Abstract
Both clear cell and sclerotic basal cell carcinomas are extremely rare pathological types of basal cell carcinoma. An unusual case of combined type is reported in this paper. Mutations in PTCH1 influencing the Shh pathway may induce the generation of clear cells with organelle degeneration and glycogen deposition, accompanied by the increased expression of type-I and III procollagen mRNA. As a result, collagen fibers in the matrix are boosted and stiffened, leading to the occurrence of clear cell sclerotic type in this patient. Hence, wide surgical resection and long-term follow-up are required for this high-risk basal cell carcinoma to prevent a recurrence.
Keywords
clear cell; sclerotic type; basal cell carcinoma
Introduction
Basal cell carcinoma (BCC) of the external nose frequently occurs on the nasal alar and tip. The cause of the disease is associated with long-term exposure to sunlight. Extended local resection currently is the preferred alternative for the treatment of basal cell carcinoma and presents a satisfactory long-term outcome [1]. The clinicopathological types of BCC can be generally divided into five types: nodular ulcer, pigmented, sclerodermatous, superficial, and fibroepithelioma. Among them, the sclerodermatous type, also known as the sclerotic type, is relatively rare; the clear cell type is extremely rare and unusual [2]. The combined type of these two pathological types remains unreported. In this paper, a case of clear cell sclerotic basal cell carcinoma is reported.
Case report
A 54-year-old man was referred to our hospital with a red patchy mass on the right nasal alar for more than 10 years. Ten years ago, the patient developed a black macule about the size of soybean on the right nasal alar without apparent inducements. With crusts and occasional bleeding on the lesion, the patient presented with itching, but not with any pain or other discomforts. Later, it gradually enlarged to a red plaque. Pathology of a skin biopsy revealed a great possibility of “basal cell carcinoma”.
Dermatological examination demonstrated a 3.0cm*2.7cm reddish-brown plaque on the right nasal alar with uneven surface and significant infiltration, as well as a few scales with elevated edges and pigmentation but without bleeding or exudation (Figure 1).
Figure 1: Basal cell carcinoma
A reddish-brown plaque on the right nasal alar with uneven surface and significant infiltration, as well as a few scales with elevated edges and pigmentation
On July 28, 2021, extended 5mm resection (depth up to subcutaneous fat layer and nasal cartilage) of nasal basal cell carcinoma and full-thickness skin graft repair (medial skin graft of the left upper arm) were performed under local anesthesia (Figure 2). Meanwhile, resected tissue was sent for pathological examination intraoperatively to guarantee a complete resection. Postoperative histopathological examination (Figures 3,4) suggested hyperkeratosis with hypokeratosis of the lesion. The tumor mass was located in the dermis and partially connected to the epidermis. It was composed of basaloid cells, arranged in a palisading pattern around, and exhibited no significant contraction gap. However, some myxoid materials were observed in some masses. Additionally, lumen differentiation, local clear cell-like change, collagen proliferation and sclerosis around the lower tumor tissue were observed. A diagnosis of clear cell sclerotic basal cell carcinoma was performed following the pathological findings.
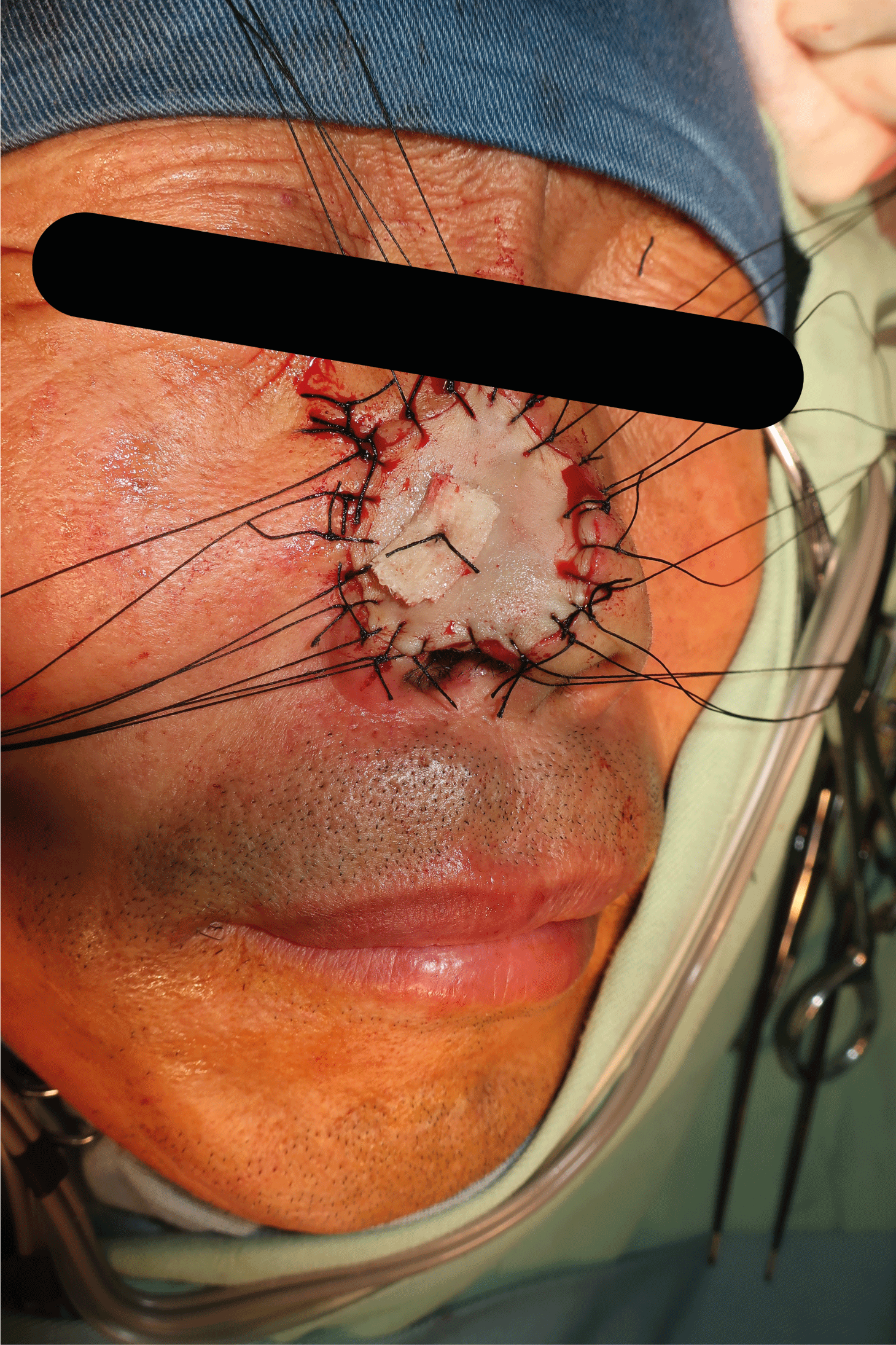
Figure 2: Extensive surgical resection surgery
Extensive surgical resection of nasal basal cell carcinoma and full-thickness skin graft repair were performed
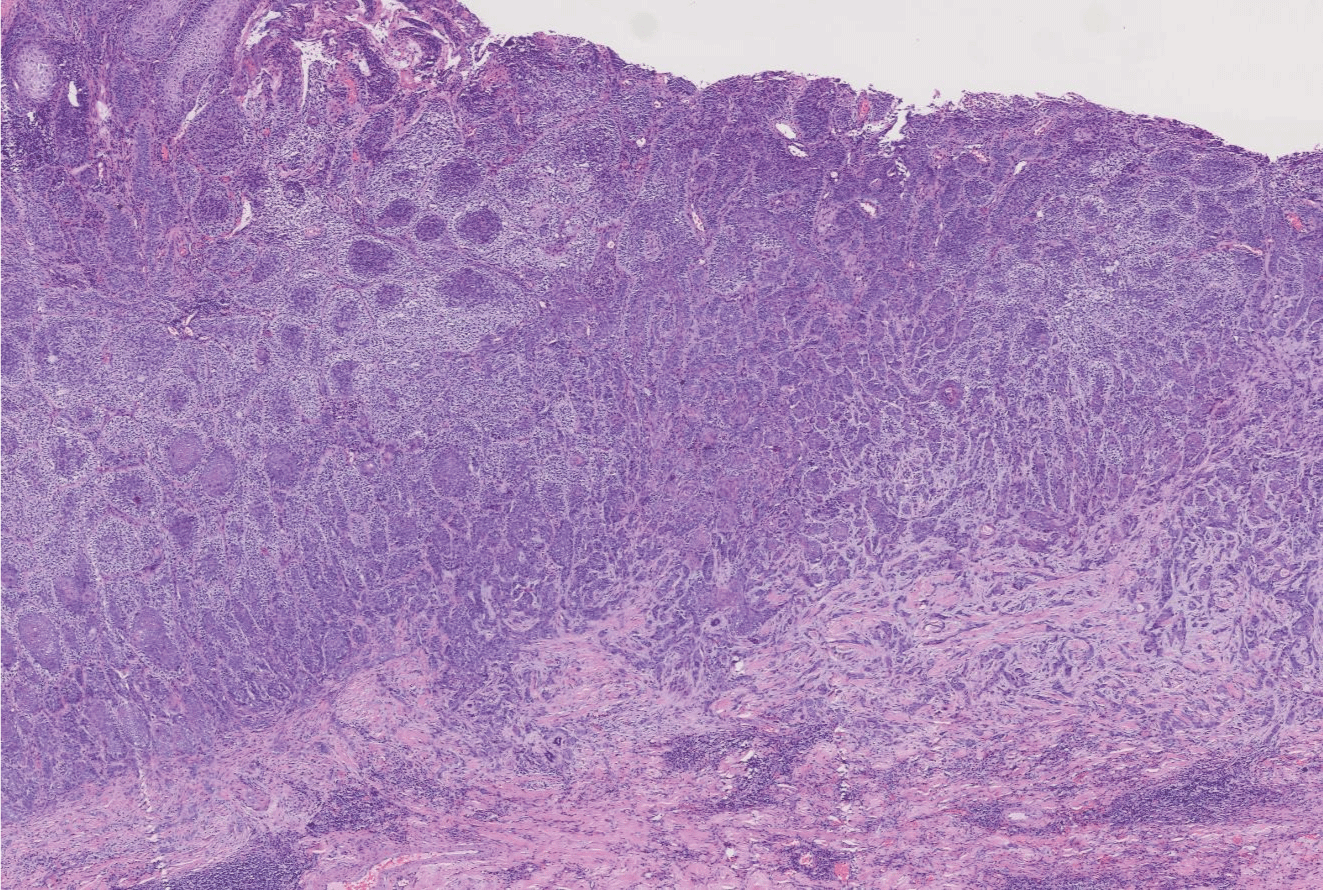
Figure 3: (H&E 200X) Lumen differentiation, local clear cell-like change, collagen proliferation and sclerosis around the tumor tissue were observed
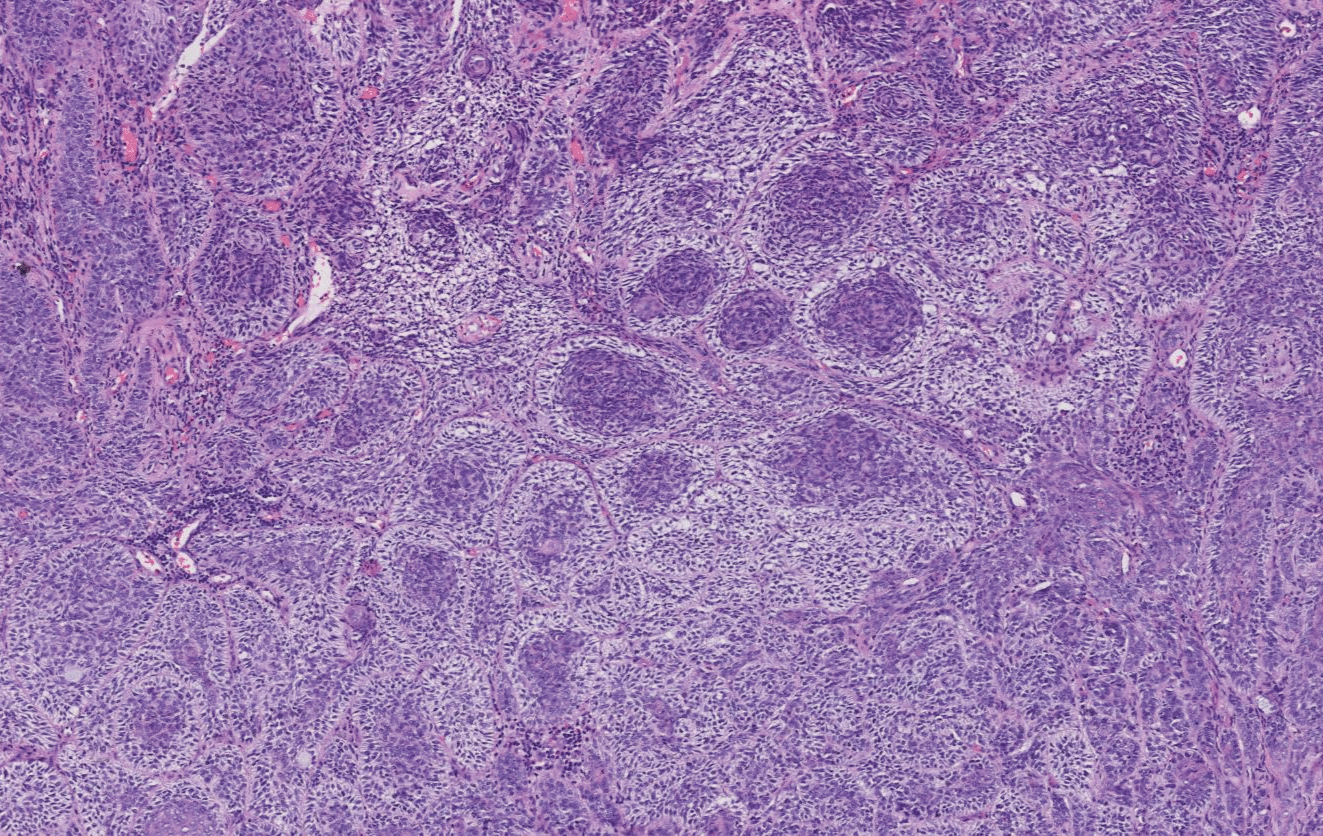
Figure 4: (H&E 400X) The transparent changes in cells under the microscope
Discussion
In 1984, Barr and Williamson first reported clear cell BCC. As of 2019, only 30 cases of clear cell BCC had been reported worldwide and were concentrated on the histopathological features of clear cell BCC. However, there was little research on molecular mechanisms and clinical behaviors of it. Some researchers believed that the membrane-bound vacuoles were the terminal products of intracellular organelles or phagosomes under an electron microscope. Thus, clear cells were considered the result of degeneration, and most cases exhibited glycogen deposition, sometimes accompanied by sialomucin deposition [3,4]. Glycogen and other substances dissolve and lose during pathological preparation, leading to the transparent changes in cells under the microscope. Tsai KK discovered PTCH1 incapacitating mutation in this type by genome mapping [5]. It was a malformation, which could cause DNA mismatch repair deficiencies and then microsatellite instability in various solid tumors. PTCH1 mutations in the Shh pathway contributed to diagnosing clear-cell BCC and determining the right treatment options.
The sclerotic type of BCC was first described by Jacob in 1827. Among 3000 cases of basal cell carcinoma counted by Botvinick in 1967, this type accounted for only 0.6% and was even more rarely reported in China [6]. In sclerotic BCC, there is a potential for increased levels of type-I and III procollagen mRNA homeostasis that could reflect increased collagen synthesis and determine the biochemical composition of the BCC stroma [7]. Up-regulation of S-phase kinase-associated protein-2 (Skp2) expression promotes the degradation of cell cycle negative regulatory proteins such as kinase inhibition protein-1 (P27kipl) through the ubiquitin-proteasome pathway, resulting in the loss of cell cycle inhibition and thus the development of this type of BCC [8].
In this case, the patient was characterized by both clear cell type and sclerotic type. Its pathogenesis could be explained as follows. First, the up-regulation of Skp2 expression degraded P27kipl after persistent UV damage to the nose, increasing the expression of type-I and III procollagen mRNA. Then, collagen fibers in the matrix were boosted and stiffened, contributing to the development of the sclerotic type. Concurrently, the abnormal regulation of the Shh pathway and cell cycle by mutated PTCH1 may degenerate some organelles to form clear cells, leading to the combination of clear cell type on the basis of sclerotic type in this patient. Owing to the rarity of the clear cell type, its pathogenesis, prognosis, and optimal treatment remain poorly described. The rate of BCC metastasis is low but pulmonary metastasis can be observed in the clear cell type [5,9]. Those with metastasis are more likely to relapse after surgical resection. The targeted immunotherapy may provide a more effective treatment strategy for metastatic BCC; two Shh pathway inhibitors, sonidegib and vismodegib, are the only FDA (Food and Drug Administration)-approved targeted therapies for patients with locally advanced BCC who are not eligible for surgery or who relapse after surgery [5]. It is in that sclerotic BCC is difficult to treat and insensitive to radiation, the extent of the tumor in histopathology is generally significantly beyond the range estimated clinically, and deeper invasion may be more aggressive. The 2019 European BCC guidelines revealed that concerning high-risk BCC, a peripheral margin of 5~15 mm should be removed based on individual tumor characteristics, and the resection margin could be appropriately reduced if the extent of reconstruction was limited; moreover, the resection depth should reach the fascia, perichondrium and periosteum in those located in the head and face [10]. Therefore, extensive surgical resection surgery and long-term follow-up are required to guard against a recurrence, as suggested by combining the two types of characteristics of BCC.
Funding Support
National key research and development program (2022YFC2504700).
References
- Mendez BM, Thornton JF (2018) Current Basal and Squamous Cell Skin Cancer Management. Plast Reconstr Surg 142: 373e-387e. [Crossref]
- Chang JM (2008) Rare histological variant of basal cell carcinoma. J Clin Dermatol 37: 406-408.
- Barr RJ, Alpern KS, Santa Cruz DJ, Fretzin DF (1993) Clear cell basal cell carcinoma: an unusual degenerative variant. J Cutan Pathol 20: 308-316. [Crossref]
- Kim DY, Cho SB, Chung KY, Kim YC (2006) Clear cell basal cell carcinoma with sialomucin deposition. Yonsei Med J 47: 870-872. [Crossref]
- Tsai KK, Khurana N, McCalmont T, Daud A, Bastian B, et al. (2019) PTCH1 Mutation in a Patient With Metastatic Undifferentiated Carcinoma With Clear Cell Change. J Natl Compr Canc Netw 17: 778-783. [Crossref]
- Botvinick I, Mehregan AH, Weissman F (1967) Morphea-like Basal Cell Epithelioma in a Child. Arch Dermatol 95: 67-68. [Crossref]
- Moy RL, Moy LS, Matsuoka LY, Bennett RG, Uitto J (1988) Selectively enhanced procollagen gene expression in sclerosing (morphea-like) basal cell carcinoma as reflected by elevated pro alpha 1(I) and pro alpha 1(III) procollagen messenger RNA steady-state levels. J Invest Dermatol 90: 634-638. [Crossref]
- Dai DM, Zhu H (2011) Expression of P27kip1 and Skp2 in basal cell carcinoma and its clinical pathological characters. J Int Oncol 38: 158-160.
- Fernandez-Acenero MJ, Cenjor C, Cordova S (2011) Clear cell basal cell carcinoma with pulmonary metastasis: case report and literature review. Am J Dermatopathol 33: 379-382. [Crossref]
- Peris K, Fargnoli MC, Garbe C, Kaufmann R, Bastholt L, et al. (2019) Diagnosis and treatment of basal cell carcinoma: European consensus-based interdisciplinary guidelines. Eur J Cancer 118: 10-34. [Crossref]




