With regard to reduction of CO2, there are many reports [1-4]. Reduction of CO2 in air is extremely important because this research can not only reduce the concentration of CO2 in the atmosphere but also can convert the CO2 into useful compounds including CO, H2 or low-molecular-weight organic compounds. Here, mini-review is described according to last year’s report [5] by author.
The author devised composites consisting of nanometre-sized titanium oxide (TiO2) particles (a) and micrometre-sized zirconium oxide (ZrO2) particles (b), and, applied them using an original method. First, particles or molecules adsorbed onto the composite surface were removed by the elimination of static electricity using an ion blower. Next, after the composites were cooled in a refrigerator for more than 20 h, they were placed into a transparent gas-barrier plastic bag with room air. Cooling in a refrigerator enabled the whole surface of the composite to be covered with a very thin layer of water via the condensation of water vapour in the air. Cleaning of the composite’s surface and formation of the thin water layer are extremely important.
Moreover, this method is unique because the reduction of CO2 is performed in air.
Figure 1 shows SEM image of the composite. The image shows that numerous nanometre-sized TiO2 particles are present on the top layer of the composite, and the core of the composite is composed of micrometre-sized ZrO2 particle.

Figure 1. SEM image of the composite.
CO2 was reduced under real solar light without the use of platinum as a co-catalyst.
The weight ratio (TiO2/ZrO2) of the composites was mainly 1:1. After (a) and (b) were pressed to form the composites, the composites were scattered onto an electric conducting material such as a copper plate. Figure 2 shows a typical photograph of the experiment under irradiation by real solar light. Experiments were performed under high room temperatures (approximately 30°C) and high humidity (60-80%). After irradiation, the concentration of formaldehyde and methanol in the bag was measured using gas-detecting tubes.
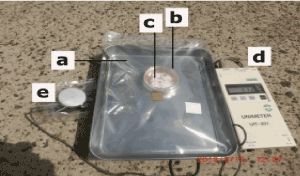
Figure 2. Experiment under irradiation by real solar light.
a: Gas-barrier plastic bag with 1000 ml of air; b: Glass laboratory dish containing the composites and Cu plate; c: Composites (TiO2/ZrO2=1:1); d: Solar-energy-detecting instrument; e: Solar-energy-detecting sensor.
The author obtained experimental results that reaction was catalytic, and, reaction site existed at interface of TiO2 and ZrO2. Then, figure 3 shows plots of formaldehyde and methanol products (μmol/ (g·300 s)) as a function of the irradiation intensity of solar light (mW/cm2).This shows that CO2 reducing reaction was enhanced strongly by the photocatalytic effort.
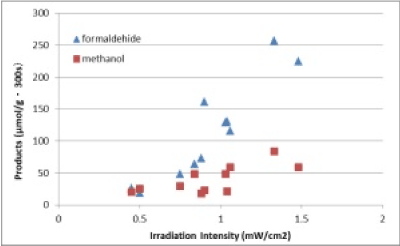
Figure 3. Plots of formaldehyde and methanol products.
The thin water layer on the composites provides a reaction medium for the catalytic reduction of CO2. And, author’s method is catalytic reaction enhanced by the TiO2 photo-catalyst. Moreover, the fact that a large amount of reduced products was obtained from CO2 and H2O in air under irradiation of only real solar light at room temperature and atmosphere pressure is a novel and important finding.
- Inoue T, Fujishima A, Konishi S, Honda K (1979) Photoelectrocatalytic reduction of carbon dioxide in aqueous suspensions of semiconductor powders. Nature 277: 637-638.
- Low J, Cheng B, Yu J (2017) Surface modification and enhanced photocatalytic CO2 reduction performance of TiO2: a review. Appl Surf Sci 392: 658-685.
- Nikokavoura A, Trapalis C (2017) Alternative photocatalysts to TiO2 for the photocatalytic reduction of CO2. Appl Surf Sci 391: 149-174.
- Yuan L, Xu YJ (2015) Photocatalytic conversion of CO2 into value -added and renewable fuels. Appl Surf Sci 342: 154-167.
- Moriya I (2017) Converting of CO2 into low-molecular-weight organic compounds with the TiO2/ZrO2 composites under solar irradiation. Sci Rep 7: 14446. [Crossref]



