Regenerative endodontic procedures (REP) on teeth with necrotic pulps and open apices require robust disinfection. The purpose of this case report is to present clinical and radiographic findings of a regenerative endodontic procedure that utilizes MTAD for single-visit disinfection of a tooth with necrotic pulp and open apex with 4-year follow-up. A maxillary central incisor of a 7-year-old girl was avulsed and replanted. The tooth developed pulpal necrosis and symptomatic apical periodontitis. After access preparation into the empty chamber, the canal was irrigated with 5.25% NaOCl followed by BioPure MTAD (Dentsply, Tulsa Dental Specialties, Tulsa, OK). Intracanal bleeding was stimulated. 3 mm of Mineral Trioxide Aggregate (MTA) was placed directly over the clot and access was permanently restored with composite. Clinical examination at 1 year and 6 months showed a closed apex with no sensitivity to percussion or palpation. After 4 years, the tooth was asymptomatic and responsive to both cold and electric pulp testing. Potential differences in angulation between preoperative and recall images were corrected with a geometrical imaging program, NIH ImageJ with TurboReg plug-in. The present case demonstrates REP of a tooth with necrotic pulp and open apex in a single visit with MTAD disinfection.
Pulpal status and degree of root development are major factors in treatment planning for teeth requiring vital pulp treatment or root canal treatment [1]. Vital pulp treatment can be performed on teeth with open and closed apices. Permanent teeth with closed apices are routinely endodontically treated with a high rate of long-term success [2]. However, teeth with immature and often divergent apices are not suitable for routine endodontic techniques due to large diameter apical foramens and thin dentinal walls susceptible to root fracture [3]. One-visit or two-visit mineral trioxide aggregate (MTA) apexification is currently used in teeth with necrotic pulps and open apices [3] with a high success rate [4]. However, this procedure addresses only technical issues involved in treatment of these teeth and does not completely eliminate the chance for root fracture [5].
There is a growing body of evidence suggesting the possibility of bringing vital tissues into the pulp space of teeth with necrotic pulps and open apices, along with continued growth of the root and thickening of the root canals walls [6]. Hargreaves et al. [3] identified three components necessary for the success of this procedure: stem cells, signaling molecules and a three-dimensional physical scaffold that can support cell growth and differentiation. Regenerative endodontic procedures are only possible when the root canal space is completely disinfected, and a microenvironment is created that is conducive to repopulation by vital tissues [7-9].
Many published regenerative endodontic cases describe a range of clinical protocols with varying irrigants, medicaments, clinical procedures, and follow-up times with little standardization [10]. Frank et al. [11] demonstrated the formation of an apical closure with repeated calcium hydroxide [Ca(OH)2] dressings. It was theorized that this continued root development is a result of the stimulation of residual papilla and root sheath cells that survived apical infection [12]. Hoshino and colleagues [13,14] have demonstrated the effectiveness of triple antibiotic paste (TAP), a mixture of ciprofloxacin, metronidazole, and minocycline, in eradicating bacteria from the infected dentin of root canals. Thereafter, Banchs and Trope [15] presented a successful case of revascularization of an immature mandibular second premolar following disinfection with sodium hypochlorite (NaOCl), CHX and TAP. While TAP has become a valuable intracanal medicament for eradication of bacteria in regeneration procedures [16], other disadvantages such as staining, stem cell viability and multiple treatment visits warrant investigation into other disinfection techniques.
BioPure MTAD (MTAD) (Dentsply, Tulsa Dental Specialties, Tulsa, OK) is a mixture of tetracycline isomer, citric acid and detergent that is bacteriostatic and shows substantivity as it can be absorbed and gradually released from tooth structure such as cementum and dentin [17].
While reports on MTAD have proven efficacy as a final irrigation with antibacterial properties, a search of literature reveals a lack of report on the use of MTAD in regeneration procedures. The purpose of this case report is to present clinical and radiographic findings of a single-visit regenerative endodontic procedure that utilizes MTAD for disinfection of an avulsed necrotic immature tooth with a 4-year follow-up.
A 7-year-old girl was seen in a private endodontic practice for evaluation and treatment of the maxillary right central incisor (#8). The patient’s mother reported that the tooth had been avulsed three days prior to the visit to the endodontic office. The patient’s orthodontist irrigated the tooth with chlorhexidine (CHX) and replanted the tooth with a total extra-oral dry time of 60 minutes. He stabilized the tooth with stainless steel wire and brackets and referred the patient for endodontic evaluation. The medical history of the patient was noncontributory. Extra oral examination revealed no abnormalities or asymmetries. Clinical examination revealed presence of orthodontic stabilization and no discoloration or fracture of tooth #8. The tooth was tender to percussion and palpation and had no response to cold or electric pulp testing (EPT). Adjacent teeth responded normally to vitality tests. Radiographic examination revealed presence of widened periodontal ligament space (Figure 1A). A follow-up examination was performed three weeks later without any change to vitality testing. On the basis of clinical and radiographic findings, a pulpal diagnosis of necrotic pulp and a periapical diagnosis of symptomatic apical periodontitis were made. A written informed consent for the procedure of regeneration with the use of MTAD was obtained from the patient’s mother. Local anesthesia was administered with lidocaine 2% 1:100,000 epinephrine and articaine 4% 1:100,000 epinephrine. After placement of rubber dam, an access cavity was prepared. Upon entry into the root canal, an empty chamber with no vital tissue or blood was noted. Working length was determined by placing a large file in the canal and confirming with a radiograph. The canal walls were lightly instrumented with large files and the canal was irrigated with approximately 10 mL of 5.25% NaOCl and dried with paper points. One mL of MTAD was placed in the canal and left for 5 minutes. The canal was then rinsed with 4 mL of MTAD and dried with paper points. A pre-bent ISO #25 K-file was extended 2 millimeters beyond the working length to stimulate bleeding to 3 millimeters below the cementoenamel junction (CEJ). Three to four millimeters of gray Mineral Trioxide Aggregate (MTA) (Dentsply, Tulsa Dental Specialties, Tulsa, OK) was gently condensed over the blood clot (Figure 1B). The tooth was then restored with a bonded composite restoration.
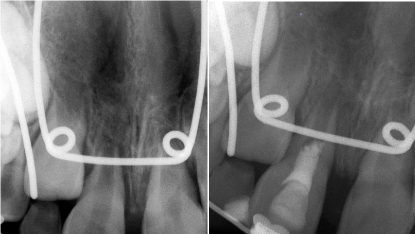
Figure 1.(A) Preoperative periapical radiograph showing tooth #8 with an open apex and periapical radiolucency. (B) Postoperative periapical radiograph following regenerative endodontic procedure and placement of MTA
The patient returned to the endodontic office after 6 months for reevaluation. Based on radiographic examination, the root apex appeared to be closing and patient reported no pain or symptoms. After 1 year and 6 months, the apex was closed, and the patient continued to be asymptomatic (Figure 2A). At 2 year follow up, tooth discoloration was noted, and walking bleach treatment was performed with sodium perborate/saline paste. After one week, shade of #8 matched patient’s adjacent dentition and access was restored with a bonded composite restoration. After 2 years and 6 months, tooth #8 continued to be asymptomatic with no sensitivity to percussion or palpation tests. Vitality tests at this time revealed a positive response to both cold and EPT. After 4 years, tooth #8 continued to respond positive to cold and EPT with no sensitivity to percussion or palpation (Figure 2B) and no crown discoloration (Figure 2C).
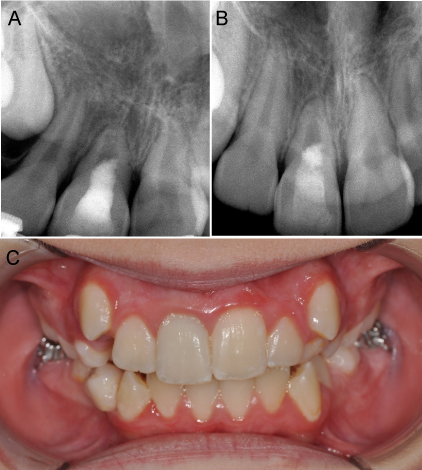
Figure 2.(A) Periapical radiograph taken at 1 year 6-month recall showing continued root thickening and apical closure. (B) Periapical radiograph taken at 4-year recall with complete apical closure. (C) Photograph taken at 4-year recall showing no discoloration of right maxillary central incisor
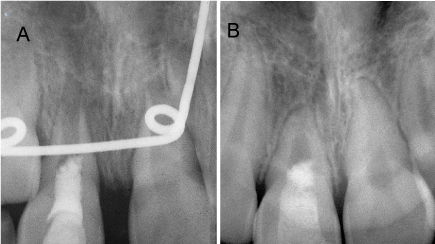
Figure 3.(A) Postoperative periapical radiograph aligned utilizing the NIH ImageJ with TurboReg plug-in. (B) Periapical radiograph taken at 4-year recall aligned utilizing the NIH Image J with TurboReg plug-in demonstrating increased root width and apical closure
Positioning and angulation were mathematically corrected using the ImageJ Software (ver 1.51; National Institutes of Health, Bethesda, MD) and the plug-in application TurboReg (Biomedical Imaging Group, Swiss Federal Institute of Technology, Lausanne, VD, Switzerland) in the method described by Bose et al. [5]. The root length and remaining dentin thickness were measured and compared from the post-operative radiograph and 4-year recall radiograph. While root length resulted in no apparent change, the remaining dentin thickness increased by 175%.
The present case report demonstrates a successful treatment option for an avulsed permanent tooth with necrotic pulp and an immature apex. It highlights the importance of case selection, complete disinfection and proper treatment to achieve continued apical development and REP of a tooth with previously necrotic pulp. Similar to many cases presented in the literature, advantages that contributed to the success of this regenerative endodontic procedure were the young age of the patient and the immature stage of tooth development with open apex, thin walls and short root. Estefan et al. [18] reported that younger age groups and wider preoperative apical diameters were better candidates for revascularization procedures resulting in greater increases in root thickness, root length and apical narrowing.
Based on the standardization of radiographs and quantitative analysis, a significant increase in the remaining dentin thickness in the apical third was detected. While the clinical relevance of root thickening or lengthening has not been confirmed, the continued thickening of the root may promote more favorable long-term outcomes in terms of fracture resistance.
In the present case, the regenerative endodontic procedure was carried out after only 3 weeks of evaluation. In cases of dental trauma, it has been shown that sensibility testing may take more time to recover than vascularity [19]. It may have been beneficial to wait longer for signs of vitality to return. However, when the treatment was initiated, no vital tissue was noted within the canal space.
In this case, MTA was placed directly over the blood clot to seal the root canal system. MTA is beneficial in regenerative endodontic procedures due to its superior seal, biocompatibility, hydrophilicity, and most importantly, bioactivity [20]. In vitro studies have demonstrated the upregulation of various cytokines and biologic markers in the presence of MTA such as interleukin (IL)-1α, IL-1β, IL-4, IL-6, IL-8, alkaline phosphatase, bone sialoprotein, osteopontin and BMP-2 [20]. However, one adverse complication found in the present case was the development of discoloration in the coronal tooth structure. This may be attributed to the placement of MTA slightly coronal to the CEJ or the lack of a collagen scaffold within the canal. Petrino et al. [21] demonstrated the advantage of a collagen matrix to control placement of MTA during regenerative endodontic procedures. The use of a collagen matrix can prevent intrusion of MTA into the canal space. The present case also demonstrated the efficacy of internal bleaching without adverse effect to the REP procedure similar to other case reports [22,23].
As demonstrated in figure 1A, a radiolucent area was noted at the periapical region of the replanted tooth which may contain the viable apical papilla. Maintenance of stem cells of the apical papilla (SCAP) is critical to promote continued root development and apical closure [24,25]. SCAP are a population of mesenchymal stem cells residing in the apical papilla of incompletely formed teeth that have the potential to differentiate into odontoblast-like cells which form root dentin [24]. SCAP are paramount to the appropriate regeneration of dental tissues. Thus, emphasis should be placed on maintaining their survival.
Successful regenerative endodontics needs the use of more robust intracanal disinfection techniques. The current use of instrumentation with NaOCl irrigation alone is insufficient to promote conditions necessary for revascularization of a tooth with necrotic pulp [3,7]. Traditional disinfection technique for endodontic regeneration procedures has involved long-term calcium hydroxide applications to form apical barriers in immature teeth [11]. Calcium hydroxide, however, may have a detrimental effect on dentin strength and may increase tooth susceptibility to root fracture [26,27]. Currently, TAP is the most widely used medicament during regenerative endodontic procedures [28]. TAP, and double antibiotic paste (DAP) which lacks the discoloring minocycline, have shown to be more effective than calcium hydroxide against E. faecalis and P. gingivalis [29].
Despite the potential beneficial effects of TAP, many disadvantages remain for the use of this compound. First, one of the most commonly reported complications of TAP is the development of tooth discoloration with unpredictable bleaching results [29]. Due to the strong association of discoloration and minocycline, a search for an alternative has been suggested [30]. Second, the use of TAP as an intracanal medicament requires multiple visits to achieve complete disinfection, which can lengthen and complicate the procedure [7]. Third, and more critical to the success of the endodontic regeneration procedure, the use of TAP has demonstrated harmful effects on the survival of SCAP. One study revealed that the use of TAP, when used at concentrations typically reported in case reports of 1 g/mL, resulted in no survival of SCAP [31]. The use of TAP, DAP or calcium hydroxide have shown negative effects on the proliferation of dental pulp stem cells on dentin which may lead to a decreased success of other vital pulp therapies [32].
MTAD is recommended as a final irrigant in nonsurgical root canal therapy due to its antibacterial property, substantivity and effectiveness in removing smear layer [17,33]. While the presence or absence of smear layer does not have much effect on the attachment of stem cells to dentin, the use of MTAD may possess additional advantages for regenerative endodontic procedures [34]. First, MTAD has demonstrated superior antibacterial efficacy in killing E. faecalis at even a dilution of up to 200 times [35]. Second, MTAD has demonstrated minimal cytotoxicity, 195 times less than 5.25% NaOCl and 50 times less than 3% H2O2 [34]. Third, the use of MTAD has demonstrated maintenance of viable dental pulp stem cells that may be beneficial for vital pulp therapy [36]. In order to further assess the use of MTAD as a disinfectant for regenerative endodontic procedures, future studies are warranted on MTAD and its effect on SCAP viability.
The present case report describes an alternative single-visit disinfection protocol for successful REP of an avulsed tooth with necrotic pulp and symptomatic apical periodontitis. The advantages of this MTAD disinfection protocol include maintenance of the SCAP with demonstrated increases in root thickness and apical closure.
The authors would like to thank the late Dr. Steven Tracey, DDS, MS for his contribution of trauma management in this case.
- Holland GR, Trowbridge HO, Rafter M (2009) Protecting the pulp, preserving the apex. In: Torabinejad M, Walton RE (Eds.) Endodontics, principles and practice. 4th ed. Saunders, Elsevier, Philadelphia.
- Salehrabi R, Rotstein I (2004) Endodontic treatment outcomes in a large patient population in the USA: an epidemiological study. J Endod 30: 846-850. [Crossref]
- Hargreaves KM, Geisler T, Henry M, Wang Y (2008) Regeneration potential of the young permanent tooth: what does the future hold? Pediatr Dent 30: 253-260. [Crossref]
- Witherspoon DE, Small JC, Regan JD, Nunn M (2008) Retrospective analysis of open apex teeth obturated with mineral trioxide aggregate. J Endod 34: 1171–1176. [Crossref]
- Bose R, Nummikoski P, Hargreaves K (2009) A retrospective evaluation of radiographic outcomes in immature teeth with necrotic root canal systems treated with regenerative endodontic procedures. J Endod 35: 1343–1349. [Crossref]
- Torabinejad M, Faras H (2012) A clinical and histological report of a tooth with an open apex treated with regenerative endodontics using platelet-rich plasma. J Endod 38: 864-68. [Crossref]
- Fouad AF (2011) The microbial challenge to pulp regeneration. Adv Dent Res 23: 285-289. [Crossref]
- Nosrat A, Li KL, Vir K, Hicks ML, Fouad AF (2013) Is pulp regeneration necessary for root maturation? J Endod 39: 1291-1295. [Crossref]
- Nair PN (2014) Endodontic biofilm, technology and pulpal regenerative therapy: Where do we go for here? Int Endod J 47: 1003-11. [Crossref]
- Hargreaves KM, Diogenes A, Teixeira FB (2014) Paradigm lost: a perspective on the design and interpretation of regenerative endodontic research. J Endod 40: S65-69. [Crossref]
- Frank AL (1966) Therapy for the divergent pulpless tooth by continued apical formation. J Am Dent Assoc 72: 87-93. [Crossref]
- Torneck CD, Smith JS, Grindall P (1973) Biologic effects of endodontic procedures on developing incisor teeth III—effect of debridement and disinfection procedures in the treatment of experimentally induced pulp and periapical disease. Oral Surg Oral Med Oral Pathol 35: 532-540. [Crossref]
- Sato I, Ando-Kurihara N, Kota K, Iwaku M, Hoshino E (1996) Sterilization of infected root-canal dentine by topical application of mixture of ciprofloxacin, metronidazole and minocycline in situ. Int Endod J 29: 118-24. [Crossref]
- Sato T, Hoshino E, Uematsu H, Noda T (1993) In vitro antimicrobial susceptibility to combinations of drugs on bacteria from carious and endodontic lesions of human deciduous teeth. Oral Microbiol Immunol 8: 172-176. [Crossref]
- Banchs F, Trope M (2004) Revascularization of immature permanent teeth with apical periodontitis: new treatment protocol? J Endod 30: 196-200. [Crossref]
- Windley W 3rd, Teixeira F, Levin L, Sigurdsson A, Trope M (2005) Disinfection of immature teeth with a triple antibiotic paste. J Endod 31: 439-443. [Crossref]
- Tay FR, Hiraishi N, Schuster GS, Pashley DH, Loushine RJ, et al. (2006) Reduction in antimicrobial substantivity of MTAD after initial sodium hypochlorite irrigation. J Endod 32: 970-975. [Crossref]
- Estefan BS, El Batouty KM, Nagy MM, Diogenes A (2016) Influence of age and apical diameter on the success of endodontic regeneration procedures. J Endod 42: 1620-1625. [Crossref]
- Bhaskar SN, Rappaport HM (1973) Dental vitality tests and pulp status. J Am Dent Assoc 86: 409-411. [Crossref]
- Torabinejad M, Parirokh M (2010) Mineral trioxide aggregate: A comprehensive literature review—Part II: Leakage and biocompatibility investigations. J Endod 36: 400-413. [Crossref]
- Petrino JA, Boda KK, Shambarger S, Bowles WR, McClanahan SB (2010) Challenges in regenerative endodontics: a case series. J Endod 36: 536-541. [Crossref]
- Belobrov I1, Parashos P (2011) Treatment of tooth discoloration after the use of white mineral trioxide aggregate. J Endod 37: 1017-1020. [Crossref]
- Miller EK, Lee JY, Tawil PZ, Teixeira FB, Vann WF Jr (2012) Emerging therapies for the management of traumatized immature permanent incisors. Pediatr Dent 34: 66-69. [Crossref]
- Huang GTJ, Sonoyama W, Liu Y, Liu H, Wang S, et al. (2008) The hidden treasure in apical papilla: The potential role in pulp/dentin regeneration and bioroot engineering. J Endod 34: 645-651. [Crossref]
- Palma PJ, Ramos JC, Martins JB, Diogenes A, Figueiredo MH, et al. Histologic Evaluation of Regenerative Endodontic Procedures with the Use of Chitosan Scaffolds in Immature Dog Teeth with Apical Periodontitis. J Endod 43: 1279-1287. [Crossref]
- Cvek M, Nord CE, Hollender L (1976) Antimicrobial effect of root canal debridement in teeth with immature root: A clinical and microbiologic study. Odontol Revy 27: 1-10. [Crossref]
- Hatibovic-Kofman S, Raimundo L, Zheng L, Chong L, Friedman M, et al. (2008) Fracture resistance and histological findings of immature teeth treated with mineral trioxide aggregate. Dent Traumatol 24: 272-276. [Crossref]
- Garcia-Godoy F, Murray PE (2012) Recommendations for using regenerative endodontic procedures in permanent immature traumatized teeth. Dent Traumatol 28: 33-41. [Crossref]
- Sabrah AH, Yassen GH, Gregory RL (2013) Effectiveness of antibiotic medicaments against biofilm formation of Enterococcous faecalis and Porphyromonas gingivalis. J Endod 39: 1385-1389. [Crossref]
- Kahler B, Rossi-Fedele G (2016) A review of tooth discoloration after regenerative endodontic therapy. J Endod 42: 563-569. [Crossref]
- Althumairy RI, Teixeira FB, Diogenes A (2014) Effect of dentin conditioning with intracanal medicaments on survival of stem cells of apical papilla. J Endod 40: 521-525. [Crossref]
- Alghilan MA, Windsor LJ, Palasuk J, Yassen GH (2017) Attachment and proliferation of dental pulp stem cells on dentine treated with different regenerative endodontic protocols. Int Endod J 50: 667-675. [Crossref]
- Mancini M, Armellin E, Casaglia A, Cerroni L, Cianconi L (2009) A comparative study of smear layer removal and erosion in apical intraradicular dentin with three irrigating solutions: A scanning electron microscopy evaluation. J Endod 35: 900-903. [Crossref]
- Ring KC, Murray PE, Namerow KN, Kuttler S, Garcia-Godoy F (2008) The comparison of the effect of endodontic irrigation on cell adherence to root canal dentin. J Endod 34: 1474-1479. [Crossref]
- Torabinejad M, Shabahang S, Aprecio RM, Kettering JD (2003) The antimicrobial effect of MTAD: an in vitro investigation. J Endod 29: 400-403. [Crossref]
- Zhang W1, Torabinejad M, Li Y (2003) Evaluation of cytotoxicity of MTAD using the MTT-tetrazolium method. J Endod 29: 654-657. [Crossref]



