Provision of enteral nutrition is an essential component of care for the critically ill patient. Feeding tubes provide the only option for enteral feeding in many cases. The placement of feeding tubes is not without risk but is a routine procedure undertaken in the clinical setting. These cases demonstrate imaging interpretation difficulties and pitfalls and surgical solutions to aberrantly placed nasogastric, percutaneous and radiological tubes.
Providing enteral nutrition can increase functional and nutritional status and improve survival and quality of life [1-4]. Gastrostomy tubes are placed for patients with reduced level of consciousness or cognition, neurological conditions, gastrointestinal obstruction, burns, malnourishment in cancer or short-bowel syndrome [5]. Temporary enteric access and feeding can be achieved with a nasogastric tube (NGT) however, they have a high failure rate owing to dislodgement or clogging. NGT placement is an unreliable medium to long-term solution for providing nutrition, medications and fluids [6]. Gaining enteral access is a routine and typically safe procedure whether it is achieved by placement of an NGT, percutaneous radiological gastrostomy (PRG), percutaneous endoscopic gastrostomy (PEG) or a surgical gastrostomy. Three cases are presented where complications have arisen with NGT, PRG and PEG insertion and a surgical intervention was required as a remedy.
A 68-year-old female with a history of hypothyroidism, hypertension, appendicectomy and lichen sclerosis presented for maxillo-facial surgery for a malignant, invasive, well to moderately differentiated submandibular, keratinising squamous cell carcinoma. The pre-operative computerised tomography (CT) scan reported a 15mm lesion with invasion into the adjacent mandible with loss of overlying medial/inner cortex extending to a depth of 10mm. There was right level II and level III cervical lymphadenopathy noted.
The patient underwent a right segmental mandibulectomy, ipsilateral neck dissection, removal of teeth, tracheostomy and a fibula free flap. Resection of the tumour was done with 12mm macroscopic margins into soft tissue. The reconstruction utilised 2 veins and an artery with the fibula graft of the right leg. Primary closure with split thickness skin graft from the right thigh. A NGT was placed intraoperatively, towards the end of the case.
The intubation was difficult. A plan was made for nasal intubation however, the nasal tube was unable to be passed through either nostril. A size 6 tube was placed orally with a C-MAC. The percentage of glottic opening (POGO) was estimated at size 3. A D-blade was used with 60% POGO with bougie.
After surgery, an erect chest X-ray was taken (Figure 1A) and later, a mobile chest X-ray (Figure1B). The NGT was seen below the left hemidiaphragm, and feeds were started. The following day, the patient was noted to be febrile, a trend that continued for several days, with an associated vasopressor requirement and increasing oxygen demand. Blood tests showed an escalation in C-reactive protein (from the 3rd day post-operation to 6th the CRP was 359, 530, 624, 635 mg/L). The patient developed an acute kidney injury. A CT-neck showed no drainable collection and that the NGT deviated left laterally (Figure 1C-E). Ooze from the tracheostomy site became evident 4 days after surgery. A CT abdomen and pelvis showed a large volume of intra-abdominal free fluid (Figure 1F). The fluid demonstrated >35-40HU density. There was a perforation of the distal oesophagus with the feeding tube traversing through the anterior oesophageal wall and terminating in the abdomen.
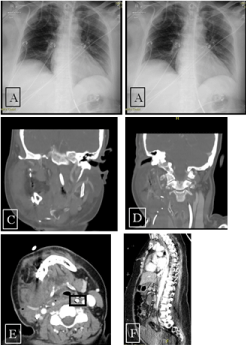
Figure 1. Erect chest radiograph. NGT projecting over the stomach. Figure 1B. Mobile AP chest radiograph. The NGT passes left of the midline with tip terminating 70mm below level of the diaphragm. Figure 1C, 1D and 1E. CT Neck: NGT with deviation to the left in oropharynx. Figure 1F. Feeding tube appears to perforate at the distal oesophagus then terminate in the upper abdomen. Large volume intra-abdominal free fluid
Five days after the maxillo-facial surgery was performed, a gastroscopy, flexible naso-endoscopy (FNE), laparotomy and formation of gastrostomy was performed. The findings included approximately 3L of feed/fluid in peritoneal cavity. The NGT appeared to have perforated at pharyngeal level and coursed via the mediastinum to abdominal cavity. The gastroscopy was normal with no evidence of the NGT in the gastrointestinal tract. The FNE was unable to visualise the point of perforation. Laparotomy facilitated removal of the tube, lavage and placement of a gastrostomy. Seven days later, a 300mL left upper quadrant residual collection was drained percutaneously.
A 38-year-old male sustained a gunshot blast to the face. Over subsequent days he underwent debridement of devitalised tissue, right eye enucleation, facial bone reconstruction, tracheostomy, craniectomy, reconstruction of the skull base, and fascial free flap.
To facilitate nutrition, radiological insertion of gastrostomy tube was performed. The procedure utilised an 18Fr gastrostomy tube over the wire through a 22Fr peel-away sheath with the aid of a 7mmx40mm angioplasty balloon. The procedure failed after 2 attempts. It was thought that overlying omentum was impeding placement of the tube. A 12Fr biliary drain placement was attempted, peritoneal contrast extravasation was detected, and the procedure abandoned. A pigtail drain remained.
Laparoscopy was performed. The pigtail drain was removed, and 4 stomach wall defects were noted (Figure 2). There was minimal contamination. Gastric acid bubbles were detected on the surface of the liver and in the abdominal cavity. Three of the defects were repaired with 3-0 vicryl and another defect was enlarged for insertion of a 24Fr Stamm gastrostomy tube. Water was injected into the balloon, a vicryl purse string deployed at the stomach surface and the tube was delivered to the skin.
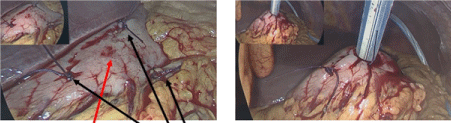
Figure 2. Defects in stomach wall encountered after unsuccessful radiological placement of PEG feeding tube repaired. The black arrow shows 3 repaired stomach wall defects. The red arrow show the stomach wall defect selected for placement of Ped tube entry. The Stamm feeding tube placed in widened stomach wall defect before delivery onto skin surface
A 63-year-old male with a medical history including gastric banding, Parkinson’s disease with severe oropharyngeal dysphagia, dementia, GORD, hiatal hernia, obstructive sleep apnoea and appendicectomy presented with a fever of unknown origin. The diagnosis was thought likely due to aspiration pneumonia based on chest X-ray findings and the history of dysphagia.
Gastroscopy showed a dilated and oedematous oesophagus. There was no transillumination at the stomach. A PEG was inserted after the second passage of the canula. Passage of the canula aspirated gas without the tip in the stomach. It was thought there was likely perforation into the colon. Intravenous antibiotics were initiated. The PEG insertion was interpreted as successful and PEG feeds were started.
Later, on the same day, the patient deteriorated. Abdominal examination was consistent with acute peritonitis, the patient became hypotensive and tachycardic. CT abdomen and pelvis demonstrated the pre-tube catheter within the anterior abdominal wall extending into the intra-abdominal space however not clearly appreciated to extend into the stomach lumen (Figure 3). There was a large volume of free fluid and gas within the peritoneal space and concern for leak into the peritoneal space.
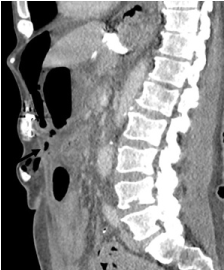
Figure 3. CT abdomen. Large volume free fluid and gas and PEG tube catheter not seen entering the gastric lumen. The arrow shows the tract created by dislodgment of the tube and the remainder of the tube in the abdominal wall
An exploratory laparotomy with removal of PEG tube, repair of gastric defect and insertion of feeding jejunostomy was performed. The findings included a dislodged PEG tube situated in subcutaneous plane, a defect in gastric body within lesser sac and 2.5L of free fluid containing feeds. The procedure involved repair of the defect in the stomach through the lesser sac, shortening of the gastric band and lavage.
Long-term use of a nasogastric tube for feeding when passage of food along the gastrointestinal tract is compromised, leads to a range of complications including lesions of the nasal wing, chronic sinusitis, GORD and aspiration pneumonia. Insertion of a PEG tube is superior [7] and although the use of enteral feeds in people with advanced dementia is common, a risk to benefit estimate is not available [8]. The range of options for placement of percutaneous feeding tubes includes radiologic, endoscopic and surgical methods. PEG tube insertion is generally considered to be a safe and effective procedure. Available evidence to recommend endoscopic over radiologic insertion of tube is yet to be established [9-11] and surgical placement can typically only be justified when it is placed during another procedure or after failure of endoscopic or radiologic attempts [12]. There is little difference in the rate of complications when a laparoscopic or open method is used when surgery is required [13]. These 3 cases demonstrate rare complications of the three commonly utilised techniques for placing enteral feeding tubes in critically ill patients.
No funding was sought to support the study
The authors declare no conflict of interest
- Cowen ME, Simpson SL, Vettese TE (1997) Survival estimates for patients with abnormal swallowing studies. J Gen Intern Med 12: 88-94. [Crossref]
- Kobayashi K, Cooper GS, Chak A, Sivak MV, Jr., Wong RC (2002) A prospective evaluation of outcome in patients referred for peg placement. Gastrointest Endosc 55: 500-506. [Crossref]
- Meisel K, Arnold RM, Stijacic Cenzer I, Boscardin J, Smith AK (2017). Survival, functional status, and eating ability after percutaneous endoscopic gastrostomy tube placement for acute stroke. J Am Geriatr Soc 65: 1848-1852. [Crossref]
- Taylor CA, Larson DE, Ballard DJ, Bergstrom LR, Silverstein MD, et al. (1992) Predictors of outcome after percutaneous endoscopic gastrostomy: A community-based study. Mayo Clin Proc 67: 1042-1049. [Crossref]
- Kurien M, McAlindon ME, Westaby D, Sanders DS (2010) Percutaneous endoscopic gastrostomy (peg) feeding. BMJ 340: c2414.
- Park RH, Allison MC, Lang J, Spence E, Morris AJ, et al. (1992) Randomised comparison of percutaneous endoscopic gastrostomy and nasogastric tube feeding in patients with persisting neurological dysphagia. BMJ 304: 1406-1409. [Crossref]
- Gomes CA Jr, Andriolo RB, Bennett C, Lustosa SA, Matos D, et al. (2015) Percutaneous endoscopic gastrostomy versus nasogastric tube feeding for adults with swallowing disturbances. Cochrane Database Syst Rev. 2015: CD008096. [Crossref]
- Sampson EL, Candy B, Jones L (2009) Enteral tube feeding for older people with advanced dementia. Cochrane Database Syst Rev 2009: CD007209. [Crossref]
- Park SK, Kim JY, Koh SJ, Lee YJ, Jang HJ, et al. (2019) Complications of percutaneous endoscopic and radiologic gastrostomy tube insertion: A kasid (korean association for the study of intestinal diseases) study. Surg Endosc 33: 750-756. [Crossref]
- Silas AM, Pearce LF, Lestina LS, Grove MR, Tosteson A, et al. 2005. Percutaneous radiologic gastrostomy versus percutaneous endoscopic gastrostomy: A comparison of indications, complications and outcomes in 370 patients. Eur J Radiol 56: 84-90. [Crossref]
- Yuan Y, Zhao Y, Xie T, Hu Y (2016) Percutaneous endoscopic gastrostomy versus percutaneous radiological gastrostomy for swallowing disturbances. Cochrane Database Syst Rev 2: CD009198. [Crossref]
- Barkmeier JM, Trerotola SO, Wiebke EA, Sherman S, Harris VJ, et al. (1998) Percutaneous radiologic, surgical endoscopic, and percutaneous endoscopic gastrostomy/gastrojejunostomy: Comparative study and cost analysis. Cardiovasc Intervent Radiol 21: 324-328. [Crossref]
- Bankhead RR, Fisher CA, Rolandelli RH (2005) Gastrostomy tube placement outcomes: Comparison of surgical, endoscopic, and laparoscopic methods. Nutr Clin Pract 20: 607-612. [Crossref]



