Abstract
Spinal cord injury (SCI) and associated neuropathic pain are among the most complex medical conditions to treat and thus therapies that are consistently effective across patient populations do not exist. The development of more effective therapies is hampered by the animal models widely used in SCI research. Currently, spinal cord research to advance the discovery and development of novel therapies primarily relies on rodent models that pose significant translational limitations due to differences in size and physiology to humans. Swine, in contrast, compare more favorably to humans with respect to spine and spinal cord anatomy and vasculature, injury-induced immune response, and functional assessments of pain. Additionally, specific breeds of swine such as the Wisconsin Miniature Swine™ (WMS™) possess thoracic spine that is more similar to the human spine in terms of length, vertebral body size, and overall shape, when compared to a conventional breed of swine. In the current pilot study, we examine the ex vivo distribution of infusate in the WMS™ spinal cord to demonstrate the reproducibility of infusions and thus the suitability of WMS™ for the development of novel SCI therapy delivery platforms such as convection enhanced delivery. Infusions of bromophenol blue dye were performed in 3 cadaver spines through the superior intervertebral space via a needle at 4 locations (C6, T2, T10 and L2). Thirty minutes later a laminectomy was performed to remove the spinal cord and measure the distance travelled by dye. Spinal cord location of the dye injection had a significant (P < 0.005) effect on the diffusion distance of the dye. The reproducibility of infusion data allowed for determination of differences even when using a small number of spines. This pilot study indicates the feasibility of using WMS™ as a platform for developing delivery devices.
Keywords
animal models, spinal cord injury, miniature swine, spinal cord injection
Introduction
The annual incidence of spinal cord injury (SCI) in the United States is approximately 17,000 with a total prevalence of around 276,000 patients [1]. Many patients experience loss of motor function, neurological deficits, and neuropathic pain (NP). While many promising molecular, gene and cell therapies are being explored for SCI and NP, advances to clinically relevant solutions are hampered by a critical gap that exists in the research and preclinical development process. Most SCI and NP research occur in small animal models, such as mice [2-5] or rat [6-9] models. While these rodent models have led to further understanding of the molecular mechanisms of SCI, no therapy shown to be safe and effective in rodent studies has advanced through clinical trials and shown convincing efficacy in treating human SCI [10].
Direct drug delivery to the spine cannot be accomplished in a translatable manner in rodent models. Small animals do not adequately model human SCI due to disproportional spinal cord surface to volume ratios. In such models, simple diffusion drives pharmaceutical delivery, bathing a large segment of the spinal cord and often producing notable effects. However, promising therapies developed in rodents when advanced to humans often fail to demonstrate a substantial effect in the human spinal cord where simple diffusion does not deliver the therapy into portions of the transverse spinal cord. Thus, a relevant large animal model of spinal cord pathology is needed to overcome this significant translational limitation.
We published an extensive review [11] that concluded swine, next to non-human primates, best model humans with respect to (1) spine and spinal cord anatomy, (2) spinal vasculature, (3) immune responses, and (4) assessment of higher neural function, and thus are most suitable for advancing the development of novel therapies and delivery systems such as convection enhanced delivery (CED) [12-14]. However, previously developed swine models of SCI that generally use conventional breeds of swine pose a practical challenge in the research setting.
Conventional breeds of swine typically reach 100 kg (220 lb.) by 4 months of age and 249–306 kg (550–675 lb.) at full maturity [15], and are especially problematic for long-term (weeks to months) studies of SCI and recovery. In contrast, miniature swine breeds such as the Wisconsin Miniature Swine™ (WMS™) range from 25–50 kg (55–110 lb.) at 4 months of age and 68–91 kg (150–200 lb.) at full maturity, approximating the size of an average human, and can be maintained at adult human sizes for several years. We recently characterized the WMS™ spine anatomy and showed its similarity to that of humans [16], particularly in aspects that are relevant to the development of a CED platform and neurocatheters.
With our current pilot study, we examine the ex vivo distribution of infusate in the WMS™ spinal cord to evaluate the reproducibility of infusions and thus the suitability of WMS™ for the development of novel SCI therapy delivery platforms.
Methods
All experiments involving animals were conducted under protocols approved by the University of Wisconsin-Madison Institutional Animal Care and Use Committee in accordance with published NIH and USDA guidelines. Three 5–6 month-old WMS™ (approx. 50 kg) bred and maintained at the Swine Research and Teaching Center (SRTC; University of Wisconsin-Madison) were euthanized by electrical stunning of the brain followed by exsanguination. The WMS™ cadaver spines (n=3) were dissected down to the bone tissue prior to infusions; this was done to more accurately guide and confirm the positioning of the needle at target sites. Needles (28G; 0.36mm) were inserted through the superior intervertebral space into the spinal cord at four locations on the spine (C6, T2, T10 and L2) and then bromophenol blue dye was infused at a constant rate (50µL over 15 seconds) into the cord (Figure 1). Thirty minutes after infusion, and following a total laminectomy, the spinal cord was carefully removed and measurements of dye diffusion were taken by 2 independent observers.
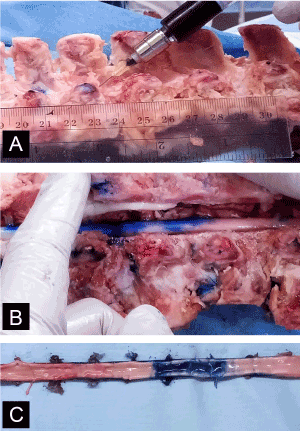
Figure 1[A] Bromophenol blue dye (50 µL over 15 seconds) was injected into WMS cadaver spines through the superior intervertebral space with a 28G (0.36mm) needle at specified locations. [B] A total laminectomy was performed and the spinal cord was removed. [C] Measurements of dye diffusion were made by two independent observers.
Statistical analysis
A fixed-effects analysis of variance (ANOVA) model was fit for the measured parameter (dye diffusion distance) using the PROC MIXED function (SAS Software (Version 8), SAS Institute Inc, Cary, NC) to test for significant effects of spinal cord location of injection on dye diffusion distance. Correlations between observations taken on the same spinal cord was modeled using a compound symmetry correlation structure. For the measured dependent variable, the model was fit using the untransformed data, and the residuals were evaluated to ensure that standard ANOVA assumptions of constant variance and normality were reasonably met. Transformation of the data was not required to improve adherence to these assumptions. Type III tests were performed to evaluate significance and least-square means were calculated. Any least-square means comparisons made subsequent to the Type III tests were adjusted using the Tukey-Kramer p-value adjustment. The data is reported as least-square mean ± SEM. Statistical significance was accepted at P < 0.05.
Results
The total WMS™ spine length was 583.7 ± 21.5 mm and is comparable to published values of humans (mean age: 72 years old (55-84 years)) and conventional swine (breed: Landrace, age: 4 months, mean weight: 40 kg), which are 569.4 mm and 569.5 mm, respectively [17]. The location of bromophenol blue dye injection had a significantly effect (P < 0.005) on the distance of dye diffusion (Figure 2). The mean distance of diffusion at C6, T2, T10 and L2 were 78.3 ± 8.7 mm, 85.7 ± 8.8 mm, 65.0 ± 8.7 mm and 61.7 ± 8.7 mm, respectively. The distance of diffusion at C6 was significantly greater than those at T10 (P < 0.05) and L2 (P< 0.01); distance of diffusion at T2 was also significantly greater than those at T10 (P<0.01) and L2 (P< 0.005).
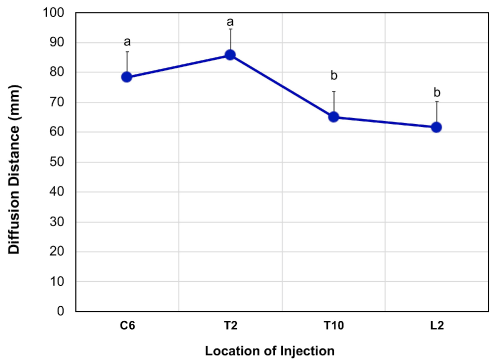
Figure 2Distance of dye diffusion 30 minutes after 50 µL injection of bromophenol blue dye into the spinal cord at C6 (n=3), T2 (n=2), T10 (n=3) and L2 (n=3) vertebrae locations with a 28G (0.36mm) needle. The diffusion distance was significantly (p<0.05) different between those at C6 and L2. The data is presented as least square mean ± SEM. Means without a common letter statistically differ (P < 0.05).
Discussion
The primary finding of the pilot study is that delivery of infusate into the WMS™ spinal cord can be done in a reproducible manner, suggesting that studies to model targeted therapy delivery parameters and distribution are feasible in this swine breed. In addition to the human-like size of the breed, the growth rate of the breed is not as rapid as that of conventional swine. This is of particularly importance to the usefulness of the breed in SCI studies, where the rapidly growing spine and spinal cord of conventional swine would not anatomically and physiologically model the comparatively static nature of the injured spine and spinal cord of a human adult. The size of the WMS™ also accommodates the use of clinical imaging modalities such as MRI, CT, and PET, which are essential for developing imaging-guided drug delivery systems.
Drug-based therapies for SCI and NP management, administered intrathecally for example, have limited efficacy in humans [19]. This is in part because the mode of delivery does not ensure that the therapies reach the targeted region in the spinal cord. Systemic delivery of a promising agent is hampered by the blood/spinal cord barrier, while intrathecal delivery does not allow sufficient diffusion of the agent to the specific site of injury. There is a large unmet need to target, visualize, and control delivery in humans to specific white and gray matter spinal cord fascicles to generate the desired treatment while avoiding undesired side effects. CED is also being pursued for many brain-based pathologies and our group has previously optimized CED parameters (e.g., backflow, infusion cloud morphology, and volume of distribution) for brain in vitro, ex vivo, and in vivo with non-human primates [13,20-23]. The advantage of CED is its potential for delivering therapeutic agents to a larger (rostral-caudal) portion of the dorsal gray matter. The interstitial space within white matter is less dense than in gray matter which makes it difficult to target specific regions within the spinal cord thus affecting the efficacy and accuracy of infusion. CED uses continuous positive pressure gradients that expands the interstitial space and increases fluid penetrance. It offers efficient, targeted, homogeneously dispersed infusions within the spinal cord using a minimally invasive procedure while preserving the health and integrity of the tissues. For example, in this study, we observed significantly greater dye diffusion distances at C6 and T2. This may be due to the greater number of white matter tracts in superior regions of the spinal cord. Longitudinal white matter tracts provide less resistance to fluid movement than the denser gray matter of the spinal cord [18]. CED may provide the ability to overcome differences in passive diffusion rates that exist in different segments of the spinal cord. However, as this is a pilot study (i.e., the study used spines from only three swine), care should be taken in making broad inferences about the results.
A reliable and translational model, such as the WMS™, is vital for advancing the development of CED into the spinal cord. The development of CED also involves the concurrent development of several other technologies and techniques. These include the development of MRI-guided minimally invasive surgical devices for guiding specially-designed neurocatheters into the spinal cord at desired locations and MRI–guidance protocols to quantitatively assess infusion into SC gray matter and minimize migration along the white matter tracts. Additionally, improved understanding of fluid dynamics within the nervous tissue is needed to refine MRI software that can predict, monitor, and alter CED infusions in real-time to create ellipsoidal treatment zones along tracts of the spinal cord [24-27].
Acknowledgements
We would like to thank Kush Patel, and Lisa Liu for their technical contributions to the study.
Funding statement
The research described in the manuscript was supported by discretionary funds from the research program of Dr. Shanmuganayagam.
References
- (2016) National Spinal Cord Injury Statistical Center. Spinal cord injury facts and figures at a glance. [Cited January 2, 2017]. [Available from: https://www.nscisc.uab.edu].
- Gaudet AD, Mandrekar-Colucci S, Hall JC, Sweet DR, Schmitt PJ, et al. (2016) Mir-155 deletion in mice overcomes neuron-intrinsic and neuron-extrinsic barriers to spinal cord repair. J Neurosci 36: 8516-8532. [Crossref]
- White SV, Czisch CE, Han MH, Plant CD, Harvey AR, et al. (2016) Intravenous transplantation of mesenchymal progenitors distribute solely to the lungs and improve outcomes in cervical spinal cord injury. Stem Cells 34: 1812-1825. [Crossref]
- Coll-Miro M, Francos-Quijorna I, Santos-Nogueira E, Torres-Espin A, Bufler P, et al. (2016) Beneficial effects of il-37 after spinal cord injury in mice. Proc Natl Acad Sci U S A 113: 1411-1416. [Crossref]
- Butenschon J, Zimmermann T, Schmarowski N, Nitsch R, Fackelmeier B, et al. (2016) Psa-ncam positive neural progenitors stably expressing bdnf promote functional recovery in a mouse model of spinal cord injury. Stem Cell Res Ther 7: 11. [Crossref]
- Miranpuri GS, Schomberg DT, Alrfaei B, King KC, Rynearson B, et al. (2016) Role of Matrix Metalloproteinases 2 in Spinal Cord Injury-Induced Neuropathic Pain.Ann Neurosci23: 25-32. [Crossref]
- Liu G, Fan G, Guo G, Kang W, Wang D, et al. (2017) FK506 Attenuates the Inflammation in Rat Spinal Cord Injury by Inhibiting the Activation of NF-κB in Microglia Cells.Cell Mol Neurobiol37: 843-855. [Crossref]
- Kloefkorn HE, Pettengill TR, Turner SM, Streeter KA, Gonzalez-Rothi EJ, et al. (2017) Automated gait analysis through hues and areas (agatha): A method to characterize the spatiotemporal pattern of rat gait. Ann Biomed Eng 45: 711-725. [Crossref]
- Wu C, Chen J, Liu Y, Zhang J, Ding W, et al. (2016) Upregulation of PSMB4 is Associated with the Necroptosis after Spinal Cord Injury.Neurochem Res41: 3103-3112. [Crossref]
- Kwon BK, Streijger F, Hill CE, Anderson AJ, Bacon M, et al. (2015) Large animal and primate models of spinal cord injury for the testing of novel therapies. Exp Neurol 269: 154-168. [Crossref]
- Schomberg DT, Miranpuri GS, Chopra A, Patel K, Meudt JJ, et al. (2017) Translational Relevance of Swine Models of Spinal Cord Injury.J Neurotrauma34: 541-551. [Crossref]
- Lonser RR, Gogate N, Morrison PF, Wood JD, Oldfield EH (1998) Direct convective delivery of macromolecules to the spinal cord.J Neurosurg89: 616-622. [Crossref]
- Miranpuri G, Hinchman A, Wang A, Schomberg D, Kubota K, et al. (2013) Convection enhanced delivery: A comparison of infusion characteristics in ex vivo and in vivo non-human primate brain tissue. Ann Neurosci 20. [Crossref]
- Lonser RR (2017) Imaging of Convective Drug Delivery in the Nervous System.Neurosurg Clin N Am28: 615-622. [Crossref]
- Schomberg DT, Tellez A, Meudt JJ, Brady DA, Dillon KN, et al. (2016) Miniature swine for preclinical modeling of complexities of human disease for translational scientific discovery and accelerated development of therapies and medical devices. Toxicol Pathol 44: 299-314. [Crossref]
- Miranpuri GS, Schomberg DT, Stan P, Chopra A, Buttar S, et al. (2018) Comparative morphometry of the wisconsin miniature swine™ thoracic spine for modeling human spine in translational spinal cord injury research. Ann Neurosci 25: 210-218.
- Busscher I, Ploegmakers JJ, Verkerke GJ, Veldhuizen AG (2010) Comparative anatomical dimensions of the complete human and porcine spine. Eur Spine J 19: 1104-1114. [Crossref]
- 18. Endo T, Fujii Y, Sugiyama SI, Zhang R, Ogita S, et al. (2015) Properties of convective delivery in spinal cord gray matter: Laboratory investigation and computational simulations. J Neurosurg Spine 30: 1-8. [Crossref]
- Vranken JH (2009) Mechanisms and treatment of neuropathic pain.Cent Nerv Syst Agents Med Chem9: 71-78. [Crossref]
- Sillay K, Hinchman A, Akture E, Salamat S, Miranpuri G, et al. (2013) Convection enhanced delivery to the brain: Preparing for gene therapy and protein delivery to the brain for functional and restorative neurosurgery by understanding low-flow neurocatheter infusions using the alaris((r)) system infusion pump. Ann Neurosci 20: 52-58. [Crossref]
- Sillay K, Schomberg D, Hinchman A, Kumbier L, Ross C, et al. (2012) Benchmarking the erg valve tip and mri interventions smart flow neurocatheter convection-enhanced delivery system's performance in a gel model of the brain: Employing infusion protocols proposed for gene therapy for parkinson's disease. J Neural Eng 9: 026009. [Crossref]
- Schomberg D, Wang A, Marshall H, Miranpuri G, Sillay K (2013) Ramped-rate vs continuous-rate infusions: An in vitro comparison of convection enhanced delivery protocols.Ann Neurosci20: 59-64. [Crossref]
- Miranpuri GS, Kumbier L, Hinchman A, Schomberg D, Wang A, et al. (2012) Gene-based therapy of parkinson's disease: Translation from animal model to human clinical trial employing convection enhanced delivery. Ann Neurosci 19: 133-146. [Crossref]
- Brady ML, Raghavan R, Block W, Grabow B, Ross C, et al. (2015) The relation between catheter occlusion and backflow during intraparenchymal cerebral infusions. Stereotact Funct Neurosurg 93: 102-109. [Crossref]
- Raghavan R, Brady M (2011) Predictive models for pressure-driven fluid infusions into brain parenchyma.Phys Med Biol56: 6179-6204. [Crossref]
- Raghavan R, Brady ML, Sampson JH (2016) Delivering therapy to target: improving the odds for successful drug development.Ther Deliv7: 457-481. [Crossref]
- Raghavan R, Odland RM (2017) Theory of porous catheters and their applications in intraparenchymal infusions. Biomed Phys Eng Express 3. [Crossref]


