Oxidative stress and free radical production is an etiology to some neurodegenerative diseases which may be preventable by prior neuronal protection using herbs. Rauwolfia vomitoria and Gongronema latifolium are medicinal herbs with antioxidant, anti-diabetic and analgesic properties among others. While R. vomitoria acts as a brain stimulant, as well as a depressant, neurotoxic effects have also been reported, which G. latifolium has shown the potential to mitigate. This study therefore investigated the effects of the combination of R. vomitoria and G. latifolium on young rats’ cerebellar cortex. Twenty young male Wistar rats (100-150 g) were divided equally into 4 groups (n=5). Oral doses of the vehicle (Tween 20™), ethanolic extracts of 200 mg/kg of R. vomitoria (RV), 200 mg/kg of G. latifolium (GL), and the combination of both (RV + GL) were given to the animals for 14 days. On day 15, the animals were sacrificed after ketamine sedation and perfusion-fixed with 10% buffered-formalin. The cerebella were excised and processed for histomorphology by silver impregnation technique and immunolabelled with anti- neuron specific enolase (NSE) and glial fibrillary acidic protein (GFAP). The histology results showed atrophied Purkinje and other neurons with increased NSE and GFAP expressions in the RV group, which were not as such in the GL and RV+GL groups, an indication of cerebellar cortical injury. In conclusion, RV was injurious to the cerebellar cortical neurons and also stimulated NSE and GFAP, but these RV-induced traumas were slightly mitigated with GL combination. This preliminary report of RV+GL combination may be considered an alternative to RV single treatment for better disease management and brain protection.
R. vomitoria, G. latifolium, cerebellar cortex, silver impregnation, neuron specific enolase, glial fibrillary acidic protein
Oxidative stress and free radical production is one of environment-induced etiology of some neurodegenerative diseases [1-3]. However, this does not rule out the hereditary aspect of some of these neurodegenerative diseases [4]. There are reports that degenerative diseases such as Alzheimer’s, Parkinson and other forms of dementia related diseases may be preventable if there is prior neuronal protection using herbs and other food types [5,6].
In Africa, the abundance of herbs and herbal products has given a promising future towards the prevention of such disease as related to neuronal degeneration. The use of such plant as Rauwolfia vomitoria and Gongronema latifolium has been on the increase due to their known medicinal values. R. vomitoria and G. latifolium show promises as antioxidants, anti-diabetics and analgesics among others [7-11].
R. vomitoria (RV), a shrub of the family Apocynaceae is commonly called serpent wood or swizzler stick and is used locally in the treatment against snake bites, fever and some nervous disorders [12]. The root bark is extensively used, and this is reported to contain alkaloids such as; ajmaline, ajmalicine, reserpine, serpentine, serpentinine, yohimbine, among others [13-15]. It is reported that RV is effective as an analgesic, anticonvulsant, and antipsychotic among others [10,16,17]. Ekong et al. [18] on the other hand reported no adverse effect on behavioural and biochemical parameters; however, adverse effects of this plant have been reported. It causes depression and Parkinsonia syndrome [19], impede motor activity behaviour, and stimulates neurodegenerative features in the cerebellum and cerebral cortical cyto-architectures [20-23]. In foetal tissues, it is reported to be hepatotoxic, cardiotoxic, as well as stimulation hypertrophy and hyperplasia of osteoblasts and osteoclasts in the femur [24-26].
Though this plant has very useful functions, its adverse effects tend to overshadow such. The potentials of this herb in combination with other viable herbs have been postulated as having better beneficial effect on the nervous system [21-23]. The benefits of this combination may be explored for health management including the prevention against neurodegenerative diseases. This study therefore explored the combination of RV with GL on the neurons of the cerebellum.
G. latifolium (GL), a climbing perennial plant of the family Asclepiadaceae, is commonly called amaranth globe or bush buck, and has both medicinal and nutritional values [27]. Its leaf is extensively used, and is reported to contain alkaloids, saponins, tannins, flavonoids and glycosides [28]. GL is reported to have hypoglycemic, anti-bacterial, antioxidant, anti-inflammatory, anti-ulcer, analgesic, anti-diabetic, anti-pyretic and cardio-protective properties [8,28-34]. These useful properties endeared the choice for its combination with RV.
It is reported that RV acts as a brain stimulant, as well as a depressant, with neurotoxic effects also reported [35,36]. GL has shown the potential to modulate some of these effects of RV [21,22]. This study therefore investigated the protective effects of the combination of RV and GL on young rats’ cerebellum.
Twenty young male Wistar rats of body weight 100-150 g were obtained from the animal facility of the Faculty of Basic Medical Sciences of the institution. The animals were randomly assigned into 4 groups (1, 2, 3 and 4) of five (5) animals each, and were allowed to acclimatize for one week before the start of the experiment. The animals were allowed 12 hours dark and light cycles, and were handled according to the guidelines for animal care by the National Institute of Health of the United States of America.
RV and GL plants were obtained from local farms in Esit Eket and Ikono, respectively, all in Akwa Ibom State, Nigeria. The roots and leaves of RV and GL respectively, were washed off dirt and the cambium teased out exposing the phloem which was subsequently excised. The phloem of the RV and the leaves of GL were air dried for one week, and were grounded into fine powder.
Both plants parts were extracted by Soxhlet method using 70-80 % alcohol. Upon complete extraction, the alcohol was completely evaporated using a steam bath, and the extracts were stored at 4 oC. The actual dose of each extracts was re-constituted with Tween 20™.
Experimental design
Group 1 rats were the control and were given the vehicle, Tween 20™ (0.5 ml). Groups 2-4 were the test groups and were administered either 200 mg/kg ethanolic extract of RV, 200 mg/kg ethanolic extract of GL or the combination of the ethanolic extracts of both RV and GL (RV + GL) respectively. All administration was orally and lasted for 14 days.
On day 15, the animals were sacrificed after ketamine hydrochlode anaesthesia. Phosphate base saline (PBS) was transcardially perfuse to eliminate blood, and thereafter perfuse-fixed with 10% neutral buffered-formalin. The whole brains were excised and preserved for 48 hours, and the cerebellum was excised and processed for histomorphology study using silver impregnation technique, and also immunolabelled with neuron specific enolase and glial fibrillary acidic protein, antibodies.
Statistical analysis
One way analysis of variance (ANOVA) was used to compare the means for the cellular density, thereafter the post-hoc test using Dunnett's multiple comparison test was carried out to find the level of significance at p < 0.05. All the results were expressed as mean + standard error of mean.
Silver impregnation
The section of the cerebellar cortex of the control group consists of three layers having dark-brown stained neurons. From the outside inwards it includes: molecular, Purkinje and granular layers. The molecular layers contained sparse small size neurons unequally distributed within the layer. The Purkinje layer had a single layer of large Purkinje cells, while the granular layer contained a dense population of small-size granule cells with intervening glomeruli (Figure 1a).
The cerebellar cortex of the RV group showed atrophic Purkinje cells, with no other apparent histopathology compared with the control (Figure 1b). The cerebellar cortex of the GL group showed no obvious histopathology compared with the control group (Figure 1c). The cerebellar cortex of the RV+GL group also showed no obvious histopathology compared with the control group (Figure 1d).
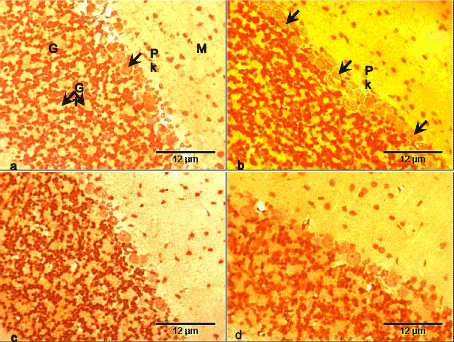
Figure 1. Photomicrographs of cerebellar cortex of the control and test groups (Bielschowsky silver impregnation, ×400):
- The cerebellar cortex of the control group consists of three layers: molecular (M) with sparse small size neurons, Purkinje with large Purkinje (Pk) neurons, and granular (G) layer with dense small size neurons and intervening glomeruli (Gl).
- The cerebellar cortex of the RV group showed atrophic Purkinje neurons (arrows).
- The cerebellar cortex of the GL group did not show obvious histopathology.
- The cerebellar cortex of the RV+GL group did not show obvious histopathology.
There was no difference in the cerebellar cortical density in all the test groups compared with the control group (Figure 2). There was significantly (p < 0.05) less average cerebellar cortical cell size in all the test groups compared with the control group (Figure 3). However, there was no difference in cell population and size among the test groups.
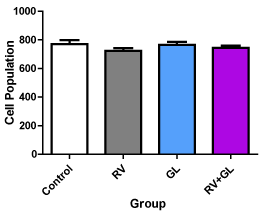
Figure 2. Average cell population of the cerebellar cortex in the experimental groups Data are presented with mean ± standard error of mean
(n=5, F = 1.064, p = 0.3920, RV = R. vomitoria, GL = G. latifolium) There is no significant difference between the test groups compared with the control group and among the test groups
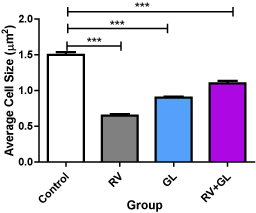
Figure 3. Average cell sizes of the cerebellar cortex in the experimental groups Data are presented with mean ± standard error of mean
(n=5, F = 166.4, p = 0.0001, RV = R. vomitoria, GL = G. latifolium) ***RV group is significantly (p < 0.001) lower than the control group There is no significant difference among the test groups
Neuron Specific Enolase (NSE)
NSE was expressed in the control group cerebellar cortex and was more pronounced in the Purkinje cell bodies (Figure 4a). There was increased expression of NSE in the Purkinje cell bodies of the RV group. The granule cells and some cells in the molecular layers also expressed NSE compared with the control group (Figure 4b). In the GL group, NSE was expressed mostly in the Purkinje cells, as well as the granule cells and cells in the molecular layer. There appear to be no difference in expression between this group and the control group (Figure 4c). There was slightly more expression of NSE especially in the Purkinje cell bodies of RV+GL group, while the granule cells and cells of the molecular layer also had NSE expression compared with the control group (Figure 4d).
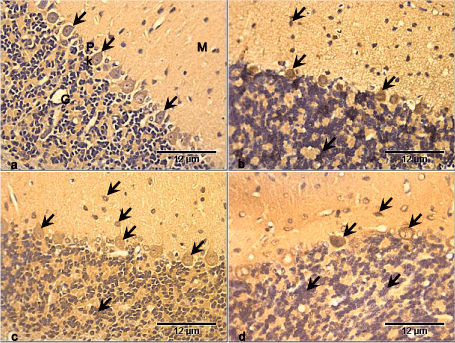
Figure 4. Photomicrographs of cerebellar cortex of the control and test groups (NSE ×400):
- NSE is expressed in the control group cerebellar cortex and is more pronounced in the Purkinje cell bodies (arrows).
- There is increased expression of NSE in the Purkinje cell bodies, granule cells and some cells in the molecular layers (arrows) compared with the control group.
- NSE is expressed mostly in the Purkinje cells, with slight expression in the granule cells and cells in the molecular layer (arrows), though there is little difference in expression compared with the control group.
- NSE is slightly more expressed especially in the Purkinje cell bodies. The granule cells and cells of the molecular layer (arrows) also expressed NSE compared with the control group.
There was significantly (p < 0.05) higher population of NSE expressed neurons in the cerebellar cortices in all the test groups compared with the control group. However, no difference was observed in NSE expressed neuronal population among the test groups (Figure 5).
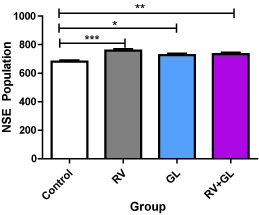
Figure 5. NSE labeled cell population of the cerebellar cortex in the experimental groups Data are presented with mean ± standard error of mean
(n=5, F = 9.511, p = 0.0008, RV = R. vomitoria, GL = G. latifolium)
*RV group is significantly (p < 0.05) lower than the control group
**RV group is significantly (p < 0.01) lower than the control group
***RV group is significantly (p < 0.001) lower than the control group
There is no significant difference among the test groups
Glial fibrillary acidic protein (GFAP)
GFAP was expressed in the cerebellar cortex of the control group. Astrocytic processes were mostly expressed in the molecular layer, with the cell bodies and processes being expressed in the granular layer (Figure 6a). There was increased GFAP expression in the RV group. Although the processes were mostly expressed in the molecular layer, the granular had more cell bodies expressing GFAP compared with control group (Figure 6b).
GFAP was expressed in the GL group mostly in the granular layer, while the molecular layer had expression in the astrocytic processes. However there appear to be no difference with the control group (Figure 6c). There was GFAP expression in the RV+GL group mostly in the granular layer, while the molecular layer had expression of the astrocytic processes. However there appear to be no difference with the control group (Figure 6d).
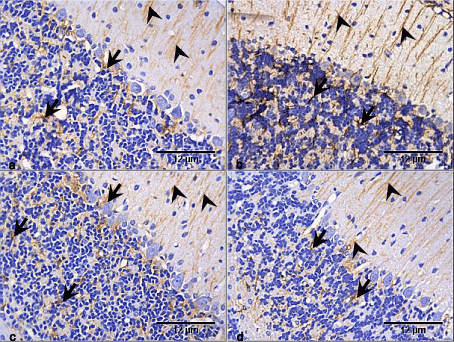
Figure 6. Photomicrographs of cerebellar cortex of the control and test groups (GFAP ×400):
- There is expression of GFAP in the cerebellar cortex of the control group with astrocytic processes (arrow heads) mostly expressed in the molecular layer, while the cell bodies (arrows) were and processes were expressed in the granular and medullary layers.
- There is increased GFAP expression in the RV group, though the processes (arrow heads) were mostly expressed in the molecular layer, the granular had more cell bodies (arrows) expressing the GFAP compared with the control group.
- GFAP is expressed in the GL group mostly in the granular layer (arrows). In the molecular, the expression is only in the astrocytic processes (arrow heads). However there appear to be no difference with the control group.
- GFAP is expressed in the RV+GL group mostly in the granular layer (arrows). The molecular layer had expression of the astrocytic processes (arrow heads), with no difference with the control group
There was significantly (p < 0.05) lower population of GFAP expressed astrocytes in the cerebellar cortex of the RV + GL group compared with the control group. However, no difference was observed in GFAP expressed astocytes population in the other test groups compared with the control group, and among the test groups (Figure 7).
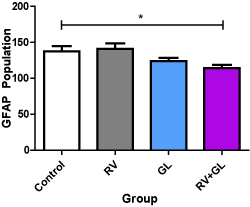
Figure 7. GFAP labeled astrocytic population of the cerebellar cortex in the experimental groups Data are presented with mean ± standard error of mean
(n=5, F = 4.208, p = 0.0225, RV = R. vomitoria, GL = G. latifolium)
*RV group is significantly (p < 0.05) lower than the control group
There is no other significant difference with the control and among the test groups
Silver impregnation is a technique that demonstrates neuronal cell bodies and processes, with little or no interference with glial cells [37,38]. It is reported to provide morphology insights into neuronal structure to detect damage or degeneration [39,40]. In the present study, the silver impregnation results showed atrophic Purkinje cells in the cerebellar cortex of the RV group, while there were slight atrophy also in the GL and RV+GL groups.
Cellular atrophy is a signal for degeneration and is known to occur when there is disruption of trophic signals to cells among other causes [41]. Some constituents of RV’s have been reported to disrupt monoamines signals in the brain [35,36,42-44], thus, disrupting their roles in arousal, emotion and cognition [45]. The disruption of these roles may form a basis for neuronal degeneration, which atrophy may be one. The present result corroborates previous reports on the toxicity of RV. RV has been reported to cause cellular damage to the cerebellum and other brain regions [20,22], although its mechanism of action is not known.
The GL group also presented slight atrophy, an indication that GL extract at the given dose may lead to cerebellar tissue trauma. But cell atrophy may be an adaptive mechanism to cope with trauma, and may not be pathological. GL has been reported with beneficial role in different body tissues [46,47], and these beneficial effect are usually physiological without associated tissue morphology study. However, Ekong et al. [22,23] reported that GL altered neurons of the cerebellum, which the present study is in line with. Slight cellular atrophy was also observed in the cerebellar cortex of the RV+GL group, also indicating trauma to this brain tissues. Ekong et al. [22] also reported similar changes with the combination, indicating that although GL may have an antagonistic effect on RV, the dosage under study may not be sufficient to prevent RV toxic effect. The present RV+GL result is also in line with the works of Ekong et al. [21] and Ekong et al. [23].
Anti-NSE which labels the cell cytoplasm and dendrites of neurons was used to study the state of the neurons. The results showed increased (p < 0.05) NSE expression in the RV, GL and RV+GL groups. NSE is a cytosolic protein that functions as brain-specific glycolytic enzyme, and plays an important role in intracellular energy metabolism [48]. It is expressed by mature neurons and cells of neuronal origin, and thus regarded as a marker of the neuronal state [49,50], but becomes markedly expressed after brain injury [51]. It is reported that RV cause neuronal injury [20,22], and this may be a reason for the marked expression of the enolase, which may be deleterious to the normal function of the cerebellar cortical cells. On the other hand, increased NSE expression in the GL and RV+GL groups, indicate injury to the neurons which the combination was unable to ameliorate successfully.
Some glial cells under certain condition also express NSE [52,53]. As it was important to rule out NSE expression by these glial cells, anti-GFAP was also studied as well. The results showed slight increased GFAP expression in the RV group, with no difference in the GL group, but lower expression (p < 0.05) in the RV+GL group. Increased GFAP expression is an indication of the up-regulation of this protein, which usually occurs when brain tissues undergo injury or at diseased state [54]. Slight increased expression of GFAP, which is an intermediate filament protein of astrocytes and ependymal cells [55-57], is indicative of the traumatic effect of RV. Marked GFAP expression is also indicative of reactive astrogliosis [58], which may be detrimental on the long run as it usually underlie neural dysfunction and pathology in certain neurological disease states [59]. GFAP expression however, appeared unaffected in the GL and the RV+GL groups, which supports previous parameter results of the present study.
RV has been reported to induce a wide range of physiological, biochemical and structural alteration in the brain [10,20,22], and its sole use by locals in psychiatry management pose a threat to the normal function of the cerebellar cortex. While GL is reported with little or no adverse effect on neural tissues, its combination has been reported to ameliorate RV-induced injury [21], which the present corroborates.
Decreased GFAP expression is invariably associated with detrimental conditions in the central nervous system [60], and could result from cytoskeletal destabilization or degradation and loss of GFAP antigenicity [61]. How-ever, it is reported that apparent decreased GFAP content reflects a decrease in GFAP expression, but may not be a decrease in the number of astrocytes [62].
RV root bark extract used in the present study is reported to contain active constituents such as reserpine, yohimbine, and ajmaline among others [13-15], and individually these constituents have powerful effects on the nervous system, which the cerebellum is part. As a combination in RV, their effects may be synergistic or antagonistic, and individually their mechanisms are needed.
The cerebellum function in the maintenance of equilibrium and muscle contraction coordination needed for carrying out movements and in the execution of the encoded instructions [63]. Alteration of the Purkinje cells which was the most affected neurons of the cerebellar cortex in this study prevents inhibitory projections to the deep cerebellar nuclei, and may lead to severe detrimental consequences. However, the combination of RV+GL may help to prevent such adverse functional and/or structural effects.
RV was observed to be toxic to the cerebellar cortical neurons as it caused cortical morphological change and stimulated marked expression of NSE and GFAP. GL on the other hand modulated the toxic effect of RV, thus protecting the cerebellar cortex from RV-induced toxicity. This preliminary report of RV+GL combination may be considered an alternative to RV single treatment for better disease management and brain protection.
- Perez-Campo R, Lopez-Torres M, Cadenas S, Rojas C, Barja G (1998) The rate of free radical production as a determinant of the rate of aging: evidence from the comparative approach. J Comp Physiol B 168: 149-158. [Crossref]
- Spencer PS, Schaumburg HH, Ludolph AC (2000) Experimental and Clinical Neurotoxicology, second ed. Oxford University Press, Oxford.
- Kennedy JL, Farrer LA, Andreasen NC, Mayeux R, St George-Hyslop P (2003) The genetics of adult-onset neuropsychiatric disease: complexities and conundra? Science 302: 822-826. [Crossref]
- Tanner CM, Ottman R, Goldman SM, Ellenberg J, Chan P, et al. (1999) Parkinson disease in twins: an etiologic study. JAMA 281: 341-346. [Crossref]
- Kris-Etherton PM, Grieger JA, Etherton TD (2009) Dietary reference intakes for DHA and EPA. Prostaglandins Leukot Essent Fatty Acids 81: 99-104. [Crossref]
- Barnard ND, Bush AI, Ceccarelli A, Cooper J, de Jager CA, et al. (2014) Dietary and lifestyle guidelines for the prevention of Alzheimer's disease. Neurobiol Aging 35 Suppl 2: S74-78. [Crossref]
- Ugochukwu NH, Babady NE (2002) Antioxidant effects of Gongronema latifolium in hepatocytes of rat models of non-insulin dependent diabetes mellitus. Fitoterapia 73: 612-618. [Crossref]
- Ugochukwu NH, Babady NE (2003 Antihyperglycemic effect of aqueous and ethanolic extracts of Gongronema latifolium leaves on glucose and glycogen metabolism in livers of normal and streptozotocin-induced diabetic rats. Life Sci 73: 1925–1938. [Crossref]
- Campbell JIA, Mortensen A, Mølgaard P (2006) Tissue lipid lowering-effect of a traditional Nigerian anti-diabetic infusion of Rauwolfia vomitoria foliage and Citrus aurantium fruit. J Ethnopharmacol 104: 379-386. [Crossref]
- Bisong S, Brown R, Osim E (2011) Comparative effects of Rauwolfia vomitoria and chlorpromazine on social behaviour and pain. N Am J Med Sci 3: 48-54. [Crossref]
- Okolie NP, Israel EEJ, Falodun A (2011) In-vitro evaluation of antioxidant potential of Rauwolfia vomitoria root extract and its inhibitory effect on lipid peroxidation as indication of aphrodisiac properties. Pharmaceutical Chem J 45: 476-480.
- Kutalek R, Prinz A (2007) African Medicinals, in: Yaniv, Z., Bachrach, U. (Eds.),Handbook of Medicinal Plants. CBS Publishers Ltd, New Delhi.
- Iwu MM, Court WE (1977) Root alkaloid of Rauwolfia vomitoria Afz. Planta Med 32: 88-94. [Crossref]
2021 Copyright OAT. All rights reserv
- Goel MK, Mehrotra S, Kukreja AK, Shanker K, Khanuja SPS (2009) In vitro propagation of Rauwolfia serpentine using liquid medium, assessment of genetic fidelity of micropropagated plants, and simultaneous quantification of reserpine, ajmaline and ajmalicine, in: Jain, S.M., Saxena, P.K. (Eds.), Methods in Molecular Biology, Protocols for In Vitro Cultures and Secondary Metabolite Analysis of Aromatic and Medicinal Plants. Humana Press, New York, 547: 17-33.
- Mensah JK, Okoli RI, Turay AA, Ogie-Odia EA (2009) Phytochemical analysis of medicinal plants used for the management of hypertension by Esan people of Edo State, Nigeria. Ethnobotanical Leaflets. 13: 1273-1287.
- Amole OO, Yemitan OK, Oshikoya KA (2009) Anticonvulsant activity of Rauvolfia vomitoria (Afzel). Afr J Pharm Pharmacol 3: 319-322.
- Bisong SA, Brown R, Osim EE (2010) Comparative effects of Rauwolfia vomitoria and chlorpromazine on locomotor behaviour and anxiety in mice. J Ethnopharmacol 132: 334-339. [Crossref]
- Ekong MB, Peter AI, Ekpene UU (2016) Co-administration of Rauwolfia vomitoria with Gongronema latifolium or Vernonia amygdalina on spatial learning, memory, and some bio-molecules. Asian J Med Sci 7: 82-87.
- Okpako DT (1991) Principles of Pharmacology: A Tropical Approach, first ed, Cambridge University Press, Cambridge.
- Eluwa MA, Idumesaro NB, Ekong MB, Akpantah AO, Ekanem TB (2008) Effects of aqueous extract of Rauwolfia vomitoria root bark on the cytoarchitecture of the cerebellum and neurobehaviour of adult male Wistar rats. Internet J Alternat Med 6(2), ispub.com/IJAM/6/2/3081.
- Ekong MB, Peter MD, Peter AI, Eluwa MA, Umoh IU, et al. (2014) Cerebellar neurohistology and behavioural effects of gongronema latifolium and Rauwolfia vomitoria in mice. Metab Brain Dis 29: 521-527. [Crossref]
- Ekong MB, Peter AI, Ekpene UU, Bassey EI, Eluwa MA, et al. (2015a) Gongronema latifolium modulates Rauwolfia vomitoria induced behavior, biochemicals, and histomorphology of the cerebral cortex. Int J Morphol 33: 77-84.
- Ekong MB, Ekpene UU, Thompson FE, Peter AI, Udoh NB, et al. (2015b) Effects of co-treatment of Rauwolfia vomitoria and Gongronema latifolium on neurobehaviour and the neurohistology of the cerebral cortex in mice. Internet J Med Update 10: 3-10.
- Eluwa MA, Udofa MT, Vulley MBG, Ekanem TB, Akpantah AO, et al. (2010) Comparative study of teratogenic potentials of crude ethanolic root bark and leaf extract of Rauwolfia vomitoria (apocynaceae) on the fetal heart. N Am J Med Sci 2: 592-595. [Crossref]
- Eluwa MA, Ekanem TB, Udoh PB, Ekong MB, Akpantah AO, et al. (2013b) Teratogenic effects of crude ethanolic extract of root bark and leaf extract of Rauwolfia vomitoria (Apocynaceae) on the femur of albino Wistar rats foetuses. J Histol 2013.
- Eluwa MA, Ekanem TB, Udoh PB, Ekong MB, Akpantah AO, et al. (2013b) Teratogenic effects of crude ethanolic extract of root bark and leaf extract of Rauwolfia vomitoria (Apocynaceae) on nissl substances of albino Wistar rats foetuses. Neurosci J [Crossref]
- Morebise O (2015) A review on Gongronema latifolium, an extremely useful plant with great prospects. Eur J Med Plants 10: 1-9.
- Eleyinmi AF (2007) Chemical composition and antibacterial activity of Gongronema latifolium. J Zhejiang Univ Sci B 8: 352-358. [Crossref]
- Adenuga W, Olaleye ON, Adepoju PA (2010) Utilization of bitter vegetable leaves (Gongronema latifolium, Vernonia amygdalina) and Garcinia kola extracts as substitutes for hops in sorghum beer production. Afr J Biotech 9(51), 8819-8823.
- Morebise O, Fafunso MA, Makinde JM, Olajide OA, Awe EO (2002) Anti-inflammatory property of the leaves of Gongronema latifolium. Phytother Res 16: s75–s77. [Crossref]
- Ogundipe OO, Moody JO, Akinyemi TO, Raman A (2003) Hypoglycemic potentials of methanolic extracts of selected plant foods in alloxanized mice. Plant Foods Hum Nutr 58: 1–7.
- Glew RS, VanderJagt DJ, Huang YS, Chuang LT, Bosse R, et al. (2004) Nutritional analysis of the edible pit of Sclerocarya birrea in the Republic of Niger (daniya, Hausa). J Food Comp Anal 17: 99-111.
- Akuodor GC, Idris-Usman MS, Mbah CC, Megwas UA, Akpan JL, et al. (2010) Studies on anti-ulcer, analgesic and antipyretic properties of the ethanolic leaf extract of Gongronema latifolium in rodents. Afr J Biotechnol 9: 2316-2321.
- Okokon JE, Umoh UF, Ekpo BA, Etim EI (2013) Antidiabetic study of combined extracts of Vernonia amygdalina, Ocimum gratissimum, and Gongronema latifolium on alloxan-induced diabetic rats. J Nat Pharm 4: 28-31.
- Iversen LL, Glowinski J, Axelrod J (1965) The uptake and storage of H3-norepinephrine in the reserpine-pretreated rat heart. J Pharmacol Exp Ther 150: 173-183. [Crossref]
- López-Muñoz F, Bhatara VS, Alamo C, Cuenca E (2004) [Historical approach to reserpine discovery and its introduction in psychiatry]. Actas Esp Psiquiatr 32: 387-395. [Crossref]
- Tolivia D, Tolivia J (1991) A new rapid silver impregnation for neuronal bodies on methacrylate sections. J Neurosci Methods 36: 139-143. [Crossref]
- Minbay FZ, Kahveci Z, Cavusoglu I (2001) Rapid Bielschowsky silver impregnation method using microwave heating. Biotech Histochem. 76: 233-237.
- Pfeifenbring S, Bunyan RF, Metz I, Röver C, Huppke P, et al. (2015) Extensive acute axonal damage in pediatric multiple sclerosis lesions. Ann Neurol 77: 655-667. [Crossref]
- Knezovic A, Osmanovic-Barilar J, Curlin M Hof PR, Simic G, Riederer P, et al. (2015) Staging of cognitive deficits and neuropathological and ultrastructural changes in streptozotocin-induced rat model of Alzheimer’s disease. J Neural Transm 122: 577-592. [Crossref]
- Strayer DS, Rubin E (2009) Cell Injury. In: Rubin E, Reisner HM. edited. Essentials of Rubin's Pathology, fifth ed. Lippincott Williams & Wilkins, Baltimore.
- Glowinski J, Axelrod J, Iversen LL (1966) Regional studies of catecholamines in the rat brain. IV. Effects of drugs on the disposition and metabolism of H3-norepinephrine and H3-dopamine. J Pharmacol Exp Ther 153: 30-41. [Crossref]
- de la Torre JC (1972) Dynamics of Brain Monoamines, first ed. Plenum Press, New York.
- Brannan T, Martinez-Tica J, Yahr MD (1991) Effect of yohimbine on brain monoamines: an in vivo study. J Neural Transm Park Dis Dement Sect 3: 81-87. [Crossref]
- Siegel GJ, Agranoff BW, Albers RW, Fisher SK, Uhler MD (1999) Basic Neurochemistry: Molecular, Cellular and Medical Aspects. sixth ed. Lippincott-Raven, Philadelphia.
- Edet EE, Akpanabiatu MI, Eno AE, Umoh IB, Itam EH (2009) Effect of Gongronema latifolium crude leaf extraction on some cardiac enzymes of alloxan induced diabetic rats. Afr J Biochem Res 3: 366-369.
- Akpan HD, Ekpo AJ (2015) Protective role of diets containing Gongronema latifolium leaves on streptozotocin-induced oxidative stress and liver damage. J Appl Pharmaceut Sci 5: 85-90.
- Iwanaga T, Takahashi Y, Fujita T (1989) Immunohistochemistry of neuron-specific and glia-specific proteins. Arch Histol Cytol 52: 13-24. [Crossref]
- McAleese SM, Dunbar B, Fothergill JE, Hinks LJ, Day IN (1988) Complete amino acid sequence of the neurone-specific gamma isozyme of enolase (NSE) from human brain and comparison with the non-neuronal alpha form (NNE). Eur J Biochem 178: 413-417. [Crossref]
- Pleines UE, Morganti-Kossmann MC, Rancan M, Joller H, Trentz O, et al. (2001) S-100 beta reflects the extent of injury and outcome, whereas neuronal specific enolase is a better indicator of neuroinflammation in patients with severe traumatic brain injury. J Neurotrauma 18: 491-498. [Crossref]
- Ogata M, Tsuganezawa O (1999) Neuron-specific enolase as an effective immunohistochemical marker for injured axons after fatal brain injury. Int J Legal Med 113: 19-25. [Crossref]
- Vinores S, Marangos P, Bonnin J, Rubinstein L (1984) Immunoradiometric and immunohistochemical demonstration of neuron-specific enolase in experimental rat gliomas. Cancer Res 44: 2595-2599. [Crossref]
- Sensenbrenner M, Lucas M, Deloulme J (1997) Expression of two neuronal markers, growth-associated protein 43 and neuron-specific enolase, in rat glial cells. J Mol Med (Berl) 75: 653-663. [Crossref]
- Smith ME, Eng LF (1987) Glial fibrillary acidic protein in chronic relapsing experimental allergic encephalomyelitis in SJL/J mice. J Neurosci Res 18: 203–208.
- Jacque CM, Vinner C, Kujas M, Raoul M, Racadot J, et al. (1978) Determination of glial fibrillary acidic protein (GFAP) in human brain tumors. J Neurol Sci 35: 147-155. [Crossref]
- Roessmann U, Velasco ME, Sindely SD, Gambetti P (1980) Glial fibrillary acidic protein (GFAP) in ependymal cells during development. An immunocytochemical study. Brain Res 200: 13–21. [Crossref]
- Venkatesh K, Srikanth L, Vengamma B, Chandrasekhar C, Sanjeevkumar A, et al. (2013) In vitro differentiation of cultured human CD34+ cells into astrocytes. Neurol India 61: 383-388. [Crossref]
- Herrmann JE, Imura T, Song B, Qi J, Ao Y, et al. (2008) STAT3 is a critical regulator of astrogliosis and scar formation after spinal cord injury. J Neurosci 28: 7231-7243. [Crossref]
- Sofroniew MV (2009) Molecular dissection of reactive astrogliosis and glial scar formation. Trends Neurosci 32: 638-647. [Crossref]
- Wilhelmsson U, Li L, Pekna M, Berthold CH, Blom S, et al. (2004) Absence of glial fibrillary acidic protein and vimentin prevents hypertrophy of astrocytic processes and improves post-traumatic regeneration. J Neurosci 24: 5016–5021. [Crossref]
- Liu C, Li Y, Lein PJ, Ford BD (2012) Spatiotemporal patterns of GFAP upregulation in rat brain following acute intoxication with diisopropylfluorophosphate (DFP). Curr Neurobiol 3: 90–97. [Crossref]
- Bondan EF, Martins MFM, Viani FC (2013) Decreased astrocytic GFAP expression in streptozotocin induced diabetes after gliotoxic lesion in the rat brainstem. Arq Bras Endocrinol Metabol 57: 431–436. [Crossref]
- Kiernan JA, Rajakumar N (2014) Barr's The Human Nervous System: An Anatomical Viewpoint, tenth ed. Lippincott, Williams & Wilkins, Baltimore.







