Despite the fact that urea breath test (UBT) for the diagnosis of Helicobacter pylori (H. pylori) has high sensitivity and specificity, the accuracy of UBT in paediatrics is less than that of adults. The aim of the current study was to prospectively determine the safety and the diagnostic accuracy of the 13C-UBT for the detection of H. pylori using the point-of-care continuous BreathID® Hp and the breath sampling bag test, BreathID®Hp Lab System, in a pediatric population. Fifty-three children performed the 13C-UBT via both the BreathID®Hp and the BreathID® Hp Lab System and were asked to provide a stool sample for conventional H. pylori antigen testing. BreathID®Hp sensitivity was 93.3% [95% CI (68.05%; 99.83%)] and specificity was 100% [95% CI (86.77%; 100.00%)] compared to stool antigen. The overall agreement in detection of H. pylori using the BreathID®Hp breath test versus the stool antigen test was 97.56% [95% CI (87.14%; 99.94%)]. BreathID®Hp Lab System sensitivity was 93.3% [95% CI (68.05%; 99.83%)] and specificity was 100% [95% CI (87.23%; 100.00%)] compared to stool antigen. The overall agreement in detection of H. pylori using the BreathID®Hp Lab System breath test versus the stool antigen test was 97.62% [95% CI (87.43%; 99.94%)]. One minor possibly related adverse event was recorded.
Conclusions: Both the BreathID®Hp and the BreathID®Hp Lab System breath sample collection devices were safe and accurate in diagnosis of H. pylori infection in children.
helicobacter pylori, urea breath test
Helicobacter pylori (H. pylori) is mainly acquired in the first five years of life and studies on the epidemiology of this infection depend on the availability of a noninvasive diagnostic test [1]. Since the main factor predisposing to infection is poor socioeconomic status [2], improved standards of living play a crucial role in declining prevalence rates. Indeed, prevalence is seen to be decreasing over time, but there are still areas of the world that are endemic. According to the Joint European/North American Society for Pediatric Gastroenterology Hepatology and Nutrition (ESPGHAN/NASPGHAN) guidelines [3], H. pylori testing is recommended in children with gastric or duodenal ulcers. Moreover, these guidelines state that post-treatment confirmation of eradication is mandatory for all children treated with antibiotics. Additionally, non-invasive testing for H. pylori is recommended as part of the investigation of children with chronic immune thrombocytopenic purpura (ITP) [3].
Biopsies taken via endoscopy and the carbon-labeled urea breath test (UBT) are considered the "gold standard" methods for the diagnosis of active H. pylori infection [4]. The Maastricht V Consensus Report recommended 13C-UBT as the best approach for the diagnosis of H. pylori infection, due to its high sensitivity, specificity, and excellent performance, especially in patients in whom endoscopy is not indicated [5]. UBTs have high accuracy and reproducibility because they are functional tests that essentially sample the entire stomach and are not prone to the same level of sampling error as biopsy-based tests, and false-positive results are uncommon [6]. The sensitivity and specificity of the breath test range from 90% to 100%, and in most cases, it is above 95% [7-9].
However, in the pediatric population, especially in young children UBT has shown variable accuracy [10,11]. In a meta-analysis including thirty-one articles and 135 studies Leal et al. [12] evaluated the diagnostic performance of the 13C-UBT in children stratified in subgroups of < 6 and ≥ 6 years of age. The results showed good accuracy in all ages combined (sensitivity 95.9%, specificity 95.7%), with high accuracy in children >6 years (sensitivity 96.6%, specificity 97.7%). The 13C-UBT test was less accurate in young children, but adjusting cut off value, pretest meal, and urea dose, this accuracy could be improved [12]. Magalhães Queiroz et al. [13] evaluated a cohort of 414 infants (ages 6 to 30 months) living in impoverished regions of two developing countries in South America. They showed excellent agreement between the results of the 13C-UBT and the stool antigen test indicating that UBT is a reliable method for the diagnosis of H. pylori infection in very young children [13]. Similar results were reported by Pacheco et al. [14].
BreathID®Hp (Exalenz Bioscience, Modiin, Israel), an FDA-cleared (2013) continuous UBT device for adults, provides several unique advantageous features. Instead of collecting and analyzing discrete breath samples, breath samples are continually evaluated by a nasal cannula, providing excellent accuracy (>99%) in detecting H. pylori and shortening the breath testing procedures. Moreover, test results are available in real-time for decision making at the point of care [15,16]. The BreathID®Hp Lab System, FDA-cleared for adults in 2016, collects breath samples into bags, which are then either tested on-site or delivered to a central laboratory. The system can perform sequential diagnosis on 10 pairs of breath collection bags within approximately 30 minutes, via a fully automated process, as opposed to the BreathID®Hp that measures only one subject at a time. In addition, it enables breath testing in locations that do not have the device itself and the test cannot be performed on site. The current study aimed to evaluate the accuracy of the respective BreathID®Hp and BreathID®Hp Lab System in children with suspected H. pylori infection.
Study design
This non-randomized, open-label clinical study was conducted at six clinical sites (five in the United States and one in Israel). The primary objective of the study was to confirm the safety of the 13C-urea and the secondary objective was to confirm the efficacy of the respective BreathID®Hp System and the BreathID®Hp Lab System in children. The results were compared to the standard of care, highly sensitive stool antigen test [17]. Eligibility criteria included children with a clinical indication compatible with H. pylori infection or seeking confirmation of post-therapy eradication, ages 3-17 years old who could provide stool samples and complete the breath test with either breath systems or both, and whose legal guardian signed the consent form. Children who underwent treatment of PPI and H2 blockers within two weeks of the 13C-UBT/stool antigen test or children who received antibiotics (unrelated to H. pylori eradication) within four weeks prior to the 13C-UBT/stool antigen test or those with an allergy to test substrates, were excluded.
The study was approved by each clinical site's Institutional Review Board or an Independent Ethics Committee and was registered at clinicaltrials.gov (NCT02905825).
Urea breath test
A solution containing 75mg 13C-urea and 4 mg citric acid was given orally. In the presence of H. pylori infection, bacterial urease splits 13C-urea into 13CO2 and ammonia. The 13C-labeled CO2 is absorbed in the blood stream and excreted by the lungs. The BreathID®Hp devices sense CO2 in exhaled breath and analyze its different isotopes based on the specific optical-radiation emission and absorption of 13CO2 and 12CO2 gases. The device calculates the change in the 13CO2/12CO2 ratio from exhaled breath before and after ingestion of 13C labeled urea and calculates the delta-over-baseline (DOB) value.
When using the BreathID®Hp, breath samples are collected via a nasal cannula that passively and continually collects the patient’s exhaled breath. The entire procedure, including the ingestion of the 13C labeled substrate and until results are provided, takes 10–30 minutes. When using the BreathID®Hp Lab System, breath analyses compare a pair of breath sample bags collected at baseline and 15 minutes after ingestion of the test substrate, instead of using the nasal cannula. The bags can store the exhaled breath samples for up to 14 days from the time of collection and up to 10 sets of bags can be sequentially analyzed, within 20 minutes.
ELISA stool antigen
Stool antigen was tested using the Premier Platinum HpSA PLUS enzyme immunoassay (EIA), which is an in vitro, qualitative assay using microwell-based enzyme immunoassay to detect H. pylori antigens present in human stool. The visual color change renders the interpretation of results objective and simple and no calculations are required. Monoclonal anti-H. pylori capture antibodies are adsorbed to microwells. Diluted patient samples and a conjugate (peroxidase conjugated to a plurality of monoclonal antibodies) are added to the wells and incubated for one hour at room temperature. A wash is performed to remove unbound material. Substrate is then added and incubated for ten minutes at room temperature. Color develops in the presence of bound enzyme. Stop solution is added and the results are interpreted visually or spectrophotometrically. A central laboratory provided the stool collection kits and analyzed the stool specimens.
Statistical analyses were performed using SAS v9.4 (SAS®, SAS Institute Cary, NC USA) software. The required significance level of findings was equal to or lower than 5%. All statistical tests were two-sided, unless defined otherwise. Where confidence limits were appropriate, the confidence level was 95%. Baseline values were defined as the last valid value prior to the breath test. For the descriptive statistics, mean values and their standard deviations were calculated.
In total, 53 children were enrolled in the study, 56.6% of whom were female, and 60.4% Caucasian. Age median was 9.0 (range: 4.0-17.8), with 28.5% of the children 6 years old or younger, 47.6% between the ages of 7-12 and 23.8% aged 13 or older. Median weight was 30 kg (range: 14.0-103.0), median height was 132.1 cm (range: 95.0-177.8) and median BMI was 18.0 kg/m2 (range 12.2-39.9).
Out of 54 children screened for participation, 53 met the eligibility criteria for performing the 13C-UBT. Of these, 11 were unwilling or unable to provide stool/breath samples as required and were withdrawn from the study (Figure 1). The remaining 42 children completed the study with breath sample bags and 41 completed the continuous test using the nasal cannula. The demographic data of children performing the BreathID®Hp and the BreathID®Hp Lab System tests are presented in table 1. The most common indications for H. pylori testing were abdominal pain (73.8%) followed by vomiting (42.8%), nausea (35.7%) and post-treatment eradication validation (30.9%) (Table 2).
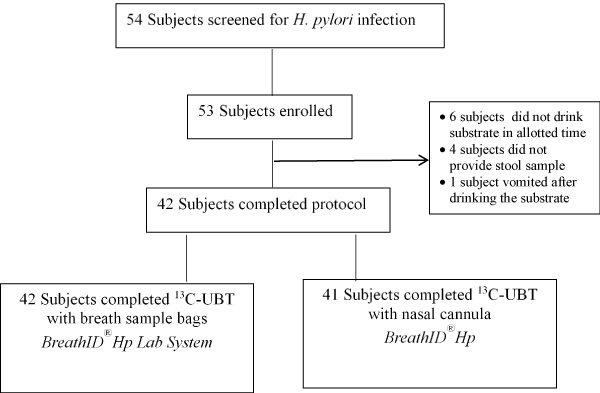
Figure 1. Subject disposition.

Figure 2. The BreathID® Lab System.
Table 1. Demographics and anthropometric measures of participating children.
|
BreathID®Hp
(nasal cannula) |
BreathID®Hp Lab System (breath sample bags) |
Gender |
Male % (n/N) |
39.0% (16/41) |
40.5% (17/42) |
Female % (n/N) |
61.0% (25/41) |
59.5% (25/42) |
Ethnic
Origin |
Caucasian % (n/N) |
63.4% (26/41) |
61.9% (26/42) |
African-American % (n/N) |
22.0% (9/41) |
23.8% (10/42) |
Asian-Pacific % (n/N) |
2.4% (1/41) |
2.4% (1/42) |
Hispanic % (n/N) |
4.9% (2/41) |
4.8% (2/42) |
Other % (n/N) |
7.3% (3/41) |
7.1% (3/42) |
Age (years) |
<12 % (n/N) |
73.2% (30/41) |
73.8% (31/42) |
≥12 % (n/N) |
26.8% (11/41) |
26.2% (11/42) |
Age (years) |
N |
41 |
42 |
Mean (SD) |
10.3 (3.93) |
10.2 (3.92) |
Median [Range] |
9.9 [4.4; 17.8] |
9.7 [4.4; 17.8] |
Weight (kg) |
N |
41 |
42 |
Mean (SD) |
41.3 (23.28) |
41.0 (23.10) |
Median [Range] |
32.0 [15.4; 102.1] |
31.5 [15.4; 102.1] |
Height (cm) |
N |
41 |
42 |
Mean (SD) |
138.6 (21.42) |
138.0 (21.49) |
Median [Range] |
137.2 [96.5; 177.8] |
137.1 [96.5; 177.8] |
BMI (kg/m2) |
N |
41 |
42 |
Mean (SD) |
19.9 (6.25) |
19.9 (6.17) |
Median [Range] |
18.0 [12.2; 39.9] |
18.0 [12.2; 39.9] |
Table 2. Indications for H. pylori testing.
Indication |
n/N (%) |
Abdominal pain |
31/42 (73.8%) |
Vomiting |
18/42 (42/8%) |
Nausea |
15/42 (35.7%) |
Post-eradication therapy |
13/42 (30.9%) |
Reflux |
9/42 (21.4%) |
Diarrhea |
4/42 (9.5%) |
Dyspepsia |
4/42 (9.5%) |
Heartburn |
3/42 (7.1%) |
IDA |
2/42 (4.8%) |
Belching |
2/42 (4.8%) |
B12 deficiency |
1/42 (2.4%) |
Discomfort |
1/42 (2.4%) |
Other: constipation, flatulence, abdominal distension, halitosis, family member with HP, fatigue, low ferritin |
8/42 (2.4% each) |
Note: some subjects had multiple indications.
Fourteen of the 42 children tested positive for the stool H. pylori Ag. BreathID®Hp sensitivity was 93.3% [95% CI (68.05%; 99.83%)] and specificity was 100% [95% CI (86.77%; 100.00%)] (Table 3). The positive predictive value (PPV) was 100% and the negative predictive value (NPV) was 96.3%. The overall agreement in diagnosis between the BreathID®Hp breath test and the stool antigen test was 97.56% [95% CI (87.14%; 99.94%)] (Table 4). The BreathID®Hp Lab System sensitivity was 93.3% [95% CI (68.05%; 99.83%)] and specificity was 100% [95% CI (87.23%; 100.00%)]. PPV was 100% and NPV was 96.43%. The overall agreement in diagnosis between the BreathID®Hp Lab System breath test and the stool antigen test were 97.62% [95% CI (87.43%; 99.94%)] (Table 5).
Table 3. Comparative results of stool antigen, BreathID®Hp and BreathID®Hp Lab System.
|
BreathID®Hp
(nasal cannula) |
BreathID®Hp Lab System
(breath sample bags) |
Stool antigen results |
HP (+) |
HP (-) |
Total |
HP (+) |
HP (-) |
Total |
HP (+) |
14 |
1 |
15 |
14 |
1 |
15 |
HP (-) |
0 |
26 |
26 |
0 |
27 |
27 |
Total |
14 |
27 |
41 |
14 |
28 |
42 |
Table 4. BreathID®Hp performance measures.
|
% (n/N) |
95% CI |
Overall Percent Agreement |
97.56% (40/41) |
[87.14%; 99.94%] |
Positive Percent Agreement |
93.33% (14/15) |
[68.05%; 99.83%] |
Negative Percent Agreement |
100.00% (26/26) |
[86.77%; 100.00%] |
PPV |
100.00% (14/14) |
[76.84%; 100.00%] |
NPV |
96.30% (26/27) |
[81.03%; 99.91%] |
Table 5. BreathID®Hp Lab System performance measures.
|
% (n/N)
|
95% CI
|
Overall Percent Agreement |
97.62% (41/42) |
[87.43%; 99.94%] |
Positive Percent Agreement |
93.33% (14/15) |
[68.05%; 99.83%] |
Negative Percent Agreement |
100.00% (27/27) |
[87.23%; 100.00%] |
PPV |
100.00% (14/14) |
[76.84%; 100.00%] |
NPV |
96.43% (27/28) |
[81.65%; 99.91%] |
One adverse event (AE) of vomiting was reported and deemed to be possibly related to the urea and citric acid but not to the device, since it occurred shortly after ingestion of the substrate. The child was withdrawn from the study. The AE rate was 1.89% (1/53) (95% CI: [0.05%; 10.07%]).
Recommendations from the evidence-based international guidelines emphasize that active H. pylori testing is the preferred modality to diagnose H. pylori infection. Additional support for this concept came when Cigna became the first large national payer in the USA to decide that it will no longer reimburse serology testing as of August 2014. In adults, the BreathID®Hp Lab System has been demonstrated to be as safe and as effective and substantially equivalent to BreathID®Hp [18]. The aim of the current manuscript was to evaluate the diagnostic performance of these two systems in a pediatric setting.
Based on the high levels of accuracy obtained when compared to stool antigen, this study confirmed that both breath sample collection systems are safe and efficacious in diagnosing H. pylori in children. Only one possibly related minor adverse event related to the test substrate, but not to the device, was recorded. The accuracy levels obtained for the pediatric population were similar to those obtained in the adult population [18,19]. Furthermore, the results are comparable with those of a previous study that showed 100% concordance between BreathID®Hp and endoscopic results in a subset of children undergoing gastroscopy [20].
Despite the fact that UBT has high sensitivity and specificity, the accuracy of UBT in pediatrics is less than that of adults [21]. The sensitivity and specificity of this method in young children was found to be 95–97% and 97–98%, respectively [12,22,23]. However, some studies have suggested that the accuracy of this method is lower in children under the age of 6 years [24,25]. Both BreathID®Hp systems proved highly accurate compared to other available non-invasive tests, including other available UBTs on the market. The BreathID®Hp and BreathID®Hp Lab System provide a safe, simple and non-invasive method of testing for H. pylori at a lower cost than endoscopy, and more pleasant than stool collection. The two BreathID®Hp breath collection systems complement each other and meet the needs of different age groups of children, providing a versatile solution for H. pylori testing. Younger children may find it difficult to inflate sample bags; passive breath collection using the nasal cannula may be more suitable for them. The BreathID®Hp Lab System allows more freedom of testing even in locations which do not have the actual analysis device. The bags are stable for 14 days, enabling sufficient time for transport and accumulation of several tests for efficiency purposes. Furthermore, both devices involve easy-to-perform analysis methods, requiring minimal training.
It should be noted, that there were several limitations in the study: it was difficult to enroll very young children and only 28.5% of the children were under 6 years of age. Additionally, confirmation of post-treatment eradication UBTs were tested on a small group of patients only (26.4%). However, the efficacy of the post-treatment UBT in adults has already been confirmed and can be extrapolation to children. Finally, due to the nature of this study on the pediatric population, the UBT outcomes were compared to those of stool antigen tests, an accepted reference standard, and not to the gold standard of histology which needs endoscopy. Recently, Best et al. [26] in a Cochrane Library Review, compared the diagnostic accuracy of UBT, serology, and stool antigen test, and concluded that UBTs had high diagnostic accuracy, while serology and stool antigen tests were less accurate for diagnosis of H. pylori infection [26].
The results of the current study support the safety and the utility of the two breath collection methods, BreathID®Hp or BreathID®Hp Lab System, in detecting the presence of H. pylori both initially and post-treatment in all ages of the pediatric population. Based on the current study results, the BreathID®Hp Systems received marketing clearance from FDA for H. pylori detection in children.
Conflict of interest
Haim Shirin received research grants and has ownership interest with Exalenz Bioscience Ltd, Israel.
Funding: The study was supported by Exalenz Bioscience Ltd.
Ethical approval
The study was approved by each clinical site's Institutional Review Board or an Independent Ethics Committee and was registered at clinicaltrials.gov (NCT02905825).
Informed consent
Informed consent was obtained from legal guardian of all individual participants included in the study.
- Suerbaum S, Michetti P (2002) Helicobacter pylori infection. N Engl J Med 347: 1175-1186. [Crossref]
- Rajindrajith S, Devanarayana NM, de Silva HJ (2009) Helicobacter Pylori infection in children. Saudi J Gastroenterol 15: 86-94. [Crossref]
- Jones NL, Koletzko S, Goodman K, Bontems P, Cadranel S, et al. (2017) Joint ESPGHAN/NASPGHAN Guidelines for the management of Helicobacter pylori in children and adolescents (Update 2016). J Pediatr Gastroenterol Nutr 64: 991-1003. [Crossref]
- Chey WD1 Wong BC; Practice Parameters Committee of the American College of Gastroenterology (2007) American College of Gastroenterology guideline on the management of Helicobacter pylori infection. Am J Gastroenterol 102: 1808-1825. [Crossref]
- Malfertheiner P, Megraud F, O'Morain CA, Gisbert JP, Kuipers EJ, Axon AT, Bazzoli F, Gasbarrini A, Atherton J, Graham DY, Hunt R, Moayyedi P, Rokkas T, et al. (2017) Management of Helicobacter pylori infection-the Maastricht V/Florence Consensus Report. Gut 66: 6-30. [Crossref]
- Howden CW, Hunt RH (1998) Guidelines for the management of Helicobacter pylori infection. Ad Hoc Committee on Practice Parameters of the American College of Gastroenterology. Am J Gastroenterol 93: 2330-2338. [Crossref]
- Chey WD (2000) Accurate diagnosis of Helicobacter pylori. 14C-urea breath test. Gastroenterol Clin North Am 29: 895-902. [Crossref]
- Graham DY, Klein PD (2000) Accurate diagnosis of Helicobacter pylori. 13C-urea breath test. Gastroenterol Clin North Am 29: 885-893. [Crossref]
- Perri F (2000) Diagnosis of Helicobacter pylori infection: which is the best test? The urea breath test. Dig Liver Dis 32: S196-S198. [Crossref]
- Kindermann A, Demmelmair H, Koletzko B, Krauss-Etschmann S, Wiebecke B, et al. (2000) Influence of age on 13C-urea breath test results in children. J Pediatr Gastroenterol Nutr 30: 85-91. [Crossref]
- Machado RS, Patricio FR, Kawakami E (2004) 13C-urea breath test to diagnose Helicobacter pylori infection in children aged up to 6 years. Helicobacter 9: 39-45. [Crossref]
- Leal YA, Flores LL, Fuentes-Panana EM, Cedillo-Rivera R, et al. (2011) 13C-urea breath test for the diagnosis of Helicobacter pylori infection in children: a systematic review and meta-analysis. Helicobacter 16: 327-337. [Crossref]
- Queiroz DM, Saito M, Rocha GA, Rocha AM, Melo FF, et al. (2013) Helicobacter pylori infection in infants and toddlers in South America: concordance between [13C]urea breath test and monoclonal H. pylori stool antigen test. J Clin Microbiol 51: 3735-3740. [Crossref]
- Pacheco SL, Ogata SK, Machado RS, Patricio FR, Pardo ML, et al. (2013) Diagnosis of Helicobacter pylori infection by means of reduced-dose (13)C-urea breath test and early sampling of exhaled breath. J Pediatr Gastroenterol Nutr 57: 607-611. [Crossref]
- Schmilovitz-Weiss H, Sehayek-Shabat V, Eliakim R, Skapa E, Avni Y, et al. (2012) Applicability of a short/rapid 13C-urea breath test for Helicobacter pylori: retrospective multicenter chart review study. BMC Gastroenterol 12: 8. [Crossref]
- Broide E, Shirin H (2015) Evaluation of Exalenz Bioscience's BreathID for Helicobacter pylori detection. Expert Rev Mol Diagn 15: 299-312. [Crossref]
- Sabbagh P, Javanian M, Koppolu V, Vasigala VR, Ebrahimpour S (2019) Helicobacter pylori infection in children: an overview of diagnostic methods. Eur J Clin Microbiol & Infect Dis 38: 1035-1045. [Crossref]
- Richter V, Gonzalez JO, Hazan S, Gottlieb G, Friedenberg K, et al. (2019) The validity of breath collection bags in detecting H. pylori using the novel BreathID®Hp Lab System: a multicenter clinical study in 257 subjects. Ther Adv Gastrointest Endosc 12: 2631774519843401. [Crossref]
- Shirin H, Kenet G, Shevah O, Wardi J, Birkenfeld S, et al. (2001) Evaluation of a novel continuous real time (13)C urea breath analyser for Helicobacter pylori. Aliment Pharmacol Ther 15: 389-394. [Crossref]
- Levine A, Shevah O, Miloh T, Wine E, Niv Y, et al. (2004) Validation of a novel real time 13C urea breath test for rapid evaluation of Helicobacter pylori in children and adolescents. J Pediatr 145:112-114. [Crossref]
- Czinn SJ (2005) Helicobacter pylori infection: detection, investigation, and management. J Pediatr 146:S21–S26.
- Kawakami E, Machado RS, Reber M, Patrício FRS (2002) 13C-urea breath test with infrared spectroscopy for diagnosing Helicobacter pylori infection in children and adolescents. J Pediatr Gastroenterol Nutr 35:39–43.
- Megraud F (2005) On behalf of the European Pediatric Task Force on Helicobacter pylori. Comparison of non-invasive tests to detect Helicobacter pylori infection in children and adolescents results of a multicenter European study. J Pediatr 146:198–203.
- Drumm B, Koletzko S, Oderda G (2000) Helicobacter pylori infection in children: a consensus statement. European Paediatric Task Force on Helicobacter pylori. J Pediatr Gastroenterol Nutr 30:207–213.
- Pathak C, Bhasin D, Khanduja K (2004) Urea breath test for Helicobacter pylori detection: present status. Trop Gastroenterol 25:156–161.
- Best LM, Takwoingi Y, Siddique S, Selladurai A, Gandhi A, Low B, Yaghoobi M, Gurusamy KS (2018) Non-invasive diagnostic tests for Helicobacter pylori infection. Cochrane Database Syst Rev 3:CD012080. doi: 10.1002/14651858.CD012080.pub2.


