Aminaphtone is a molecule affecting endothelin-1 and adhesion molecules expression, which preserves capillary walls integrity. We report 50 cases of patients who were tested for albuminuria less than 30 days before the start and after the suspension of aminaphtone therapy, 75 mg twice a day for 91-251 days, without changing concomitant medications with ACEi or ARB.
Baseline albuminuria ranged from 34 to 2011 mg/L. In 40 patients it showed a reduction between -9 and -662 mg/L (median -50), and an average percent decrease of -35.9 + 21.4%. In other 10 cases, albuminuria variations ranged between +12 and +60 mg/L (median +24) with an average percent change +17.6 + 24.5%.
The albuminuria variations showed no correlation with therapy length, gender, age, diabetes, ACEi and/or ARB treatment, changes in creatinine or estimated glomerular filtration rate (eGFR). A difference was observed in the average percent changes of albuminuria between patients with baseline eGFR greater (N = 32) or lower (N = 18) than 45 ml/min: -33.0+21.8% vs. -11.3+38.5%, respectively (P <0.02).
Our observations seem to indicate an action of aminaphtone on albuminuria reduction in earlier stages of kidney disease, perhaps through a different mechanism from that of drugs effective on the renin-angiotensin-aldosterone system.
Albuminuria; Aminaphtone; Endothelium; Microangiopathy.
Albuminuria, defined as urinary excretion albumin greater than 30 mg in 24 hours, is an early biomarker of organ damage in patients with diabetes or hypertension [1, 2]. Even in the absence of diabetes mellitus, albuminuria is a parameter that defines not only the prognosis of nephropathy, but also the increase of the cardiovascular risk [3, 4]. Albuminuria, therefore, can be considered the renal epiphenomenon of a generalized endothelial damage. The control of albumin excretion in hypertensive patients, with or without diabetic nephropathy, is equally important as blood pressure control. An anti-proteinuric effect can be achieved as a result of the control of blood pressure or metabolic syndrome, and by some medications directly acting on the glomerular capillaries hemodynamics, such as inhibitors of the angiotensin converting enzyme (ACEi) and angiotensin receptor blockers (ARB) [5, 6].
A further approach could target endothelial dysfunction, defined as the imbalance between endothelium-derived vasodilator and vasoconstrictor substances, which plays an important role in the pathogenesis of vascular diseases, like diabetic microangiopathy. Increasing circulating levels of adhesion molecules (in the early stage of microangiopathy) and of endothelin-1, an endothelial-derived vasoconstrictor peptide with mitogenic, pro-oxidative, pro-inflammatory and pro-fibrotic properties (in a more advanced stage of microangiopathy), have shown to be relevant to the pathophysiology of diabetic vasculopathy, hypertension, coronary and systemic atherogenesis [7]. So, it seemed logical to counteract the over-production of endothelin-1 using a selective endothelin-1-receptor antagonist, like avosentan. This drug demonstrated to decrease proteinuria in diabetic patients; however, it also increased morbidity and mortality by inducing fluid retention [8].
It has recently been reported a case of albuminuria reduction in a patient with type 1 diabetes after a two months therapy with another drug, aminaphtone [9]. Aminaphtone is a synthetic molecule derived from 4-aminobenzoic acid, that antagonizes the pathological events related to inflammatory disorders of the vascular wall through biochemical mechanisms related to the production of endothelin-1 and the expression of adhesion molecules [10,11]. It is able to control a wide range of genes engaged in inflammation, homeostasis, body fluid levels, response to hypoxia, cell division, cell-to-cell signaling. Interacting with different transcription factors, it down-regulates the biosynthesis and release of cytokines and chemokines [12]. Studies on human endothelial cells (grown in vitro) have revealed that the activity of aminaphtone is also present at very low concentrations (2-6 mg/ml) [10, 11].
Aminaphtone is effective in treating the inflammation in pathologic processes sustained by loss of integrity of capillaries and production of endothelin-1. It has been used for the therapy and prevention of various diseases, such as gums or nose hemorrhage, bleeding in patients under oral anticoagulant therapy, the syndrome of cyclic idiopathic edema, the Schamberg disease (multiple purpuric lesions of the lower limbs), chronic venous diseases, lymphatic stasis, capillary fragility induced by chronic renal failure [13-20].
Following the report of a case of albuminuria reduction after treatment with aminaphtone, we performed a retrospective review of cases of albuminuric patients referred to our clinics and treated with the same drug. Here we report 40 cases in which there has been a decrease in albuminuria after a course of therapy with aminaphtone.
Out of a population of about 1500 patients referred to the clinics of Nephrology of Taranto, Castellaneta and Massafra (Italy), 50 adult subjects met the following requirements:
- They had been treated with aminaphtone, 75 mg orally twice a day, from July 2015 to July 2016;
- They presented albuminuria, tested no more than 30 days before the start of therapy with aminaphtone, and tested again when the drug was still taken, or no later than 30 days after its suspension;
- During the observation period, they had not undergone variations of other concomitant medications with possible effects on albuminuria, such as ACEi or ARB;
- During the observation period, they had not suffered from fever or urinary tract infections.
To avoid errors due to the collection of 24-hours urine, we defined as albuminuric the patients with albumin concentration higher than 30 mg/L on a sample of early morning urine.
The Table 1 contains patients’ data, reasons and durations of aminaphtone therapy, renal disease, major comorbidities, renal function expressed either as plasma creatinine or as glomerular filtration rate estimated by the simplified MDRD formula (eGFR) [21], ACEi or ARB therapies, and the values of albuminuria before and after the therapy with aminaphtone.
Table 1. Patients’ data, durations of aminaphtone therapy, major comorbidities, renal function expressed either as plasma creatinine or as glomerular filtration rate estimated by the simplified MDRD formula (eGFR), ACEi or ARB therapies, and values of albuminuria before (basal) and after (final) the therapy with aminaphtone. (Abbreviations: DIA = diabetic nephropathy; NAS = nephroangiosclerosis; MN = membranous nephropathy; AMY = amyloidosis; IgA = IgA nephropathy).
Patient |
Age |
Gender |
Reasons for aminaphtone prescription |
Length of therapy |
Renal disease |
Hypertension |
Creatinine |
eGFR |
ACEi |
ARB |
Albuminuria |
Albuminuria |
basal |
final |
|
[yrs] |
|
|
[days] |
|
|
[mg/dl] |
[ml/min] |
|
|
[mg/dl] |
[mg/dl] |
|
|
|
|
|
|
|
|
|
|
|
|
|
AA |
54 |
F |
lymphedema |
186 |
DIA |
YES |
0,90 |
69,3 |
YES |
YES |
326 |
142 |
AD |
77 |
M |
nosebleed |
175 |
FGS |
YES |
1,33 |
55,4 |
NO |
YES |
884 |
908 |
AF |
67 |
M |
nosebleed |
147 |
DIA |
YES |
1,10 |
71 |
YES |
NO |
1640 |
1112 |
AF |
80 |
M |
nosebleed |
176 |
DIA |
YES |
1,98 |
34,7 |
YES |
NO |
138 |
152 |
AG |
41 |
M |
lymphedema |
159 |
IgA |
NO |
1,14 |
75,2 |
NO |
YES |
180 |
137 |
BF |
51 |
F |
lymphedema |
161 |
DIA |
YES |
0,67 |
98,6 |
NO |
YES |
100 |
15 |
CB |
78 |
M |
purpura |
119 |
DIA |
YES |
2,01 |
34,3 |
YES |
YES |
460 |
410 |
CC |
60 |
M |
nosebleed |
228 |
MN |
YES |
0,98 |
82,9 |
YES |
NO |
1980 |
1318 |
CF |
86 |
M |
nosebleed |
175 |
NAS |
YES |
1,94 |
35 |
NO |
YES |
61 |
112 |
CG |
67 |
M |
nosebleed |
205 |
DIA |
YES |
1,22 |
63 |
NO |
YES |
2011 |
1640 |
CI |
77 |
F |
purpura |
168 |
DIA |
YES |
0,83 |
70,9 |
NO |
YES |
70 |
56 |
CN |
58 |
M |
purpura |
147 |
FGS |
YES |
1,20 |
66,1 |
NO |
YES |
80 |
61 |
CV |
73 |
M |
purpura |
187 |
DIA |
YES |
1,12 |
68,3 |
YES |
NO |
1090 |
541 |
DC |
72 |
M |
purpura |
196 |
NAS |
YES |
1,85 |
38,4 |
NO |
YES |
420 |
444 |
DO |
74 |
M |
nosebleed |
212 |
DIA |
YES |
1,80 |
39,4 |
NO |
YES |
196 |
77 |
DP |
55 |
M |
purpura |
169 |
IgA |
YES |
1,15 |
70,2 |
YES |
NO |
50 |
36 |
DR |
79 |
F |
lymphedema |
195 |
DIA |
YES |
0,51 |
123,6 |
NO |
YES |
50 |
41 |
DS |
69 |
F |
lymphedema |
129 |
DIA |
YES |
0,61 |
103,4 |
YES |
YES |
60 |
31 |
FA |
68 |
M |
purpura |
157 |
AMY |
YES |
1,11 |
70 |
NO |
YES |
346 |
304 |
FC |
61 |
F |
purpura |
161 |
DIA |
YES |
1,87 |
29,1 |
NO |
YES |
143 |
125 |
FR |
68 |
F |
nosebleed |
184 |
DIA |
YES |
2,92 |
17 |
NO |
YES |
78 |
60 |
GA |
82 |
F |
nosebleed |
168 |
DIA |
YES |
1,43 |
37,3 |
YES |
NO |
100 |
50 |
GN |
71 |
M |
purpura |
174 |
DIA |
YES |
2,50 |
27,2 |
YES |
NO |
300 |
322 |
GV |
69 |
M |
purpura |
166 |
IgA |
YES |
1,13 |
68,4 |
YES |
YES |
585 |
597 |
LU |
78 |
M |
nosebleed |
107 |
NAS |
YES |
1,37 |
53,4 |
YES |
NO |
152 |
28 |
LV |
64 |
M |
lymphedema |
106 |
DIA |
YES |
1,56 |
47,9 |
YES |
YES |
227 |
189 |
LV |
69 |
M |
lymphedema |
196 |
DIA |
YES |
1,20 |
63,8 |
YES |
NO |
84 |
52 |
MM |
81 |
F |
purpura |
194 |
NAS |
YES |
1,14 |
48,6 |
NO |
YES |
109 |
36 |
MM |
67 |
F |
purpura |
174 |
DIA |
YES |
1,16 |
49,5 |
NO |
YES |
62 |
49 |
MN |
67 |
M |
nosebleed |
187 |
DIA |
YES |
0,90 |
89,5 |
YES |
YES |
315 |
246 |
NL |
58 |
F |
lymphedema |
245 |
DIA |
YES |
0,88 |
70,1 |
NO |
YES |
70 |
46 |
NM |
89 |
M |
purpura |
91 |
NAS |
YES |
1,50 |
46,8 |
YES |
NO |
206 |
126 |
NR |
71 |
F |
nosebleed |
188 |
DIA |
YES |
1,20 |
47,1 |
NO |
YES |
74 |
39 |
PA |
78 |
F |
lymphedema |
119 |
DIA |
YES |
1,30 |
42,1 |
NO |
YES |
34 |
12 |
PA |
75 |
F |
purpura |
251 |
DIA |
YES |
2,10 |
24,4 |
NO |
YES |
143 |
198 |
PG |
69 |
M |
lymphedema |
152 |
DIA |
YES |
0,80 |
101,9 |
YES |
NO |
137 |
93 |
PM |
72 |
F |
purpura |
133 |
DIA |
YES |
3,90 |
12 |
YES |
NO |
380 |
410 |
PM |
62 |
F |
purpura |
154 |
DIA |
YES |
2,70 |
19 |
YES |
NO |
1220 |
1280 |
PP |
71 |
F |
purpura |
187 |
DIA |
YES |
1,94 |
27 |
NO |
YES |
102 |
114 |
PS |
59 |
F |
nosebleed |
154 |
DIA |
YES |
1,18 |
49,8 |
NO |
YES |
80 |
69 |
RE |
73 |
F |
lymphedema |
201 |
DIA |
YES |
1,59 |
33,8 |
NO |
YES |
328 |
212 |
RG |
65 |
M |
purpura |
96 |
DIA |
YES |
4,18 |
15,3 |
NO |
YES |
404 |
81 |
SG |
69 |
M |
purpura |
173 |
NAS |
YES |
1,00 |
78,7 |
NO |
YES |
200 |
182 |
SM |
58 |
M |
lymphedema |
181 |
MN |
YES |
1,80 |
41,4 |
YES |
NO |
938 |
638 |
TC |
72 |
M |
lymphedema |
135 |
NAS |
NO |
0,91 |
87 |
NO |
NO |
411 |
189 |
TF |
77 |
M |
purpura |
103 |
DIA |
YES |
0,99 |
77,9 |
NO |
YES |
211 |
191 |
TG |
72 |
M |
purpura |
179 |
DIA |
YES |
0,90 |
88,2 |
YES |
YES |
174 |
123 |
VA |
80 |
M |
lymphedema |
124 |
MN |
YES |
3,41 |
18,6 |
YES |
NO |
997 |
945 |
VE |
72 |
M |
nosebleed |
210 |
DIA |
YES |
1,19 |
63,9 |
NO |
YES |
220 |
161 |
ZI |
79 |
M |
lymphedema |
140 |
DIA |
YES |
1,45 |
49,9 |
NO |
YES |
88 |
27 |
The duration of therapy with aminaphtone ranged between 91 and 251 days (median 171). Aminaphtone was prescribed by general practitioners or by specialists different from the nephrologist, fundamentally for 3 groups of motivations: recurrent nosebleeding, purpuric lesions of the skin, lower limbs edema, usually interpreted as lymphedema. No patients, not even subjects with advanced renal failure, suffered from important adverse effects of aminaphtone, aside from a few cases of transient nausea, headache or abdominal pain.
The albuminuria variations are represented in Figure 1.
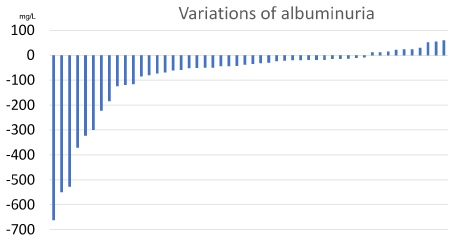
Figure 1. Changes in albuminuria after treatment with 75 mg b.i.d. aminaphtone in 50 patients [baseline value = zero].
Forty out of the 50 patients showed an albuminuria reduction.
In the 40 cases where it decreased, baseline albuminuria range was 34-2011 mg/L [median 188]; the range of final albuminuria was 12-1640 mg/L (median 132), with a variation after aminaphtone between -662 and -9 mg/L (median -50). The treatment lasted between 91 and 245 days (median 168). The average percent decrease (+SD) of albuminuria was of -35.9 + 21.4%.
In the other 10 cases, baseline albuminuria range was 61-1220 mg/L (median 340); the range of final albuminuria was 112-1280 mg/L (median 366); the variation of albuminuria after aminaphtone ranged between +12 and +60 mg/L (median 24). The treatment lasted between 133 and 251 days (median 175), a duration not significantly different from that of patients with decreased albuminuria. The average percent change (± SD) of albuminuria was +17.6 + 24.5%.
The albuminuria variations showed no correlation with gender, age, diabetes, treatment with ACEi and/or ARB, with the days of aminaphtone therapy, or with the changes in serum creatinine or eGFR at the end of the observation period.
Almost all patients (48 out of 50) had hypertension. Also the 2 patients with normal blood pressure showed a decrease of albuminuria.
There was a significant difference in basal plasma creatinine and eGFR in patients in which albuminuria decreased compared to those where it increased. The range of baseline creatinine was, respectively, 0.61-4.18 mg/dl (median 1.17) and 1.13-3.90 mg/dl (median 1.96), P <0.005. The range of eGFR was respectively 123.6-15.3 ml/min (median 63.9) and 68.4-12.0 ml/min (median 31.0), P <0.003.
Figure 2 shows the scatterplot of percent changes of albuminuria versus baseline creatinine and eGFR.
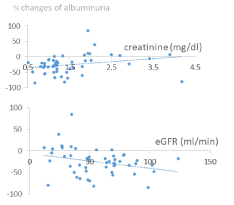
Figure 2. Scatterplots of the percent changes of albuminuria vs baseline creatinine [top box] and vs eGFR [lower box] [N = 50].
It can be pointed out that, below the values of 1.5 mg/dl for creatinine and above 45 ml/minute for eGFR, almost all the changes of albuminuria were negative. Using these values as cut-offs to separate the patients into 2 groups, it can be observed (Figure 3) that between the group with creatinine <1.5 mg/dl (N = 32) and that with creatinine >1.5 mg/dl (N = 18) the average percent changes in albuminuria differ significantly (P <0.003): -34.8% (+22.4) vs. -8.0% (+35.7), respectively. Similarly, between the two groups with eGFR greater (N = 32) and lower (N = 18) than 45 ml/min, the average percent variations of albuminuria differ significantly (P <0.02): -33.0% (+21.8) vs. -11.3% (+38.5), respectively.
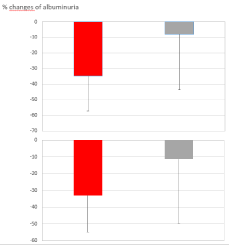
Figure 3. Top box: percent changes of albuminuria in patients with creatinine lower (N = 32, red) and higher (N = 18, gray) than 1.5 mg/dl (mean and SD, P <0.003). Lower box: percent changes of albuminuria in patients with eGFR higher (N = 32, red) and lower (N = 18, gray) than 45 ml/minute (mean and SD, P <0.02).
In the present paper we report 40 cases of albuminuria reduction in outpatients attending our Nephrology clinics, after treatment with aminaphtone, taken at a dose of 75 mg twice a day continuously for a period that ranged from 3 to 8 months. In other 10 patients an antiproteinuric effect of the drug was not observed.
Almost all of the observed patients suffered from hypertension, while a large percentage had type 2 diabetes (34 of 50). In addition, most patients were on drugs affecting the renin-angiotensin-aldosterone system (RAAS). The albuminuria variations showed no correlation with gender, age, diabetes, treatment with an ACEi or ARB, duration of therapy with aminaphtone. It is remarkable that the reduction in albuminuria was obtained either in diabetic or in non-diabetic patients.
The degree of renal function, expressed both as plasma creatinine or as eGFR estimated with the simplified MDRD formula [21], was the only parameter that demonstrated to have an influence on the antiproteinuric effect. It is possible that a more severe renal dysfunction (chronic kidney disease beyond the stage IIIa, with eGFR < 45 ml/minute) blunted the response to the drug, which instead was present primarily in patients with mild to moderate renal impairment.
Taking into account that the prevalence of chronic kidney disease in stages I to IIIa in Italy is about 6.2% of the adult population [22, 23], in this country we have more than 2 million people affected by kidney disease, and many of them, who are albuminuric, could benefit from the aminaphtone therapy.
Since this is a report of cases based on retrospective analysis of data, it does not shed light on the mechanism by which the aminaphtone could exert an antiproteinuric action. We can assume that this drug, unlike ACEi and ARB drugs, which act mainly on glomerular hemodynamics [5, 6], could affect the expression of adhesion molecules, endothelin-1 and pro-inflammatory molecules at the endothelium level [12].
In conclusion, our observations seem to indicate a direct action of aminaphtone on albuminuria reduction, at least in the initial stage of kidney disease, perhaps through a different mechanism from that of drugs traditionally used for their activity on the RAAS.
Of course, as our data are retrospective, they do not allow firm conclusions, but the foundations are laid to prepare prospective randomized, controlled, double-blind trials, for example comparing patients receiving placebo or drugs active on the RAAS versus patients who add to these drugs also aminaphtone, in order to demonstrate a possible additional effect of decreasing albuminuria.
Not applicable.
2021 Copyright OAT. All rights reserv
The authors declare that there is no conflict of interest regarding the publication of this article.
- Matsushita K, Ballew SH, Coresh J (2016) Cardiovascular risk prediction in people with chronic kidney disease. Curr Opin Nephrol Hypertens 25: 518-523. [Crossref]
- Leoncini G, Ratto E, Viazzi F, Pontremoli R (2007) Ruolo della microalbuminuria nella valutazione del rischio globale nel paziente con ipertensione essenziale. G Ital Nefrol. 6: 565-573.
- Sleight P (2000) The HOPE Study [Heart Outcomes Prevention Evaluation]. J Renin Angiotensin Aldosterone Syst 1:18-20. [Crossref]
- Kalaitzidis RG, Karasavvidou DP, Tatsioni A, Pappas K, Katatsis G, et al. (2015) Albuminuria as a marker of arterial stiffness in chronic kidney disease patients. World J Nephrol. 4: 406-414. [Crossref]
- Toto RD, Adams-Huet B, Fenves AZ, Mitchell HC, Mulcahy W, et al. (1996) Effect of ramipril on blood pressure and protein excretion rate in normotensive nondiabetic patients with proteinuria. Am J Kidney Dis. 28: 832-840. [Crossref]
- Slagman MC, Navis G, Laverman GD (2010) Dual blockade of the renin-angiotensin-aldosterone system in cardiac and renal disease. Curr Opin Nephrol Hypertens. 19:140-52.
- Bertini M (2015) “Endothelial Protector Drugs” and Diabetes: Is there a Role for these drugs? J Diabetes Obes 1:1-3.
- Mann JF, Green D, Jamerson K, Ruilope LM, Kuranoff SJ, et al. (2015) Avosentan for overt diabetic nephropathy. J Am Soc Nephrol 21: 527-535.
- Romano C, Tamburella C, Costa M, Messina M, Fassari AL, et al. (2014) Aminaphtone therapy in patients with type 1 diabetes and albuminuria: a case report. J Med Case Rep. 8: 443. [Crossref]
- Lemma S (2006) Novel mode of action of the Aminaphtone : down-regulation of a E-selective expression in ECV-304 cells. Int. Angiology 25 suppl 1: 189.
- Scorza R, Santaniello A, Salazar G, Lenna S, Colombo G, et al. (2008) Aminaphtone, a derivative of 4-aminobenzoic acid, downregulates endothelin-11 production in ECV 304 cells: an in vitro study. Drugs R D 9: 251-257. [Crossref]
- Salazar G, Bellocchi C, Todoerti K, Saporiti F, Piacentini L, et al. (2016) Gene expression profiling reveals novel protective effects of Aminaphtone on ECV304 endothelial cells. Eur J Pharmacol 782: 59–69. [Crossref]
- Godoy JMP, Batigália F, Mendes RN, Paiva JV, Oliveira JD (2003) Aminaphtone in the treatment of nosebleed. Rev bras hematol hemoter 25: 65–71.
- Godoy JMP, Paludetto G, Testoni BR, Sano PY (2010) Aminaphtone for Light Bleeding in Patients Under Oral Anticoagulation. Open Cardiovasc Med J 4: 146–147. [Crossref]
- Godoy JMP (2008) Aminaphtone in idiopathic cyclic oedema syndrome. Phlebology. 23: 118-119. [Crossref]
- Godoy JMP, Batigália F (2009) Aminaphtone in the control of multiple purpuric lesions of the lower extremities. Thromb J 7: 8.
- De Anna D, Mari F, Intini S, Gasbarro V, Sortini A, et al. (1989) Effects of therapy with aminaftone on chronic venous and lymphatic stasis. Minerva Cardioangiol 37: 251-254. [Crossref]
- Doni A, Mondaini F, Somigli M, Comparini L, Gavazzi M, et al. (1983) Modification of primary hemostasis and capillary fragility induced by aminaftone in chronic renal insufficiency. Clin Ter 107: 45-50. [Crossref]
- R. Scorza (2009) Aminaphtone enhances iloprost beneficial effect in patients with systemic sclerosis and recurrent ulcers. ACR/ARHP Annal Scientific Meeting Philadelphia PA (USA). October 16-21.
- Gelso E, Corradetti R (2004) Etiopathogenesis of the chronic venous insufficiency: actuality of aminaphtone in microcircle alterations. Intern. Journal on Drugs and Therapy. 21: 1-8.
- Levey AS, Coresh J, Greene T, Stevens LA, Zhang YL, et al. (2006) Chronic Kidney Disease Epidemiology Collaboration. Using standardized serum creatinine values in the modification of diet in renal disease study equation for estimating glomerular filtration rate. Ann Intern Med 145: 247-54. [Crossref]
- Ministero della Salute (2014) (Italian Health Ministry): Documento di Indirizzo per la Malattia Renale Cronica.
- Garofalo C, Liberti ME, Sagliocca A, Michini C, Palmisano R, et al. (2012) Epidemiologia e prognosi della malattia renale cronica in Italia. G Ital Nefrol. 29 Suppl 58: 3-11.



