Since the advent of biological products as medications for rheumatoid arthritis (RA), dramatic progress has been made in RA treatment. The goal of the drugs existing prior to the introduction of biological products was to prevent exacerbation of RA and maintain the status quo. Biological products have allowed us to aim for various remission states (clinical, structural, functional, quality of life outcome, serological, and immunological). However, the prices of biological products are prohibitive, such that not all patients with RA are able to afford treatments by these drugs. Biosimilars are drug products whose efficacy and safety have been experimentally and clinically proven to be equivalent to preceding biological products, and their prices are lower than those of the preceding drugs. Currently, the development and sale of biosimilars for treating RA are being promoted worldwide. This paper reviews the actual status of biosimilars for RA, provides new evidence of differences in adverse events in some cases where biosimilars are switched, regardless of appropriate efficacy, and describes the future prospects of biosimilars.
Biosimilar; Rheumatoid arthritis; R &D; market; Medical insurance; Medical cost; Biologics
History of the development of advanced therapeutic drugs for rheumatoid arthritis
Figure 1 and 2 shows the trend of the sale of advanced therapeutic drugs for rheumatoid arthritis (RA) currently available commercially in Japan. After the release of infliximab in 2003, biological products were introduced to the market with great frequency, and biological products with different mechanisms of action and dosing regimens became available (the use of rituximab for RA is not approved in Japan, unlike in overseas countries). Ten years after the release of the first biological product in Japan, the sale of Janus kinase inhibitors was promoted, and biosimilars (BSs) were also actively marketed.
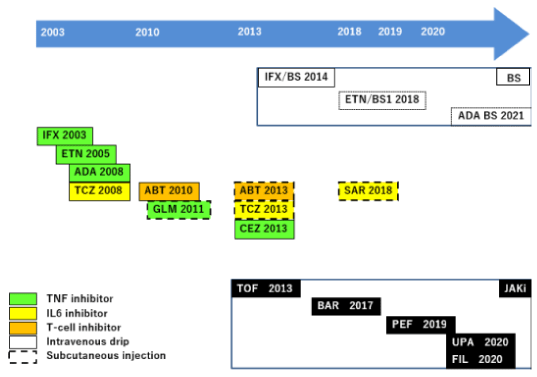
Figure 1. History of marketing biological products, biosimilars, and targeting synthetic DMARDs to treat rheumatoid arthritis (Japan). IFX, infliximab; ETN etanercept; ADA, adalimumab; TCZ, tocilizumab; ABT, abatacept; GLM, golimumab; CEZ, certolizumab pegol; SAR, sarilumab; BS, biosimilar; JAKi, JAK inhibitor; TOF, tofacitinib; BAR, baricitinib; PEF, peficitinib; UPA, upadacitinib; FIL, filigotinib
Figure 2. Number of changes in the manufacturing process after marketing of preceding biotechnology-applied RA drugs (RPs). Even in cases of RPs, the manufacturing process of biological products is altered multiple times after sales release. Reasons for such alterations include changing the source of cell culture medium, developing a new purification process, transferring to a new production facility, etc. [1].
What are BSs?
BSs are pharmaceutical agents with a grade and quality equivalent to already approved effective biotechnology drugs (reference products [RPs]) and with safety and efficacy clinically proven to be equivalent to those of RPs. These BSs are developed and approved after the RP patent expires. BSs are refined or produced using cell culture techniques, similar to those used to produce RPs, and are composed of recombinant proteins, polypeptides, and their derivatives, the characteristics of which can be analyzed by a series of analytical methods (e.g., conjugates).
The BS approval process
A grade and quality equivalent to those of RPs are required to approve the BSs produced. BS approval goes through a process that includes the following four steps: 1) quality characteristic analysis, i.e., analysis of similarity in physical and chemical properties, biological activity, etc. (e.g., primary amino acid sequence structure is the same); 2) nonclinical studies (e.g., toxicity study to confirm differences in impurities); 3) pharmacokinetic /pharmacodynamic studies (e.g., drug half-life, human antibody production, cross-over study); and 4) clinical studies (e.g., phase III study to verify the efficacy or safety equivalent to RP).
Quality characteristics of BSs
Prior to the implementation of clinical studies, the following grade/quality equivalence tests (quality tests) should be performed: 1) structure: peptide mapping, amino acid sequence and composition analysis, sugar composition and sugar chain structure analysis, etc.; 2) physical and chemical properties, such as molecular weight, electrophoresis, high-performance liquid chromatography (HPLC), isoforms; 3) immunochemical properties: enzyme-linked immunosorbent assay (ELISA), western blotting, etc.; 4) purity/impurities: ELISA, HPLC, electrophoresis, etc.; 5) biological activity: bioassay; 6) contamination: viral test, mycoplasma test, sterile test, microbial limit test, etc. The similarities between the RP and BS need to be established.
However, a biological product is a mixture of (1) active constituents: objective substance/objective substance-related agents (deaminated substance or oxidized substance with biological activity among changed objective substances), as well as impurities; (2) Impurities, including (2-1) objective substance-derived impurities (changed objective substances that have no characteristics comparable to the objective substance; precursors and decomposition or degradation products of the objective substance, etc.), and (2-2) manufacturing process-derived impurities (cell base material, cell culture medium, and impurities and infectious factors from the extraction process, isolation, processing, or purification). It is impossible to produce BSs with the same active constituents and impurities as those of the RP; nevertheless, BSs should be as close as possible to RPs in this aspect.
Similarities between biological products in terms of their characteristics
A particular RP indeed varies from one production lot to another. In addition, the manufacturing processes of RPs have often been altered for the purposes of manufacturing cost reduction and collection rate improvement [1]. Such alterations include changes in the manufacturer of the culture medium, change to a new purification process, and release to a new manufacturing site. It is not possible to produce proteins with identical quality characteristics if the method or site of manufacturing is altered. Production of RPs, in this case, refers to the production of RP analogs in a broad sense.
Risk management plan (RMP) and postmarketing surveillance
It is necessary to confirm the safety of BSs in terms of immunogenicity and other aspects and formulate the RMP (a plan to evaluate safety consistently from development to the post marketing stage, including post marketing surveillance).
BSs approved for RA
As of July 2021, BSs of three originator TNF inhibitors are approved for use in RA; some are also approved for use in diseases other than RA. These BSs are provided by multiple companies (Table 1: Biosimilar TNF inhibitors approved for the treatment of RA in major countries, expressed by development codes). These BSs are commercially available at prices 50%–70% lower than those of RPs [2].
Table 1. Types and indications of BSs approved in Japan (July 2021)
|
Development code |
Year of recognition |
Indication |
Infliximab |
CT-P13 1)2)3) |
2013 |
Rheumatoid arthritis, Behçet's disease,
Psoriasis, Ankylosing spondylitis, Crohn's
disease, Ulcerative colitis |
SB2 1)2) |
2016 |
PF-06438179/GP1111 1)2)3) |
2017 |
NI-071 3) |
2017 |
ABP710 2) |
2019 |
Etanercept |
SB4 1)2) |
2016 |
GP2015 1)2) |
2016 |
Rheumatoid arthritis, Juvenile idiopathic arthritis |
LBEC0101 1) |
2018 |
YLB113 1)3) |
2019 |
ABP501 1)2)3) |
2016 |
BI-6955012) |
2017 |
GP2017 1)2) |
2018 |
SB5 1)2) |
2018 |
Adalimumab |
FKB327 1)2)3) |
2018 |
Rheumatoid arthritis, Psoriasis, Ankylosing spondylitis, Crohn's disease |
ENIA11 3) |
2018 |
PF-06410293 1)2) |
2019 |
MSB11022 1) |
2019 |
CT-P13 1) |
2020 |
LBAL 3) |
2021 |
Development of BSs (experimental)
The development process of BSs will be explained below using etanercept (ETN)-BS (LBEC0101), whose development the author was involved in [3]. To produce BS, its comparison with an RP to demonstrate equivalence in grade and quality through the aforementioned quality characteristic test is key. Among various parameters, particular importance is attached to antigen-dependent cell-mediated cytotoxicity (ADCC) activity. This is because carbohydrate chains bound to the antibody cannot be replicated, whereas the BS is produced as a drug product with the same amino acid structure as that of the RP, to a great extent, excluding lot-to-lot differences in the carbohydrate chains in the RP. Carbohydrate chains bound to effector cells with Fcγ receptors (e.g., NK cells, macrophages, and neutrophils) activate effector cells and attack target cells that are targeted by antibodies (cells that produce TNF and elicit inflammation) and other cells by another route mediated by effector cells (ADCC activity). ADCC activity can be measured by a reporter gene assay. The evidence that BS has a similar ADCC activity to the RP is indispensable for approving the BS.
Equivalence in the incidence rates of anti-drug antibodies and neutralizing antibodies is also required for approval; electrochemiluminescence (ECL) is usually used to compare the BS and RP in this regard.
Development of BSs (clinical)
In the development of a BS, clinical studies are required to demonstrate the efficacy and safety of the RPs. The equivalence of BSs to RPs is judged by whether a null hypothesis can be rejected using sample data. If the sample size is large, the difference approximates the equivalence limit; however, a large sample size requires significant time and cost. In this regard, setting the target number of cases will be explained using ETN-BS as an example [3]. The minimum number of cases required to determine equivalence (difference between the means of two groups) is obtained from the following equation: N = 2X(Zα+Zβ)2 × SD2/⊿2, where α is the significance level at 5%, but 0.025 is used here for a one-tailed test, β is the probability of missing a significant difference, Zα and Zβ represent the % of a standard normal distribution, SD is the variability from previous studies, ⊿ is the expected intergroup difference, and the below-mentioned equivalence margin is 0.6. As the minimum target number of cases was calculated to be 115, a simulated data set of ETN was prepared with reference to existing literature, setting 100 cases each for the BS and RP. Then, the minimum number of cases that would provide 90% or higher equivalence was calculated again, and the number of cases in each group was determined to be 187 for a clinical study, including a 20% dropout rate.
Since demographic characteristics vary among different patients in a real-world clinical setting, we first determined explanatory variables to analyze differences. As the estimate of efficacy, y, can be calculated from the explanatory variable X, parameter β, and error ℇ, the equation y = Xβ + ℇ was used. We set four explanatory variables (X), i.e, history of conventional synthetic disease-modifying rheumatic drug (csDMARD) use, nationality, pretreatment value of disease activity score (DAS28), and history of biological DMARD (bDMARDs) use, and calculated parameter Xβ from the product and inverse matrix of n and n rows of X1–X4 using SAS (SAS Institute, Cary, NC, USA). To the value obtained, ℇ was added, and yBS and yRP were calculated for BS and RP, respectively, and the estimated difference between the administration groups (yBS - yRP) was obtained. The 95% confidence interval (CI) was calculated as 95% CI ± K (% of t distribution 2.5%) × standard error. If the CI is within the preliminarily set margin and crosses 0, the equivalence between the BS and RP can be proven. This study used DAS28-ESR for clinical evaluation, and the margin was set at ± 0.6, as the responsiveness of DAS by the European Alliance of Associations for Rheumatology is rated as good, moderate, and no response at 0.6. The 95% CI was calculated to be -0.377–0.078, proving the equivalence of the BS and RP (Figure 3A).
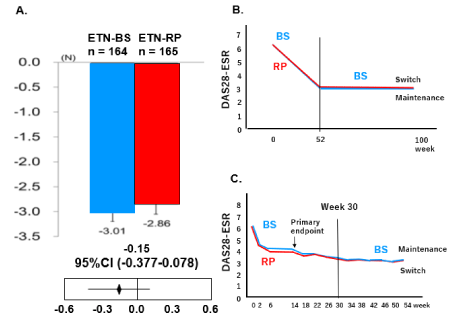
Figure 3. ETN-RP and ETN-BS were equivalent in efficacy as determined by DAS28-ESR (A) [3]. There was no difference in efficacy when ETN-RP was switched to ETN-BS (B) [4]. There was no difference in efficacy when IFX-RP was switched to IFX-BS (C) [5].
Studies on switching from RPs to BSs
Studies on switching from RPs are usually performed on the occasion of or after the approval of BSs [2]. Such clinical studies are performed to demonstrate the appropriate efficacy and safety of BSs upon switching from RPs and facilitate the wide use of BSs. Switching studies have also been performed for BSs approved in Japan [4,5], demonstrating no issues in the efficacy and safety of switching from RPs to BSs (Figure 3B, 3C).
Studies on switching between BSs
Although many clinical studies have shown that switching from RPs to BSs is appropriate [2,4,5], no study has reported BS-to-BS switching. Therefore, we performed a study on switching from an ETN-BS (development code LBEC1010) to another ETN-BS (development code YLB113) [7], which included 102 patients with RA on LBEC1010 who satisfied the remission criteria [6,7] in terms of DAS28-C-reactive protein (CRP) for at least 12 weeks. Statistical analysis included 93 patients who were followed up for at least 12 weeks after switching. There was no significant difference in efficacy between the initial ETN-BS therapy and succeeding ETN-BS therapy. However, with reference to adverse events in the second therapy, six cases of pain at needle insertion, one case of generalized itching, and one case of injection site redness were reported. Since there was no difference in pH between the two agents (6.0–6.6 in LBEC1010 vs. 6.1–6.5 in YLB113), the pain at needle insertion was not attributable to the pH of the agent.
However, although LBEC1010 did not contain citrate buffer solution, it was added to the YLB113formulation. In cases of subcutaneous injection, pain typically occurs unless the concentration of citrate buffer solution is less than 7.3 mM [8]; however, the corresponding concentration in YLB113 was higher than this level at 15.3 mM. In addition, while the needle gauge was 29G for LBEC1010, the needle was thicker (27G G) for YLB113, which might also contribute to pain at needle insertion for YLB113 (the needle thickness was identical in all cases in studies on switching from RPs). The observed generalized itching and injection site redness could be hypersensitivity reactions elicited by different proteins or additives since biological products are protein-based [3]. The two ETN-BS agents examined in this study were not identical in the manufacturing process and methods. causing differences in the incidence of hypersensitivity reactions. When switching from one BS to another in the future, precaution will need to be taken to avoid adverse reactions due to differences between BS.
Actual status on BS use for RA
Figures 4 and 5 shows an international comparison of the percentages of TNF inhibitor BS use to RPs to treat RA. In countries with high percentages of BS use, RPs are replaced by BS in more than 90% cases, whereas the percentage of BS use is less than 10% in Japan [9]. Most countries have put in place specific supply-side policies to promote access to BSs. However, few countries have implemented specific incentives targeting physicians [10]. The presence of key opinion leaders, local guidelines, and gainsharing arrangements appeared to play a role in BS markets in Sweden [11]. The reason for BS market expansion in Europe may be that the EULAR does not distinguish between RPs and BSs in recommendations for RA treatment [12]. Another reason is the unique approach to BSs by respective countries. For instance, in Norway, a country leading in the use of BSs, the government bulk-purchases BSs at the lowest prices through annual bidding and provides BSs to medical institutions. Therefore, the government performs quality assurance clinical studies (NOR SWITCH) to increase BS credit and promote smooth switching to BSs. Consequently, the fact that the efficacy and safety of BSs are appropriate is widely reported to people in the country to promote the expansion of BS use [13].
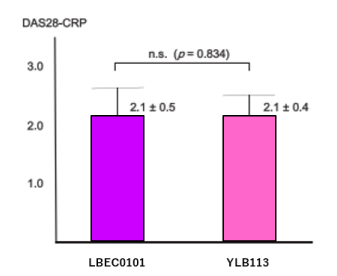
Figure 4. DAS28-CRP at 12 weeks after switching from etanercept BS (LBEC0101) to another ETN-BS (YLB113) [7].
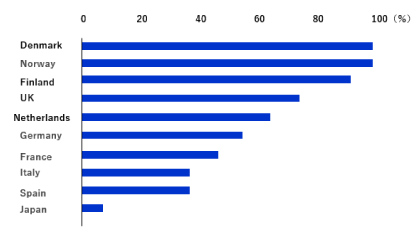
Figure 5. International comparison of the percentages of the use of TNF inhibitor BSs to RPs [9].
In some countries, the use of BSs is promoted by incentives. For example, in the UK, BSs with the lowest prices are recommended by the National Institute for Health and Care Excellence (NICE), and 1% of the cost of expensive drugs, excluding tax, is given to the prescribing physicians [14]. In Germany and France, there is a reference price system for each hospital, and less expensive drugs are preferred by this system. In France, formulation changes to BSs are feasible in pharmacies, but it seems that this policy is not effective in promoting switching to BSs [14]. In contrast, in Germany, goals for BS percentage are set in each state, and physicians who exceed 125% of the budget will have to pay the full amount exceeding 115%, unless its validity is shown [14]. In addition, according to data from the International Generic and Biosimilar Medicines Association (IGBA), physicians who have achieved these goals receive a part of the cost reduced by BS use.
In Japan, support for BSs, optimization of BS medical costs, and the 2020 policy for increasing BS use were discussed in Cabinet meetings in 2017. The initial step for introducing BSs in the medical remuneration system was taken in the BS recommendation policy. Further attention should be paid to movements related to these policies. In 2021, another new BS was approved [15]. Further attention
should be paid to movements related to these policies.
- Schneider CK (2013) Biosimilars in rheumatology: The wind of change. Ann Rheum Dis 72: 315-318. [Crossref]
- Moots R, Azevedo V, Coindreau JL, Dörner T, Mahgoub E, et al. (2017) Switching between reference biologics and biosimilars for the treatment of rheumatology, gastroenterology, and dermatology inflammatory conditions: considerations for the clinician. Curr Rheumatol Rep 19: 37. [Crossref]
- Matsuno H, Tomomitsu M, Hagino A, Shin S, Lee Jet al. (2018) Phase III, muticentre, double-Blind, randomised, parallel-group study to evaluate the similarities between LBEC0101 and etanercept reference product in terms of efficacy and safety in patients with active rheumatoid arthritis inadequately responding to methotrexate. Ann Rheum Dis 77: 488-494. [Crossref]
- Park MC, Matsuno H, Kim J, Park SH, Lee SH et al. (2019) Long-term efficacy, safety and immunogenicity in patients with rheumatoid arthritis continuing on an etanercept biosimilar (LBEC0101) or switching from reference etanercept to LBEC0101: An open-label extension of a phase iii multicentre, randomised, double-blind, parallel-group study. Arthritis Res Ther 21:122. [Crossref]
- Matsuno H, Matsubara T (2019) A randomized double-blind parallel-group Phase III study to compare the efficacy and safety of NI-071 and infliximab reference product in japanese patients with active rheumatoid arthritis refractory to methotrexate. Mod Rheumatol 29: 919-927. [Crossref]
- Inoue E, Yamanaka H, Hara M (2007) Comparison of disease activity score (DAS)28-erythrocyte sedimentation rate and DAS28- C-reactive protein threshold values. Ann Rheum Dis 66: 407-409.
- Matsuno H (2020) Efficacy and safety of the switch from etanercept biosimilar to another biosmilar in patients with rheumatoid arthritis. Clin Rheumatol Rel Res 32: 245-250.
- Usach I, Martinez R, Festini T, Peris JE (2019) Subcutaneous injection of drugs: Literature review of factors influencing pain sensation at the injection site. Adv Ther 36: 2986-2996. [Crossref]
- Matsuno H (2010) Current status of biosimilar. Prog Med 41: 35-41.
- Evelien M, Arnold GV, Isabelle H, Dylst P, Godman B, et al. (2017) Policies for biosimilar uptake in Europe: An overview. PLoS One 12: 1-17. [Crossref]
- Evelien M, Steven S, Per T (2019) Diferent policy measures and practices between swedish counties. infuence market dynamics: Part 1-biosimilar and originator infiximab in the hospital setting. BioDrugs 33: 285-297.
- Smolen JS, Landewé RBM, Bijlsma JWJ, Burmester RG, Dougados M, et al. (2019) EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 Update. Ann Rheum Dis 79: 685-699. [Crossref]
- Jørgensen KK, Olsen IC, Goll G. et al. NOR-SWITCH study group. Switching from originator infliximab to biosimilar CT-P13 compared with maintained treatment with originator infliximab (NOR-SWITCH): A 52-week, randomised, double-blind, non-inferiority trial. Lancet 389: 2304-2316. [Crossref]
- Market Access 2020 | Breaking barriers: Biosimilars (pharmaphorum.com)
- Matsuno H, Kang YM, Okada M, Lee SI, Park SH. et al. (2021) Comparison of the efficacy and safety of LBAL, a candidate adalimumab biosimilar, and adalimumab reference product in patients with active rheumatoid arthritis inadequately responding to methotrexate: A 52-week phase III randomized study. Clin Exp Rheumatol (Online ahead of print.) [Crossref].
Editorial Information
Editor-in-Chief
Andy Goren
University of Rome, Italy
Article Type
Review Article
Publication history
Received date: August 05, 2021
Accepted date: August 25, 2021
Published date: September 01, 2021
Copyright
©2021 Matsuno H. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
©2021 Matsuno H (2021) Role of biosimilars in rheumatoid arthritis - Process from R & D to marketing and trends in the world. Clin Case Rep Rev, 7: DOI: 10.15761/CCRR.1000509.

Figure 1. History of marketing biological products, biosimilars, and targeting synthetic DMARDs to treat rheumatoid arthritis (Japan). IFX, infliximab; ETN etanercept; ADA, adalimumab; TCZ, tocilizumab; ABT, abatacept; GLM, golimumab; CEZ, certolizumab pegol; SAR, sarilumab; BS, biosimilar; JAKi, JAK inhibitor; TOF, tofacitinib; BAR, baricitinib; PEF, peficitinib; UPA, upadacitinib; FIL, filigotinib

Figure 2. Number of changes in the manufacturing process after marketing of preceding biotechnology-applied RA drugs (RPs). Even in cases of RPs, the manufacturing process of biological products is altered multiple times after sales release. Reasons for such alterations include changing the source of cell culture medium, developing a new purification process, transferring to a new production facility, etc. [1].

Figure 3. ETN-RP and ETN-BS were equivalent in efficacy as determined by DAS28-ESR (A) [3]. There was no difference in efficacy when ETN-RP was switched to ETN-BS (B) [4]. There was no difference in efficacy when IFX-RP was switched to IFX-BS (C) [5].

Figure 4. DAS28-CRP at 12 weeks after switching from etanercept BS (LBEC0101) to another ETN-BS (YLB113) [7].

Figure 5. International comparison of the percentages of the use of TNF inhibitor BSs to RPs [9].
Table 1. Types and indications of BSs approved in Japan (July 2021)
|
Development code |
Year of recognition |
Indication |
Infliximab |
CT-P13 1)2)3) |
2013 |
Rheumatoid arthritis, Behçet's disease,
Psoriasis, Ankylosing spondylitis, Crohn's
disease, Ulcerative colitis |
SB2 1)2) |
2016 |
PF-06438179/GP1111 1)2)3) |
2017 |
NI-071 3) |
2017 |
ABP710 2) |
2019 |
Etanercept |
SB4 1)2) |
2016 |
GP2015 1)2) |
2016 |
Rheumatoid arthritis, Juvenile idiopathic arthritis |
LBEC0101 1) |
2018 |
YLB113 1)3) |
2019 |
ABP501 1)2)3) |
2016 |
BI-6955012) |
2017 |
GP2017 1)2) |
2018 |
SB5 1)2) |
2018 |
Adalimumab |
FKB327 1)2)3) |
2018 |
Rheumatoid arthritis, Psoriasis, Ankylosing spondylitis, Crohn's disease |
ENIA11 3) |
2018 |
PF-06410293 1)2) |
2019 |
MSB11022 1) |
2019 |
CT-P13 1) |
2020 |
LBAL 3) |
2021 |





