Abstract
Paracoccidioidomycosis (PCM) is the most important systemic mycosis in South America, causing many deaths in the countries where it occurs. The disease is caused by the thermo-dimorphic fungi Paracoccidioides brasiliensis and Paracoccidiodes Lutzii, found in nature under a mycelial form (M) and as a yeast (Y) in host tissues. Transition between these two forms depends on the growth temperature, being M at 25°C and yeast at 37°C. This work aimed to evaluate the effect of osmotic stress, lack of glucose and amino acid deficiency in P. brasiliensis dimorphism. Here we show that yeast to mycelium transition is inhibited, at room temperature, during osmotic stress and amino acid deprivation conditions, but fungal cells maintain its growth rate and viability. Our data add to extensive available literature indicating that P. brasiliensis dimorphism does not depends exclusively on growth temperature, and its morphogenesis can be influenced by multiple factors.
Key words
Paracoccidioides brasiliensis, dimorphism, osmotic stress, amino acid deprivation
Introduction
Paracoccidioides brasiliensis was isolated for the first time in 1908, by Adolfo Lutz, and is the etiologic agent of paracoccidioidomycosis (PCM), the more prevalent systemic mycosis in Latin America, characterized by chronic granulomatous lesions [1,2]. The disease is caused by the thermo-dimorphic fungi Paracoccidioides brasiliensis and Paracoccidiodes Lutzii [3]. In nature, these pathogens are found as mycelium but its ecological niche is not yet completely clear [4,5]. Under adverse conditions (humidity and nutrient decrease) these fungi produce conidia, which can infect human host causing mild to severe disease [6].
P. brasiliensis is a dimorphic fungi that grows slowly as mycelium (M) at room temperature, between 23°C and 28°C, and convert to yeast form (Y) between 35 and 37° C, in vitro and at infected tissues. Mycelial colonies are small, irregular and formed by thin hyphae. Yeast colonies contain spherical or oval cells, multiplying by bi-polar or multipolar budding [4]. Morphological transition in this fungus, M form to Y form, is an essential step to successful establishment of infection and is primarily dependent on surrounding temperature [7-10].
Dimorphism is an adaptation process to environmental changes, observed in several pathogenic fungi [11], and involves the transformation of filamentous cells (hyphae or pseudo-hyphae) in yeast cells and vice-versa. In dimorphic fungi, temperature is the main morphological switch inducer [12] is essential to pathogenicity [11]. In this study, we aimed to evaluate the role of osmotic and nutritional stress (absence of glucose or amino acids) on Paracoccidioides brasiliensis dimorphism.
Material and methods
P. brasiliensis strain
The P. brasiliensis was maintained in Yeast Peptone Dextrose – YPD solid media (1% yeast extract, 2% peptone; 2% dextrose 2% (pH 6.3)), at 25°C for the mycelial form and at 37ºC for yeast.
Osmotic and nutritional stresses
Pb18 isolate was grown in YPD liquid medium under constant agitation at 37°C. After reaching OD600 = 0.5, cells were centrifuged at 4,000 rpm during 10 min at RT, culture media was removed, and cells were washed with sterile PBS. Cells were then added to SD (Synthetic Defined) liquid media (YNB – Yeast Nitrogen Base – Difco plus 2% glucose), supplemented or not, with NaCl (Sigma) at different concentrations (250 mM, 500 mM and 1 M). Alternatively, osmotic stress was performed using 1M KCl (Sigma) or 1M Sorbitol (Sigma) in SD. Yeast cells suspended in the indicated media (2 mL) were added to 24 wells plates and incubated at 25° C to analyze Y to M conversion or added to 96 well plate to other analysis. For glucose deprivation experiments, we used SD liquid media without glucose (S media or S(-dex)), and for amino acid starvation SD was prepared with YNB without amino acids and ammonium sulfate (Difco).
Growth and dimorphism analysis
P. brasiliensis growth and dimorphism was evaluated during 11 days by microscopic observation, with periodic inspection of cells stained with lactophenol cotton blue. Fungal growth rate was determined by measuring the cell density at 600 nm on 96 well plate.
Cell viability
To determine the yeast cells viability we measured the metabolic activity by using MTT assay. Twelve days after stress exposure, cell suspension (100 uL) from each tested well in a 24 wells plates were removed and added to a 96 well plate. MTT (3-(4,5-Dimethylthiazol-2-yl)-2,5-Diphenyltetrazolium Bromide - Sigma) 0.5mg/mL (final concentration) was added to each well and plate was incubated during 18h. After incubation, the medium containing MTT was removed, and dimethyl sulfoxide (100 μL) was added to solubilize the MTT formazan product. MTT formazan formation was measured at 490 nm. Background formazan values were determined using wells containing media (SD, SD + NaCl, S or SD w/o aa) and MTT. Data were analyzed employing Two-Way ANOVA and Bonferroni post-test.
Results and discussion
Extensive studies on P. brasiliensis and other dimorphic fungi has demonstrated that morphological transition between M and Y phases is an essential step to successful infection establishment, and is triggered by temperature changes, from 25°C to 37°C [7-9]. Here we show that 1 M NaCl block P. brasiliensis hyphae formation at 25°C, when compared to the same cells incubated in SD without NaCl (Figure 1). We aimed to verify the minimum NaCl concentration of which inhibits Y to M conversio nand as showed in figure 2 we demonstrate that dimorphism inhibition also occurs in the presence of reduced NaCl concentrations (250 mM and 500 mM), however in these conditions we were able to see some hyphae, 11 days after our analysis start point. In accordance to our findings, previous work has shown that Na+ ions influence Candida albicans dimorphism, inhibiting germinative tube formation [13]. Considering these findings, we asked about if this P. brasiliensis osmotic response is specific to Na+ ions and, as shown in figure 2, dimorphic transition was also inhibited by 1M KCl and 1M Sorbitol, at room temperature. These data corroborate a previous study demonstrating that 0.3 M NaCl and 2% Sorbitol inhibits its growth rate, and reinforces many findings suggesting that P. brasiliensis dimorphism is not exclusively triggered by thermal conditions [10].
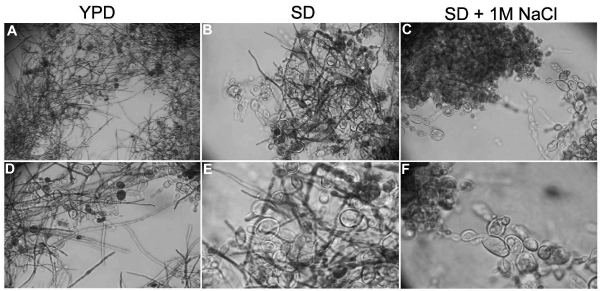
Figure 1. Osmotic stress induced by NaCl blocks P. brasiliensis conversion from Y to M form. P. brasiliensis yeast cells convert from Y to M form four days after experiment start in YPD and SD media (A, B, D and E) but this conversion is blocked in SD supplemented with 1M NaCl (SD + NaCl - C and F). The assay was repeated independently more than 5 times. A – 10X magnification; B, C and D - 40X magnification. E and F - 100X magnification.
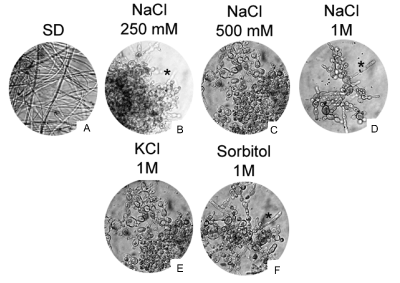
Figure 2. Osmotic stress induced by different NaCl concentrations, as well as KCl and Sorbitol, blocks P. brasiliensis dimorphism. P. brasiliensis yeast cells grown in the presence of 250 mM or 500 mM NaCl does not convert efficiently from Y to M (B and C), as occur in the presence of 1M NaCl (D), 1M KCl (E) or 1M sorbitol (F), when compared to the same cells grown in SD media (A). Asterisks in B, D and F indicates some yeast cells at initial steps to M form trasition. The assay was repeated independently more than 5 times. 40X magnification
Since osmotic environment is important to dimorphic switch in P. brasiliensis, we hypothesized that other stressors can act as dimorphism influencers. The yeast cells were then subjected to the glucose starvation (S media) but we do not notice any change in dimorphic transition in this condition. In contrary, amino acids deprivation (SD w/o aa) resulted in Y to M conversion inhibition, at room temperature, but we still observe some cells initiating transition suggesting that amino acids absence result in delayed transition (Figure 3).
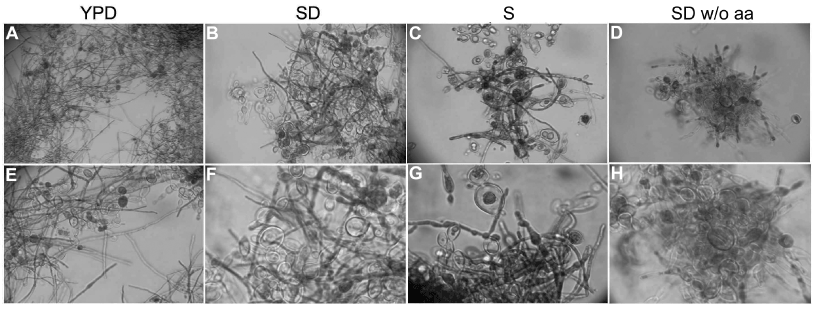
Figure 3. Amino acid starvation inhibit P. brasiliensis dimorphism. P. brasiliensis yeast cells grown on minimal media without without amino acids (SD w/o aa) showed inhibition of conversion from Y to M form, when compared to the same cells grown in YPD, SD ou SD media without glucose (S). The assay was repeated independently more than 5 times. A – 10X magnification; B, C, D and E – 40X magnification; F, G and H -100X magnification.
Our observations on dimorphism inhibition could result from reduced P. brasiliensis viability upon stress, but this was not the case, since Pb18 cells density during osmotic and nutritional stress increased (Figure 4A), indicating an active metabolism. Contrasting, only cells submitted to amino acid deprivation showed reduced growth rate when compared to the other stress conditions, stopping cellular multiplication 2 days after analysis start point (Figure 4A). The reduced growth of amino acid deprived P. brasiliensis indicates that amino acids are essential to fungal growth, and, dimorphic switch as showed in figure 3. The metabolic activity in stressed P. brasiliensis was also measured by MTT assay, 12 days after stress start point. Using this approach, we were able to confirm that P. brasiliensis cells were viable during the analyzed time points (Figure 4B). It is important to note, that the observed growth and cellular viability during stress conditions can result from metabolic utilization of stored nutrients, acquired by P. brasiliensis cells during the initial growth in YPD, just before the conversion assay start point; therefore, yeast cells were able to maintain cellular homeostasis during analyzed period.
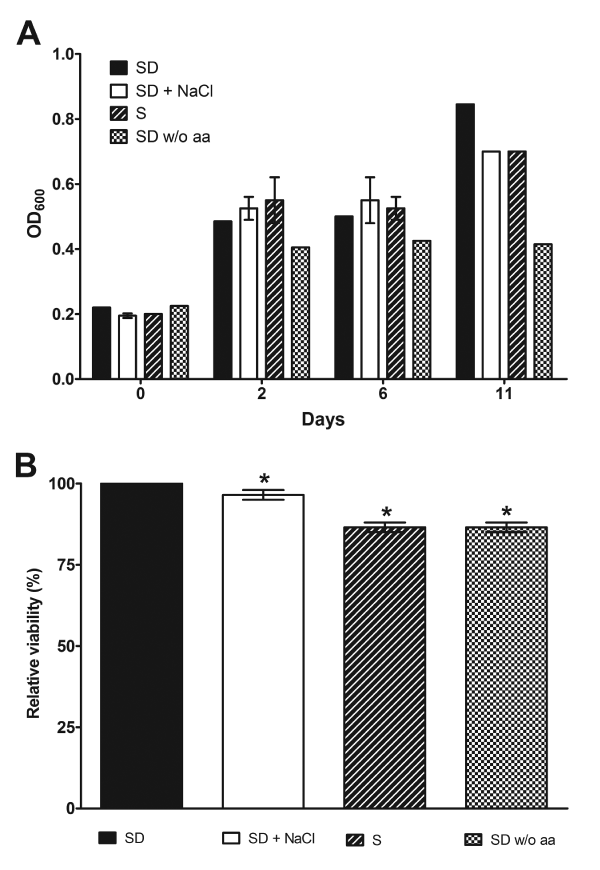
Figure 4. P. brasiliensis growth and viability is preserved upon osmotic and nutritional stress. A) P. brasiliensis growth was evaluated during stress (1M NaCl, glucose and amino acids deprivation) by spectrophotometric measurement at OD600, showing that cells have normal growth during tested conditions. B) P. brasiliensis metabolic activity was assessed quantitatively using MTT reduction assay and we observed a little decrease in viability upon stress, being most cells still viable. Metabolic activity in SD media was taken as 100%. Results shown were the average of three independent experiments ± SD. * p< 0.05 when compared with SD media.
Here we demonstrate that P. brasiliensis dimorphism is not exclusively triggered by temperature, since osmotic stress induced by different concentrations of NaCl, KCl and Sorbitol, as well as the lack of amino acids, are able to inhibit morphogenesis, blocking yeast to mycelium transition, as previously suggested by Cano and Vidal [14].
Conclusion
We show that P. brasiliensis dimorphism does not depends solely on temperature as other environmental factors, such as the osmotic environment, glucose and amino acids availability, are essential to dimorphic transition and potentially pivotal to infection establishment. The molecular processes underlying our findings are subject of future work.
Acknowledgements
We thank Dr. Patricia S. Cisalpino by P. brasiliensis Pb18 strain and PRPq-UFMG (Pro-reitoria de Pesquisa da Universidade Federal de Minas Gerais) for support.
Author contributions
VSA conceived and designed the experiments. LHSQ, VFT and VSA performed the experiments. VSA and LHSQ analyzed the data and VSA wrote the paper.
2021 Copyright OAT. All rights reserv
Conflict of interests
The authors have declared that in the conflict interests exist.
References
- Prado M, Silva MB, Laurenti R, Travassos LR, Taborda CP (2009). Mortality due to systemic mycoses as a primary cause of death or in association with AIDS in Brazil: a review from 1996 to 2006. Mem Inst Oswaldo Cruz 104: 513-521. [Crossref]
- Marques SA (2013) Paracoccidioidomycosis: epidemiological, clinical, diagnostic and treatment up-dating. An Bras Dermatol 88: 700-711. [Crossref]
- Teixeira Mde M, Theodoro RC, Oliveira FF, Machado GC, Hahn RC, et al. (2014) Paracoccidioides lutzii sp. nov.: biological and clinical implications. Med Mycol 52: 19-28. [Crossref]
- Bocca AL, Amaral AC, Teixeira MM, Sato PK, Shikanai-Yasuda MA, et al. (2013) Paracoccidioidomycosis: eco-epidemiology, taxonomy and clinical and therapeutic issues. Future Microbiol 8: 1177-1191. [Crossref]
- Brummer E, Castaneda E, Restrepo A (1993) Paracoccidioidomycosis: an update. Clin Microbiol Rev 6: 89-117. [Crossref]
- Restrepo A, McEwen JG, Castaneda E (2001) The habitat of Paracoccidioides brasiliensis: how far from solving the riddle? Med Mycol 39: 233-241. [Crossref]
- Nemecek JC, Wuthrich M, Klein BS (2006) Global control of dimorphism and virulence in fungi. Science 312: 583-588. [Crossref]
- Rappleye CA, Goldman WE (2006) Defining virulence genes in the dimorphic fungi. Annu Rev Microbiol 60: 281-303. [Crossref]
- San-Blas G, Nino-Vega G, Iturriaga T (2002) Paracoccidioides brasiliensis and paracoccidioidomycosis: molecular approaches to morphogenesis, diagnosis, epidemiology, taxonomy and genetics. Med Mycol. 40: 225-242. [Crossref]
- Kanetsuna F, Carbonell LM, Azuma I, Yamamura Y (1972) Biochemical studies on the thermal dimorphism of Paracoccidioides brasiliensis. J Bacteriol 110: 208-218. [Crossref]
- Boyce KJ, Andrianopoulos A (2015) Fungal dimorphism: the switch from hyphae to yeast is a specialized morphogenetic adaptation allowing colonization of a host. FEMS Microbiol Rev 39: 797-811. [Crossref]
- Klein BS, Tebbets B (2007) Dimorphism and virulence in fungi. Curr Opin Microbiol 10: 314-319. [Crossref]
- Northrop FD, Ljubojevic S, Davies JM (1997) Influence of Na+ and anions on the dimorphic transition of Candida albicans. Microbiology 143: 3757-3765. [Crossref]
- Cano MI, de Aguiar MS (1991) [Utilization of aminoacids in the study of the growth of Paracoccidioides brasiliensis. Influence on dimorphism]. Rev Inst Med Trop Sao Paulo 33: 319-324. [Crossref]




