vaccine, and a delay greater than 7 days during induction phase and 15 during maintenance period between immunizations. Patient who discontinued the study were followed until death or one year after inclusion date. Primary objectives of the study were to evaluate safety and immunogenicity of racotumomab-alum in pediatric patients.
The rational for selection of the dose in this study was supported in the study published by Cacciavillano et al, when they demonstrate the immunogenicity and low toxicity profile of racotumomab at maximum dose of 0.4 mg per dose. Also, there was some evidence from compassionate use in Juan Manuel Marquez pediatric hospital using 0.5 mg per dose with good toxicity profile and clinical response (data not published).
The trial was approved by each hospital Ethics Review Board before submitted to Cuban State Center for Quality Control of Medicines and Medical Devices for authorization. The trial was conducted according to Good Clinical Practice guidelines and principles of Declaration of Helsinki. Written informed consent was obtained from each patient included in the trial. An independent contract research organization (CRO): National coordinating center for Clinical Trials was responsible for conducting the trial under GCP complain. This trial is registered at the Cuban Registry of Clinical Trials (www.registroclinico.sld.cu ID: RPCEC00000206)
Patient’s selection
This trial included patients between 1 and 19 years old, with histological confirmation of NeuGcGM3 expressing neuroblastoma, nefroblastoma or Ewing’s sarcoma, with more than 30 days and less than 60 days to the end of last oncospecific therapy, with performance status ≥ 50% according Karnofsky or Lansky indexes, life expectancy more than 3 months, and adequate renal, hepatic and hematological function.
Patients participating in other clinical trials, with medical history of encephalopathy, seizures, or severe allergy, unbalanced chronic diseases, fever or infections, immunosuppressed or HIV positive, or treated with immunotherapy in last six months, were excluded.
Study assessments
Safety was the primary endpoint of this trial. In every vaccine administration adverse event detected by interrogation, medical examination, and programed laboratory test, were registered during the study period. Adverse events were graded according to Common Terminology Criteria for Adverse Events (NCI-CTCAE version 4.0). Clinical investigators determined causality relation of each adverse event to the vaccine according to causality algorithms.
Immunogenicity was evaluating as secondary endpoint. The antibody response against racotumomab monoclonal antibody and the purified ganglioside NeuGcGM3 was determined by a solid-phase ELISA, as formerly reported. The inverse of the highest serum dilution giving optical density (OD) values ≥ 0.25 and twice the value of the pre-immune serum was considered as the antibody titer. Assays were performed in triplicate for each sample and the coefficient of variation (CV) was less than 15%.
Expression of NeuGCGM3 in paraffin-embedded tumor samples was evaluated by immunohistochemistry before entering the study, using 14F7 Mab (IgG1), a highly specific anti-NeuGcGM3 ganglioside antibody. These evaluations were performed at Research Department of National Institute of Oncology and Radiobiology, using a previously reported technique [16].
Statistical analysis
For the analyses of patient’s characteristics, treatment exposure, adverse events, descriptive methods were used. Hypothesis of the study stated that vaccine treatment will be safe if severe vaccine-related adverse events proportion is under 0.4.
Patient’s characteristics
Between November 2016 and May 2018, 24 patients were evaluated to enter the trial. Fourteen patients were excluded due to death (1), inclusion in other clinical trial (1), tissue sample not available (2), and more than 60 days after the end of specific therapy. Ten NGcGM3 tumor expressing patients were included in the trial. Thera was an inclusion mistake, one patient with negative expression of NGcGM3 that was discontinued after first dose of racotumomab (Figure 1).
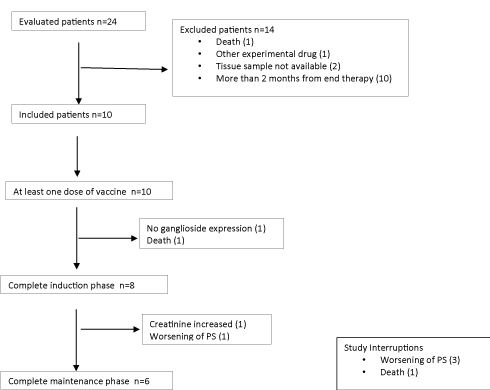
Figure 1. CONSORT Diagram.
Patient’s characteristics are shown in Table 1. Most frequent tumor type was nefroblastoma (40.0%), followed by neuroblastoma and Ewing’s sarcoma (30 % each one). Most prevalent sex was male in 70 % of the patients. Mean age of the included patients was 8 years, with a range from 6 to 12 years. Eighty percent of the included patients did not refer comorbidities, only 2 patients had history of bronchial asthma. There were different disease stages across the study group. In 90% of the patients, a tumor resection was performed, and in 50% this resection was complete. According to objective response after first line oncospecific therapy, 40% of the patients achieved a complete response.
Table 1. Patient’s demographic data.
Regarding treatment adherence, 80 % of the patients received 5 doses of the vaccine or more. Two patients did not complete induction phase, one of the discontinued the trial for negative expression of NeuGcGM3 (inclusion mistake), and other patient due to worsening of performance status. In 60 % of the patients treatment time was between 6 and 18 months (Table 2).
Table 2. Treatment adherence.
Safety
Racotumomab-related adverse events are shown in Table 3. Ten patients that received at least one dose of the vaccine, were included in safety analysis. Thirty-one adverse events were reported, 21 types of events in nine patients (90%). Only 13 adverse events were classified as related to vaccine by clinicians. Most frequent vaccine related adverse events were erythema (9.7%), local rash (6.4%) and nasal discharge (6.4%) pri. All of them were graded as mild according to NCI-CTCAE.
Table 3. Frequency of vaccine-related adverse events.
Seven serious adverse events were reported in three patients during the study, none of them were related to racotumomab. These events were: respiratory failure (2), pleural effusion (1), dyspnea (1), radiculitis (1), paraparesia (1) and atrophy of left lower limb (1). There was three deaths reported during the study period, two of them as a result of serious adverse events (respiratory failure) and the third due to progression of the disease. None of them were related to the study treatment.
There were not serious adverse events related to vaccine, we can estimate an upper level of toxicity of 36.9% with 99% of CI, therefore the study hypothesis was confirmed, racotumomab vaccine is safe because toxicity is under 40%.
Immunological response
Antibody response of IgG isotype against racotumomab was evaluated in ten patients with pre and post–immune sera. All vaccinated patients developed antibody response against racotumomab. High antibody titers were obtained, ranged from 1:400 to 1:102400 (Figure 2). Antibody response against NGcGM3 was evaluated in eight patients with pre and post-immune blood samples. This antibody response was low, with maximum titers of 1:100 for IgM isotype and 1:50 for IgG isotype (Figure 3).
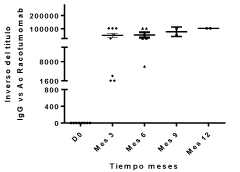
Figure 2. Antibody response against racotumomab.
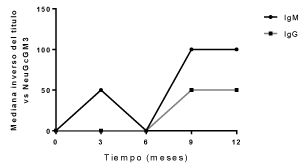
Figure 3. Antibody response against NeuGCGM3.
In this phase I study in NeuGcGM3 expressing pediatric tumors we demonstrate that racotumomab-alum vaccine is safe and able to induce an immunological response. This is our first approach to treat pediatric patients with anti-ganglioside-based immunotherapy. Other drugs against gangliosides have been successfully used in pediatric population. Dinutuximab is a chimeric monoclonal antibody that causes Antibody dependent cell cytotoxicity (ADCC) and complement-dependent cytotoxicity (CDC) by binding to GD2, a tumor associated antigen. This drug demonstrated a benefit in 2-years event free survival for postconsolidation treatment of high-risk neuroblastoma. There has been reported grade 3=4 adverse events in 10- 20 % of the treated patients. GD2 is present in melanocytes and peripheral nervous system, in contrast with NeuGcGM3 that is not expressed in normal cells [17].
Cacciavillano et al., published a phase I study with racotumomab in neuroblastoma and other refractory malignancies in pediatric population. They evaluated three level of doses and confirmed safety and immunogenicity of the vaccine. Most common local effects were erythema, induration, and local pain. No serious adverse events were related with the study treatment. No dose-limiting toxicity was found. High antibody titers against racotumomab were found in 85% of evaluable patients, and 9 of these patients elicited anti neuGcGM3 antibodies.
In our study antibody response against NeuGcGM3 was inferior to previous reported data in adult patients. Maybe some aspects such as tumor type, clinical stage, vaccine dose, concurrent specific therapies, can affect NeuGcGM3 specific antibodies. Further research is needed on this topic in pediatric population.
A phase II clinical trial with racotumomab combined with metronomic chemotherapy in high-risk neuroblastoma patients is ongoing in Argentina. Main objective of the study is to evaluate immune response developed in vaccinated patients with one year treatment. Secondary objectives are evaluating the effect in minimally disseminated disease at bone narrow, and to describe vaccine related-adverse events [18].
Even when racotumomab does not have yet evidence of efficacy in pediatric population, its safety profile is very nice, without serious adverse events related to the treatment. This favorable safety profile allows to combine racotumomab with other therapeutics weapons and keep its administration during a longer period than chemotherapy. Next steps in clinical development of racotumomab-alum vaccine in pediatric patients would confirm efficacy data in NeuGcGM3 expressing tumors.
- 1. Terrier P, Llombart-Bosch A, Contesso G (1996) Small round blue cell tumors: Prognostic factors correlated to Ewing’s sarcoma and neuroectodermal tumors. Semin Diagn Pathol 13: 250-257. [Crossref]
- 2. Fernandez LE, Gabri MR, Guthmann MD, Gomez RE, Gold S, et al. (2010) NGcGM3 ganglioside: A privileged target for cancer vaccines. Clin Dev Immunol 2010: 814397. [Crossref]
- 3. Irie A, Suzuki A (1998) CMP-N-acetylneuraminic acid hydrolase is exclusively inactive in humans. Biochem Biophys Res Comun 248: 330–333. [Crossref]
- 4. Scursoni AM, Galluzzo L, Camarero S, Lopez J, Lubieniecki F, et al. (2011) Detection of N-glycolyl GM3 ganglioside inneuroectodermal tumors by immunohistochemistry: An attractive vaccine target for aggressive pediatric cancer. Clin Dev Immunol 2011: 245181. [Crossref]
- 5. Scursoni AM, Galluzzo L, Camarero S, Pozzo N, GabriMR, et al. (2010) Detection and characterization of N-glycolyated gangliosides in Wilms tumor by immunohistochemistry. Pediatr Dev Pathol 13: 18–23. [Crossref]
- 6. Hernandez AM, Vazquez AM (2015) Racotumomab-alum vaccine for the treatment of non-small-cell lung
- cancer. Exp Rev Vaccines 14: 9–20. [Crossref]
- 7. Neninger E, Diaz RM, de la Torre A, Rives R, Diaz A, et al. (2007) Active immunotherapy with 1E10 anti-idiotype vaccine inpatients with small cell lung cancer: Report of a phase I trial. Cancer Biol Ther 6: 145–150. [Crossref]
- 8. Alfonso S, Diaz RM, de la Torre A, Santiesteban E, Aguirre F, et al. (2007) 1E10 anti-idiotype vaccine in non-small cell lung cancer: Experience in stage IIIb/IVpatients. Cancer Biol Ther 6: 1847–1852. [Crossref]
- 9. Diaz A, Alfonso M, Alonso R, Saurez G, Troche M, et al. (2003) Immune responses in breast cancer patients immunized with an anti-idiotype antibody mimicking NeuGc-containing gangliosides. Clin Immunol 107: 80–89. [Crossref]
- 10. Guthmann MD, Castro MA, Cinat G, Venier C, Koliren L, et al. (2006) Cellular and humoral immune response to N Glycolyl GM3 elicited by prolonged immunotherapy with an anti idiotypic vaccine in high risk and metastatic breast cancer patients. J Immunother 29: 215-223. [Crossref]
- 11. Alfonso M, Diaz A, Hernandez AM, Perez A, Rodriguez E, et al. (2002) An anti-idiotype vaccine elicits a specific response to N-glycolyl sialicacid residues of glycoconjugates in melanoma patients. J Immunol 168: 2523-2529. [Crossref]
- 12. Alfonso S, Valdes A, Santiesteban R (2014) A Randomized, Multicenter, Placebo-Controlled Clinical Trial of Racotumomab-Alum Vaccine as Switch Maintenance Therapy in Advanced Non-Small Cell Lung Cancer Patients. Clin Cancer Res 20: 3660-3671. [Crossref]
- 13. Hernandez M, Neninger E, Ortiz RA, Camacho K, Amador RM, et al. (2016) Switch maintenance therapy with racotumomab or nimotuzumab vs docetaxel for NSCLC patients. Ann Oncol 27: S1093P.
- 14. Sampor C, GuthmannMD, Scursoni A, CacciavillanoW, Torbidoni A, et al. (2012) Immune response to racotumomab in a child with relapsed neuroblastoma. Front Oncol 2: 195. [Crossref]
- 15. Cacciavillano W, Sampor C, Venier C, Gabri M, Mar a T.G. de D avila, et al. (2015) A Phase I Study of the Anti-Idiotype Vaccine Racotumomab in Neuroblastoma and Other Pediatric Refractory Malignancies. Pediatr Blood Cancer Wiley Periodicals, 62: 2120-2124. [Crossref]
- 16. Blanco R, Quintana Y, Blanco D, Cedeño M, Rengifo CE, et al. (2013) Tissue Reactivity of the 14F7Mab Raised against N-Glycolyl GM3 Ganglioside in Tumors of Neuroectodermal, Mesodermal, and Epithelial Origin. J Biomark 2013: 602417. [Crossref]
- 17. Ploessl C, Pan A, Maples KT, Lowe DK (2016) Dinutuximab: An Anti-GD2 Monoclonal Antibody for High-Risk Neuroblastoma. Ann Pharmacother 50: 416-422. [Crossref]
- 18. Racotumomab in patients with high-risk neuroblastoma. ClinicalTrials.gov Identifier NCT02998983.



