Purpose: Although cataract is the leading cause of blindness worldwide, there are no pharmacological agents to prevent or reverse the disease. Since oxidative stress is implicated in the pathogenesis of cataracts, and hydrogen sulfide (H2S) exhibits antioxidant and neuroprotective properties, the primary objective of this study was to investigate the ability of H2S-releasing compounds to prevent time-dependent loss of transparency in the cultured bovine lens.
Methods: Freshly isolated bovine lenses were cultured in DMEM containing H2S-producing compounds, diallyl trisulfide (DATS), GYY4137 or ascorbic acid (AA; used as positive control). Lens opacity was determined by measuring transmittance (using Synergy H1 hybrid reader) and by visual inspection of cultured lenses up to 120 h. Total glutathione (GSH) content and superoxide dismutase (SOD) activity were determined in lens homogenates using Cayman Chemical Assay kits.
Results: After 120 hours of incubation, DATS (10-7 and 10-6 M) significantly (p<0.001; n=6) attenuated the time-dependent decline in transmittance by 16.91 ± 0.01% and 28.54 ± 0.15%, while GYY4137 (10-7 and 10-6 M) also reduced transmittance by 22.05 ± 0.02% and 2.45 ± 0.32%, respectively. Similarly, DATS (10-7 M and 10-6 M) and GYY4137 (10-7 M and 10-6 M)-treated lenses exhibited relatively clear grids after 120 hours, compared to control and AA (10 mM)-treated lenses. DATS (10-6 M) and GYY4137 (10-7 M) significantly (p<0.001; n=5) reversed time-dependent decline in total GSH content by 69.69 ± 0.01% and 80.52 ± 0.17% and SOD activity by 3.23 ± 2.05% and 19.31 ± 0.89%, respectively.
Conclusion: H2S-releasing compounds prevented time-dependent cultured lens opacification by an action that depends, at least in part, on the integrity of the antioxidant defense pathways, ex vivo.
antioxidants, cataracts, hydrogen sulfide, lens opacification, glutathione, superoxide dismutase
Hydrogen sulfide (H2S) is a gaseous signaling molecule with neuroprotective effects due to its antioxidant, anti-inflammatory, and anti-apoptotic properties in some diseases associated with the central nervous system. H2S is endogenously biosynthesized from four major pathways which involve the following enzymes: cystathionine β-synthase (CBS), cystathionine γ-lyase (CSE), and 3-mercaptopyruvate sulfur transferase (3-MST) coupled with cysteine aminotransferase (CAT) utilizing L-cysteine as a substrate [1-3], and 3MST coupled with d-amino acid oxidase (DAO) utilizing d-cysteine as the substrate [4]. In addition to an endogenous pathway for the biosynthesis of H2S from L-cysteine, polysulfides have also been shown to generate H2S in in vivo systems [5]. Among the polysulfides in garlic, diallyl disulfide (DADS), diallyl trisulfide (DATS), and S-allylcysteine (SAC) can produce H2S in biological systems with DATS and DADS exhibiting a superior ability to generate H2S [6]. In addition to DATS, GYY4137 (morpholin-4-ium-4-methoxyphenyl (morpholino) phosphinodithioate), is an inorganic, water soluble prodrug for the in situ production of H2S. The prodrug GYY4137 could release H2S at a constant rate and maintain steady concentrations, compared to the sulfide salt, sodium hydrosulfide (NaHS) [6].
Oxidative stress has been implicated in the pathogenesis of cataract [7-9]. Destabilized state of crystallins and disrupted ionic environment resulting from depletion UV-filters, α-crystallin, glutathione (GSH) levels, and other antioxidant systems have been reported to result in gradual opacification of lenses [7,10-15]. Moreover, the neuroprotective effects of H2S have been described in ocular tissues by several investigators [16-20]. Deficiency of CBS due to CBS gene mutation has been linked to with ocular disorders such as ectopia lentis, myopia, and cataracts [21]. Futhermore, inhibition, proteolytic degradation, and downregulation of CSE resulted in lenticular GSH depletion and increased risk of cataract formation, suggesting a significant physiological role of H2S in the eye [22-23]. In cultured bovine lenses, the substrate for endogenous production of H2S, L-cysteine attenuated time-dependent cataract formation with a corresponding decline in GSH content and SOD enzyme activity [24]. Accordingly, we designed experiments in the present study using lenses that were cultured ex vivo to observe the time-dependent loss of lens transparency. The aim of the present study was, therefore, to determine whether H2S-releasing compounds (DATS and GYY4137) can prevent the time-dependent loss of transparency in the cultured bovine lenses. Since ascorbic acid (AA) is an endogenous antioxidant that is present in aqueous humor and lens in high concentrations [25-26] and its deficiency is correlated to cataract severity [27-29], it was used as a positive control for these studies. Parts of this manuscript have been presented in abstract format [30].
Lens culture
Bovine eyeballs from J. F. O’Neal Packing Co, Omaha, Nebraska were transported to the laboratory in an ice pack within one hour of enucleation. These eyeballs were dissected, and lenses were extracted and transferred into Kreb’s buffer solution. Dulbecco's Modified Eagle's medium (DMEM) that contains antibiotic (10,000 IU/mL penicillin and 10,000 µg/mL streptomycin) (5 mL/500 mL DMEM) was used as the culturing medium. Twelve-well plates (Thermo Fisher Scientific) were filled with 3 mL of either DMEM with AA (positive control), or DMEM and test compounds (DATS or GYY4137). The lenses were then transferred from Kreb’s buffer solution into each well. The cultures were incubated in a CO2 incubator (Napco e series CO2 incubator model 5100) at 5% CO2 and 350C; where plates were replenished with respective media enriched with the treatment compound every 24 hours.
Assessment of opacity
Transmittance was measured at 420 nm using a plate reader (Synergy H1 hybrid reader; Bio Tek Instruments, Inc) at zero, three, and six hours after culture on the first day followed by 24 hours measurements up to 120 hours. There is evidence that maximum decrease in transmittance is achieved at the 420 nm wavelength, rendering it as an acceptable indicator for the development of opacification in cultured bovine lenses [24]. No transmittance change from initial measurement (0 hour) or an increase in transmittance compared to untreated lenses at 120 hours was taken as prevention of time-dependent loss of lens transparency. Lenses were also evaluated for cataract formation visually and by taking picture against a black grid. Visually assessing lenses for opacity is a classical method that has been used to assess cataract [31]. Visual assessments were conducted at the same time interval along with transmittance measurement.
Biochemical studies
Total glutathione (GSH) content and total superoxide dismutase (SOD) activity of homogenized lenses were evaluated.
Total glutathione content: After 120 hours of culture, each lens was halved (1 g size) and homogenized, deprotonated and supernatant was collected and stored at -200C. The supernatant containing GSH (reduced) and GSSG (oxidized GSH) was assessed as per the protocol (Cayman Chemical, Item no. 703002).
Total superoxide dismutase activity: Similarly, after 120 hours of culture, each lens was halved (1 g size) and homogenized, and supernatant was collected and stored at -800C. The supernatant was assessed for total SOD activity (both cytosolic and mitochondrial) as per the protocol for SOD assay kit (Cayman Chemical, Item no. 706002).
Results are described as arithmetic mean ± standard error of the mean (SEM). Significance of differences between groups was evaluated using two-way analysis of variance (ANOVA) and one-way ANOVA followed by Dunnett’s test (Graph Pad Prism 6), where p values of less than 0.05 were considered statistically significant.
Prevention of time-dependent loss of lens transparency
DATS: The polysulfides, DADS, DATS, and SAC have been shown to release H2S in both in vivo and in vitro systems [5-6,32]. Among these polysulfides, DATS was reported to exhibit a superior ability to releasing H2S in biological media [6]. In the present study, we examined the pharmacological actions of different concentrations of DATS in protecting lenses from time-dependent loss of lens transparency. The lower concentrations of DATS (10-7 M and 10-6 M) attenuated time-dependent decrease in transmittance up to 120 hours. Both concentrations of DATS significantly (p<0.001; n=6) increased transmittance by 16.91 ± 0.01% and 28.54 ±0.15% respectively at 120 hours (Figures 1A). In contrast, the higher concentrations of DATS tested (10-5 M and 10-4 M) did not protect lenses from the time-dependent loss in transparency. The protective action of low concentrations of DATS (10-7 M and 10-6 M) was superior to that of the positive control, AA (10 mM), which was only effective up to 24 hours (Figures 1B). These observations were corroborated by optical clarity of lenses treated with DATS (10-7 M and 10-6 M) up to120 hours (Figure 1C).
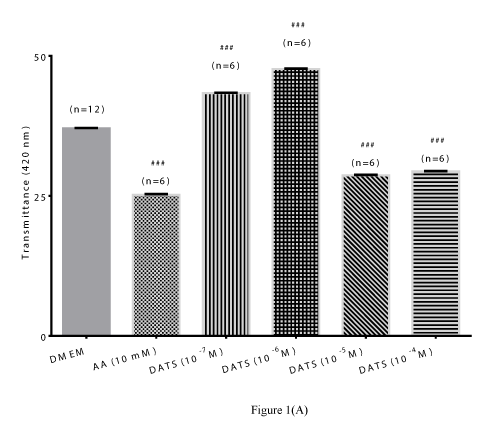
Figure 1 (A)
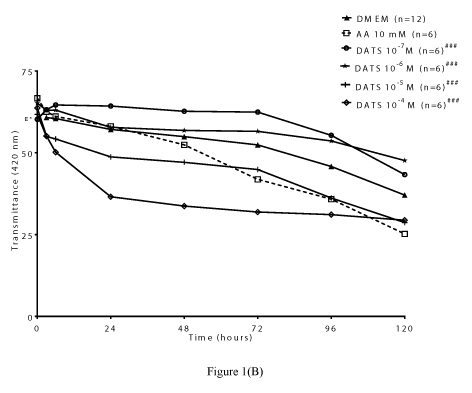
Figure 1(B)
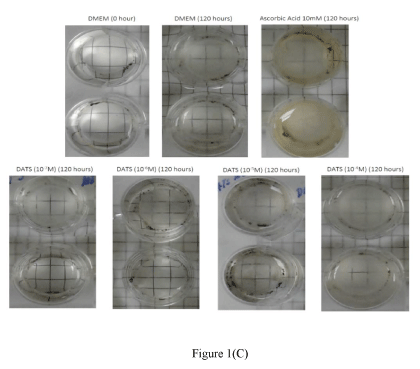
Figure 1(C)
Figure 1. Preventive effect of DATS on time-dependent loss of transparency in cultured bovine lenses. Figure 1A: Effect of DATS on transmittance (402 nm) after 120 hours. Results expressed as mean ± SEM. One-way ANOVA ###p<0.001, significantly different from lenses cultured in DMEM after 120 hours. (n) Denotes number of observations.
Figure 1B. Effect of DATS on transmittance (420 nm) during cataractogenesis up to 120 hours. Two-way ANOVA ###p<0.001, significantly different from lenses cultured in DMEM up to 120 hours. (n) Denotes number of observations. Figure 1C. Optical clarity of lenses treated with different concentrations of DATS after 120 hours; Top panel: controls, bottom panel: lenses cultured in DATS (10-7 M to 10-4 M).
GYY4137: GYY4137 is an inorganic, water soluble prodrug used for the in situ production of H2S [6]. In the present study, we also examined the ability of GYY4137 to protect lenses from time-dependent change in transmittance (420 nm). We observed that GYY4137 (10-7 M and 10-6 M) attenuated the time-dependent decrease in transmittance up to 120 hours. Both lower concentrations of GYY4137 significantly (p<0.001; n=6) increased transmittance by 22.05 ± 0.20% and 2.45 ± 0.32%, respectively after 120 hours (Figures 2A). In comparison with DATS, the higher concentrations of GYY4137 (10-4 M and 10-3 M), also failed to reverse the time-dependent decrease in transmittance. As observed with DATS, the protective action of lower concentrations of GYY4137 (10-7 M and 10-6 M) was also superior to that of AA (10 mM) (Figures 2B). In support of the earlier observation on transmittance by GYY4137 (10-7 M and 10-6 M), lenses displayed a relatively higher optical clarity at 120 hours compared to untreated lenses and AA (10 mM)-treated (Figure 2C).
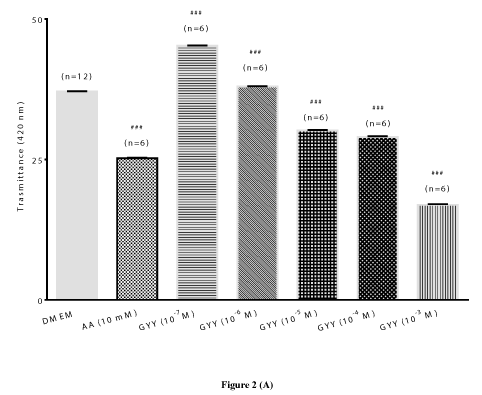
Figure 2 (A)
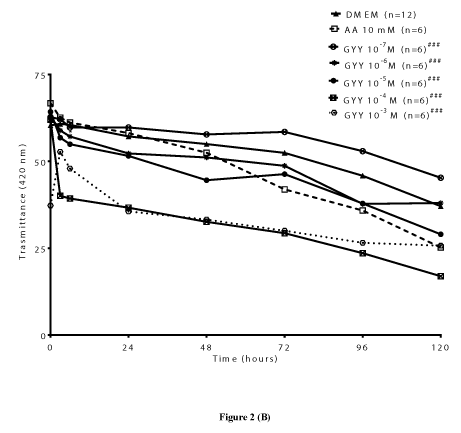
Figure 2 (B)
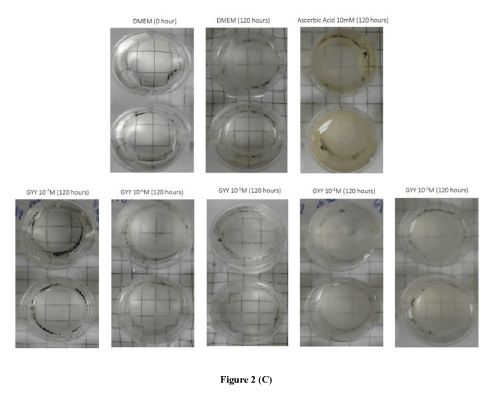
Figure 2 (C)
Figure 2. Preventive effect of GYY4137 (GYY) on time-dependent loss of transparency in cultured bovine lenses. Figure 2A. Effect of GYY on transmittance (420 nm) after 120 hours. Results expressed as mean ± SEM. One-way ANOVA ###p<0.001, significantly different from lenses cultured in DMEM after 120 hours. (n) Denotes number of observations. Figure 2B. Effect of GYY on change in transmittance (420 nm) during cataractogenesis up to 120 hours. Two-way ANOVA ###p<0.001, significantly different from lenses cultured in DMEM up to 120 hours. (n) Denotes number of observations. Figure 2C: Optical clarity of lenses treated with different concentrations of GYY after 120 hours. Top panel: controls, bottom panel: lenses cultured in GYY (10-7 M to 10-3 M).
Role of antioxidants
Total glutathione content: To assess the contribution of oxidative stress in the pharmacological responses elicited by the H2S-releasing compounds, a series of experiments were performed to measure GSH content and SOD activity in bovine lenses in the absence and presence of DATS and GYY4137. There was a significant (p<0.001; n=5), time-dependent decrease (46.08 ± 0.12%) in lenticular total GSH content of untreated lenses after 120 hours. This decline in total GSH content was significantly (p<0.001; p=5) reversed by DATS (10-6 M; 69.69 ± 0.01 %) and GYY4137 (10-7 M; 80.52 ± 0.17%) (Figure 3A and Table 1). Similarly, the lenticular total SOD activity significantly (p<0.001; n=5) decreased by 42.02 ± 8.87% over 120 hours, compared to untreated lens at 0 hour. This decline in SOD activity was also significantly (p<0.001) reversed by both DATS (10-6 M) and GYY4137 (10-7 M) by 3.23 ± 2.05% and 19.31 ± 0.89%, respectively (Figure 3B and Table 1).
Table 1. Effect of hydrogen sulfide producing compounds on total glutathione content and total superoxide dismutase enzyme activity in cultured bovine lens homogenates
Result expressed as mean ± SEM; one way ANOVA ***p<0.001 significantly different from untreated lenses at 0 hour, ### p<0.001 significantly different from untreated lenses at 120 hours. DMEM, Dulbecco's Modified Eagle's medium; GSH, glutathione; SOD, superoxide dismutase. One unit (U) is amount of superoxide dismutase (SOD) needed to induce 50% dismutation of superoxide radical.
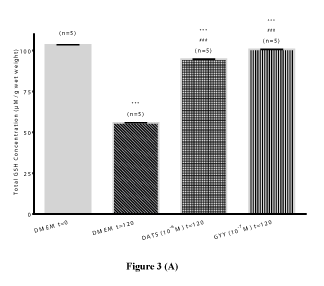
Figure 3 (A)
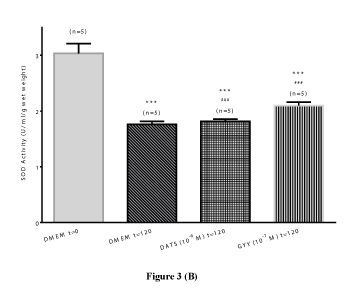
Figure 3 (B)
Figure 3. Preventive effect of hydrogen sulfide producing compounds on time-dependent loss in total glutathione (GSH) content (Figure 3A) and loss in superoxide dismutase (SOD) activity (Figure 3B) in cultured bovine lens homogenates. Results expressed as mean ± SEM. One way ANOVA ***p<0.001, significantly different from untreated lenses at 0 hour, ###p<0.001 significantly different from untreated lenses at 120 hours.
In the past few years, H2S that is synthesized endogenously from L-cysteine by CBS, CSE and 3-MST, has been reported to act as a signaling molecule that regulates cellular redox homeostasis [1-3,33-34]. In the mammalian eye, enzymes responsible for the biosynthesis of H2S (CBS, CSE and 3-MST) have been identified in ocular tissues [35-39]. Defects in CBS and CSE have been linked to cataracts, adding more evidence to the physiological role of H2S in the eye [21-22,40-41]. Taken together, evidence available supports a significant role for H2S in the pathophysiology and pharmacology of ocular tissues.
There is evidence that H2S possesses neuroprotective, antioxidant, anti-inflammatory and anti-apoptotic properties in several CNS disease models [3,42-43] and ocular tissues [16,19,43]. For instance, H2S-releasing compounds attenuated excitatory amino acid neurotransmission in bovine retina, in vitro [16] and attenuated RGC loss [19] and retinal apoptosis [43] in experimental rat glaucoma models. Since oxidative stress has been reported to play a major role in the pathogenesis of age-related cataract, several studies have been aimed at mitigating cataract formation by using antioxidants to target various antioxidant systems [44-50]. Mechanisms reported to account for the antioxidant activity of H2S include free radical scavenging reaction [51], increase in GSH content [24,52] and SOD activity [24]. Therefore, in this study, we further examined the role of SOD and GSH on H2S-mediated protection from time-dependent lens opacification in bovine lenses, ex vivo.
ln the present study, we found that both H2S-releasing compounds tested, DATS and GYY4137 protected lenses from time-dependent degradation in opacity. Comparing the effect elicited by these different H2S-releasing compounds, the lower concentrations of DATS and GYY4137 were more effective at attenuating time-dependent decline in transmittance than those elicited by the higher concentrations after 120 hours. Indeed, at the highest concentrations tested, both H2S-releasing compounds did not protect lenses from time-dependent loss in transparency. Interestingly, lower concentrations (10-6M and 10-5M) of L-cysteine, the substrate for endogenous biosynthesis of H2S were more potent at preventing cataract formation in cultured bovine lenses than that exhibited by higher concentrations (10-3M) of the amino acid [24]. The increased ability of low concentrations of DATS and GYY4137 to prevent time-dependent loss of lens opacity may be due to several factors such as the rate of H2S release from these compounds, physical/chemical characteristics of the agents (e.g hydrophobicity and /or hydrophilicity), and the presence of transporters. To the best of our knowledge, this is the first report in literature of the protective action of DATS and GYY4137 against cataract formation in an ex vivo study.
AA is an endogenous antioxidant that is present in high concentrations in the aqueous humor and lens [25-26] and its deficiency has been reported to correlate to cataract severity [27-29]. AA plays a pivotal antioxidant role in the lens, where it mitigates oxidative stress by serving as free radical scavenger and a filter against ultraviolet light [53-54]. In experimental animals, AA has been reported to attenuate cataract formation in selenite-induced cataract formation in New Zealand albino rabbits [55] and Sprague–Dawley rat pups [50,56]; and galactose-induced cataract formation in guinea pigs [57], in vivo. It was interesting to note that the lower concentrations of both DATS and GYY4137 were more potent than that elicited by AA (used as a positive control), which exhibited a modest protection up to 24 hours only. It is conceivable that H2S donors mitigate cataract formation by mechanisms that are distinct from that elicited by AA, thereby affirming the multifactorial etiology of cataract.
The lens has a sturdy defense system against oxidation [58-60]. Yet, depletion of the antioxidant defense systems has been associated with lens opacification [7,60-63]. In the present study, when compared to untreated lenses (at time 0 hour), lenticular total GSH content and total SOD activity were significantly (p<0.001) reduced by about 46% and 42% respectively, after 120 hours of incubation. In support of these observations, Heruye et al (2019) reported a similar time-dependent loss in GSH content and SOD activity in cultured bovine lenses, ex vivo [24]. Taken together, these observations support the view that the time-dependent opacification was accompanied with compromises in antioxidant defense system. It was interesting to note that both DATS and GYY4137 significantly (p<0.001) reversed time-dependent decline in total GSH content by 70% and 80%, respectively. Furthermore, these H2S-releasing compounds, DATS and GYY4137, blocked time-dependent reduction in total SOD activity by 3% and 19% respectively. Similarly, lower concentrations of L-cysteine have been shown to attenuate time-dependent loss in SOD activity and GSH content in cultured bovine lenses, ex vivo [24]. These results indicate that the loss of total GSH content and reduction in SOD activity may have contributed, at least in part, to the pharmacological actions of these H2S-releasing compounds on compounds on lens opacification.
The slow releasing H2S-compound, GYY4137 was the most prominent in elevating lenticular non-enzymatic antioxidant (GSH) and enzymatic antioxidant activity (SOD) than DATS, while it was not as prominent in protecting lenses from time-dependent loss of transparency. A similar observation was made by other investigators in astrocytes where these H2S-releasing compounds enhanced glutamate transporter-1 activity and elevated GSH production as with varying capabilities [63]. It appears that that the differences in responses elicited by the two H2S -releasing compounds in the present study may be due to their mechanism/s of action on enzymatic and non-enzymatic pathways. It is feasible that multiple and distinctive pathways are involved in the time-dependent cataract formation and upregulation of total GSH content and total SOD activity.
Although cataract is a leading cause of blindness worldwide, there are no FDA-approved pharmacological treatments that can mitigate or reverse this ocular disease in humans. Surgery has been the treatment of choice for decades, presenting a major treatment barrier in developing nations with limited access to proper healthcare. The finding that H2S-releasing compounds can mitigate cataract formation suggests a potential prophylactic role for these compounds in cataractogenesis. Interestingly, H2S-releasing compounds such are DATS are abundant in nature, rendering them accessible to developing nations that lack easy access to cataract surgery. Therefore, data from the present study support the development of new treatment modalities that can mitigate or reverse cataracts.
We conclude that H2S-releasing compounds can mitigate the time-dependent loss in opacity in cultured bovine lenses up to a period of 120 hours. The pharmacological actions produced by these compounds are due, at least in part, to their interaction with the antioxidant defense pathways in the cultured bovine lens.
All the authors made significant contribution to the study.
Creighton University, School of Pharmacy and Health professions, Department of Pharmacy Sciences, Pharmaceutical Sciences Master Program.
Authors have no conflict of interest
- Abe K, Kimura H (1996) The possible role of hydrogen sulfide as an endogenous neuromodulator. J Neurosci 16: 1066-1071. [Crossref]
- Shibuya N, Tanaka M, Yoshida M, Ogasawara Y, Togawa T, et al. (2009) 3-Mercaptopyruvate sulfurtransferase produces hydrogen sulfide and bound sulfane sulfur in the brain. Antioxid Redox Signal 11: 703-714. [Crossref]
- Chen WL, Niu YY, Jiang WZ, Tang HL, Zhang C, et al. (2015) Neuroprotective effects of hydrogen sulfide and the underlying signaling pathways. Rev Neurosci 26: 129-142. [Crossref]
- Cao X, Ding L, Xie ZZ, Yang Y, Whiteman M, et al. (2019) A Review of hydrogen sulfide synthesis, metabolism, and measurement: is modulation of hydrogen sulfide a novel therapeutic for cancer? Antioxid Redox Signal 31: 1-38. [Crossref]
- Benavides GA, Squadrito GL, Mills RW, Patel HD, Isbell TS, et al. (2007) Hydrogen sulfide mediates the vasoactivity of garlic. Proc Natl Acad Sci USA 104: 17977-17982. [Crossref]
- Zheng Y, Yu B, De La Cruz LK, Choudhury MR, et al. (2018) Toward hydrogen sulfide-based therapeutics: critical drug delivery and developability issues. Med Res Rev 38: 57-100. [Crossref]
- Spector A (1995) Oxidative stress-induced cataract: mechanism of action. FASEB J 9: 1173-1182. [Crossref]
- Kaur J, Kukreja S, Kaur A, Malhotra N, Kaur R (2012) The oxidative stress in cataract patients. J Clin Diagn Res 6: 1629-1632. [Crossref]
- Liu XF, Hao JL, Xie T, Malik TH, Lu CB, et al. (2017) Nrf2 as a target for prevention of age-related and diabetic cataracts by against oxidative stress. Aging Cell 16: 934-942. [Crossref]
- Robertson JM, Donner AP, Trevithick JR (1989) Vitamin E intake and risk of cataracts in humans. Ann N Y Acad Sci 570: 372-382. [Crossref]
- Truscott RJ (2005) Age-related nuclear cataract-oxidation is the key. Exp Eye Res 80: 709-725. [Crossref]
- Williams DL (2006) Oxidation, antioxidants and cataract formation: A literature review. Vet Ophthalmol 9: 292-298. [Crossref]
- Hains PG, Truscott RJ (2007) Post-translational modifications in the nuclear region of young, aged, and cataract human lenses. J Proteome Res 6: 3935-3943. [Crossref]
- Hains PG, Truscott RJ (2008) Proteomic analysis of the oxidation of cysteine residues in human age-related nuclear cataract lenses. Biochim Biophys Acta 1784: 1959-1964. [Crossref]
- Michael R, Bron AJ (2011) The ageing lens and cataract: a model of normal and pathological ageing. Philos Trans R Soc Lond B Biol Sci 366: 1278-1292. [Crossref]
- Opere CA, Monjok EM, Kulkarni KH, Njie YF, Ohia SE (2009) Regulation of [3H] D-aspartate release from mammalian isolated retinae by hydrogen sulfide. Neurochem Res 34: 1962-1968. [Crossref]
- Ohia SE, Robinson J, Mitchell L, Ngele KK, Heruye S, et al. (2018) Regulation of aqueous humor dynamics by hydrogen sulfide: potential role in glaucoma pharmacotherapy. J Ocul Pharmacol Ther 34: 61-69. [Crossref]
- Mikami Y, Shibuya N, Kimura Y, Nagahara N, Yamada M, et al. (2011) Hydrogen sulfide protects the retina from light-induced degeneration by the modulation of Ca2+ influx. J Biol Chem 286: 39379-39386.
- Huang S, Huang P, Liu X, Lin Z, Wang J, et al. (2017) Relevant variations and neuroprotecive effect of hydrogen sulfide in a rat glaucoma model. Neuroscience 341: 27-41. [Crossref]
- Du J, Jin H, Yang L (2017) Role of hydrogen sulfide in retinal diseases. Front Pharmacol 8: 588.
- Mudd SH, Levy HL, Kraus JP (2001) Disorders of transsulfuration. In: Scriver CR, Beaudet AL, Sly WS, Valle D, editors. The metabolic and molecular bases of inherited disease. New York: McGraw-Hill; 2001: 2007-2056.
- Sastre J, Martin JA, Gomez-Cabrera MC, Pereda J, Borrás C, et al. (2005) Age-associated oxidative damage leads to absence of gamma-cystathionase in over 50% of rat lenses: relevance in cataractogenesis. Free Radic Biol Med 38: 575-852. [Crossref]
- Heruye SH, Maffofou Nkenyi LN, Singh NU, Yalzadeh D, Ngele KK et al. (2020) Current trends in the pharmacotherapy of cataracts. Pharmaceuticals 13: 15. [Crossref]
- Heruye SH, Maffofou Nkenyi LN, Singh NU, Munt D, Njie-Mbye YF, et al. (2019) Standardization of a new method for assessing the development of cataract in cultured bovine lenses. J Pharmacol Toxicol Methods 98: 106592. [Crossref]
- Varma SD, Chand D, Sharma YR, Kuck JF Jr., Richards RD (1984) Oxidative stress on lens and cataract formation: role of light and oxygen. Cur Eye Res 3: 35-57. [Crossref]
- Senthilkumari S, Talwar B, Dharmalingam K, Ravindran RD, Jayanthi R, et al. (2014) Polymorphisms in sodium-dependent vitamin C transporter genesand plasma, aqueous humor and lens nucleus ascorbate concentrations in an ascorbate depleted setting. Exp Eye Res. 124: 24-30. [Crossref]
- Tessier F, Moreaux V, Birlouez-Aragon I, Junes P, Mondon H (1998) Decrease in vitamin C concentration in human lenses during cataract progression. Int J Vitam Nutr Res 68: 309-315.
- Ravindran RD, Vashist P, Gupta SK, Young IS, Maraini G, et al. (2011) Inverse association of vitamin C with cataract in older people in India. Ophthalmology 118: 1958-1965.
- Lim JC, Caballero Arredondo M, Braakhuis AJ, Donaldson PJ (2020) Vitamin C and the lens: New insights into delaying the onset of cataract. Nutrients 12: 3142. [Crossref]
- Opere CA, Heruye SH, Njie-Mbye YF, Ohia SE (2021) Regulation of cataract formation in bovine lenses by dallyl trisulfide. 3rd International Pharmscience R&D Virtual meeting https://vimeo.com/514208215.
- Cicchetti DV, Sharma Y, Cotlier E (1982) Assessment of observer variability in the classification of human cataracts. Yale J Biol Med 55: 81-88. [Crossref]
- Zhao W, Zhang J, Lu Y, Wang R (2001) The vasorelaxant effect of H2S as a novel endogenous gaseous KATP channel opener. EMBO J 20: 6008-6016. [Crossref]
- Xie ZZ, Liu Y, Bian JS (2016) Hydrogen sulfide and cellular redox homeostasis. Oxid Med Cell Longev 2016: 6043038. [Crossref]
- Beltowski J (2019) Synthesis, metabolism, and signaling mechanisms of hydrogen sulfide: An overview. Methods Mol Biol 2007:1-8. [Crossref]
- Pong WW, Stouracova R, Frank N, Kraus JP, Eldred WD (2007) Comparative localization of cystathionine beta-synthase and cystathionine gamma lyase in retina: differences between amphibians and mammals. J Comp Neurol 505: 158-165. [Crossref]
- Kulkarni KH, Monjok EM, Zeyssig R, Kouamou G, Bongmba ON, et al. (2009) Effect of hydrogen sulfide on sympathetic neurotransmission and catecholamine levels in isolated porcine iris-ciliary body. Neurochem Res 34: 400-406. [Crossref]
- Salvi A, Bankhele P, Jamil JM, Kulkarni-Chitnis M, Njie-Mbye YF, et al. (2015). Pharmacological actions of hydrogen sulfide donors on sympathetic neurotransmission in the bovine anterior uvea, in vitro. Neurochemical Res 41: 1020-1028. [Crossref]
- Takir S, Semiz AT, Uydes Dogan BS (2021) Hydrogen sulfide is synthesized endogenously in both retinal artery and retina mostly via CSE. Exp Eye Res 13: 108443. [Crossref]
- Kozich V, Sokolová J, Morris AAM, Pavlíková M, Gleich F, et al. (2020) E-HOD consortium (2020) Cystathionine β-synthase deficiency in the E-HOD registry-part I: pyridoxine responsiveness as a determinant of biochemical and clinical phenotype at diagnosis. J Inherit Metab Dis 44: 677-692. [Crossref]
- Fatima S, Hafeez A, Ijaz A, Asif N, Awan A, et al. (2018) Classical homocystinuria in a juvenile patient. J Coll Physicians Surg Pak 28: 488-489.
- Paul BD, Snyder SH (2018) Gasotransmitter hydrogen sulfide signaling in neuronal health and disease. Biochem Pharmacol 149: 101-109.
- Kumar M, Sandhir R (2018) Hydrogen sulfide in physiological and pathological mechanisms in brain. CNS Neurol Disord Drug Targets 17: 654-670.
- Erisgin Z, Ozer MA, Tosun M, Ozen S, Takir S (2019) The effects of intravitreal H2S application on apoptosis in the retina and cornea in experimental glaucoma model. Int J Exp Pathol 100: 330-336. [Crossref]
- Zigler JS, Qin C, Kamiya T, Krishna MC, Cheng QF, et al. (2003) Tempol-H inhibits opacification of lenses in organ culture. Free Rad Biol Med 35:1194-1202. [Crossref]
- Dubey S, Deep P, Singh AK (2016) Phytochemical characterization and evaluation of anticatarct potential of seabuckthorn leave extract. Vet Opthamol 19: 144-148. [Crossref]
- Dubey S, Saha S, Kaithwas G, Saraf SA (2015) Effect of standardized fruit extract of Luffa cylindrica on oxidative stress markers in hydrogen peroxide induced cataract. Indian J Pharmacol 47: 644-648. [Crossref]
- Dubey S, Saha S, Saraf SA (2015) In vitro anti-cataract evaluation of standardised Abies pindrow leaf extract using isolated goat lenses. Nat Prod Res 29: 1145-1148. [Crossref]
- Nakazawa Y, Oka M, Bando M, Takehana M (2015) Hesperetin prevents selenite-induced cataract in rats. Mol Vis 21: 804-810. [Crossref]
- Nakazawa Y, Oka M, Tamura H, Takehana M (2016) Effect of hesperetin on chaperone activity in selenite-induced cataract. Open Med (Wars) 11: 183-189. [Crossref]
- Nakazawa Y, Nagai N, Ishimori N, Oguchi J, Tamura H. (2017) Administration of antioxidant compounds affects the lens chaperone activity and prevents the onset of cataracts. Biomed Pharmacother. 95: 137-143. [Crossref]
- Whiteman M, Armstrong JS, Chu SH, Jia-Ling S, Wong BS, et al. (2004) The novel neuromodulator hy-drogen sulfide: an endogenous peroxynitrite 'scavenger'? J Neurochem 90: 765-768. [Crossref]
- Kimura H (2011) Hydrogen sulfide: its production, release and functions. Amino Acids 41: 113-121. [Crossref]
- Reddy VN, Giblin FJ, Lin LR, Chakrapani B (1998) The effect of aqueous humor ascorbate on ultraviolet-B-induced DNA damage in lens epithelium. Invest Ophthalmol Vis Sci 39: 344-350.
- Ohta Y, Niwa T, Yamasaki T (2001) Effect of prolonged marginal ascorbic acid deficiency on lenticular levels of antioxidants and lipid peroxide in guinea pigs. Int J Vitam Nutr Res 71: 103-109. [Crossref]
- Peighmbarzadeh SZ, Tavana M (2014) Attenuation of experimental cataract by vitamin c in rabbits. WALIA J 30: 204-207.
- Jahadi Hosseini HR, Aminlari M, Khalili MR (2008) Prevention of selenite-induced cataract by L-cysteine and vitamin C in rats. Iran Red Crescent Med J 10: 281-287.
- Yokoyama T, Sasaki H, Giblin FJ, Reddy VN (1994) A physiological level of ascorbate inhibits galactose cataract in guinea pigs by decreasing polyol accumulation in the lens epithelium: a dehydroascorbate-linked mechanism. Exp Eye Res 58: 207-218. [Crossref]
- Augusteyn RC (1981) Protein modification in cataract: possible oxidative mechanisms. In Mechanisms of Cataract Formation in the Human Lens. Duncan, G. (Ed.), Academic Press, New York; 1981:72–115.
- Wagner BJ, Margolis JW, Garland D, Roseman JE (1986) Bovine lens neutral proteinase preferentially hydrolyses oxidatively modified glutamine synthetase. Exp Eye Res 43: 1141-1143. [Crossref]
- Jahngen-Hodge J, Cry D, Laxman E, Taylor A (1992) Ubiquitin and ubiquitin conjugates in human lens. Exp Eye Res 55: 897-902. [Crossref]
- Andley UP, Liang JJ-N, Lou MF (2002) Biochemical Mechanisms of age-related cataract. In Principle, Practices in Ophthal-mology. P Albert, DM, Jakobiec, F.A. (Eds.), WB Saunders; Philadelphia, USA, 2002; 1428-1449.
- Lou MF (2003) Redox regulation in the lens. Prog Retin Eye Res 22: 657-682. [Crossref]
- Lu M, Hu LF, Hu G, Bian JS (2008) Hydrogen sulfide protects astrocytes against H2O2-induced neural injury via enhancing glutamate uptake. Free Radic Biol Med 45: 1705-1713.








