Aim: Coronavirus Disease (COVID-19) is caused by the newly emerged coronavirus SARS-CoV-2. To prevent further spread of SARS-CoV-2 and to provide a possible prophylaxis and treatment option we report about our experience on the application of two preparations with a clinical complete recovery of five patients, including two breastfed babies, from a Coronavirus disease with severe symptoms.
Material and methods: Sedeprovid (ImmunoD® CLS℗) were applied twice a day and AlphaH℗ 20 ml concentrate was consumed once a day in the morning. The course of disease and symptoms before, during and after treatment were recorded.
Results: Prior to treatment, the patients suffered from severe symptoms of confirmed COVID-19 infections, including cough, inappetence, tiredness, bone and body pain, loss of taste and smell and body temperature of ≥39ºC for several days.
Within 24 hours after application of ImmunoD® CLS℗ and AlphaH℗ a significant reduction of symptoms and a drop down of maximal body temperature was recorded. A complete recovery with normal body temperature and normal or close to normal activity was documented after 48 hours. No side effects were reported from the patients or the parents.
Conclusion: The combination of ImmunoD® CLS℗ and AlphaH℗ might offer a good treatment regimen for COVID-19 infected patients with moderate and severe disease progression before entering or after leaving the ICU ward. In addition, this protocol might be used as a prophylactic therapy option for healthcare providers.
We strongly recommend the instant confirmation of these results in a controlled randomized trial for a possible rapid benefit of COVID-19 infected patients.
COVID-19, sedeprovid, viral infection, vitamin d, vitamin c
Severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) is the novel avian virus strain first detected in Wuhan, China, that causes coronavirus disease 2019 (Covid-19) [1]. Since initial detection more than 2.1 million cases of Covid-19 have been confirmed worldwide (Johns Hopkins University 2020). Initial reports from China and Italy suggest high mortality rates and stressed intensive care unit (ICU) capacities due to SARS-CoV-2 [2,3]. Worldwide mortality of confirmed COVID-19 patients has reached 6.7% (143278 deaths / 2144719 coronavirus cases on April 16th).
Strong evidence exists that vitamin D has a potential antimicrobial activity and its deficiency has deleterious effects on general well-being and longevity [4,5]. Especially in viral infection the current vitamin D level can play an important role on the disease course and outcome [6,7].
Latest literature reviews present evidence that Vitamin D supplementation could reduce risk of Influenza and COVID-19 infections and deaths [8].
We recently reported a newly developed dimeric compound, namely cholecalciferol-N-acetylgalactosamine-Vitamin D binding protein (VitD~dgVDBP) and its positive and non-toxic effects after intravenous injection compared to sham group in mice [9].
In addition, VitD~dgVDBP (Il-42 / Sedeprovid) showed a higher macrophage activation and a lower oxidative burst than Vitamin D free dgVDBP and VDBP. That may result from a synergistic effect through a better presentation / exposition of protein bound Vitamin D to macrophages [10].
This special Vitamin D form has shown to have no side effects yet, even in high doses, neither as oral nor as iv.-application form, both in animals [9] and humans [11,12].
Furthermore, investigations demonstrated a significant improvement of macrophage function [10]. Preliminary data confirmed attenuated clinical courses of infectious diseases like influenza, HIV and Borna virus infections.
Oxidative stress is mostly involved in inflammation, especially during viral lung epithelial infection, generating free radicals like nitrogen oxide (NO·), superoxide anion radical (O2·-) and hydroxyl radicals (OH·) by immune cells [13]. Oxidative stress modulates expression of toll-like receptor 3 during respiratory syncytial virus infection in human lung epithelial A549 cells. Free radicals interfere immediately with the surrounding to modify and inactivate virus and/or bacteria by the specific oxidation of the lipid membrane and/or proteins called lipid peroxidation [14]. An overwhelm of free radicals is normally controlled by antioxidative reacting enzymes like superoxide dismutase and peroxidases forming reactive oxygen and nitrogen species like peroxynitrite, hydrogen peroxide and lipid peroxides. Any overwhelm of both, free radicals and reactive oxygen and nitrogen radicals, are also able to destroy and/or inactivate the surrounding tissue, cells and organic substances after viral respiratory infection [15]. We have demonstrated that a combination of alpha-ketoglutarate, Vitamin C, 5 –HMF and carnosine (alphaH) decreased effectively the modification of proteins during exposition of cigarette-generated free radicals in vivo and in vitro [16]. Furthermore, we also demonstrated a substantial better outcome during and after surgery in favor of alphaH supplemented lung cancer patients decreasing oxidative modification of lipids and proteins and increasing their energy performance [17].
The aim of applying the combination of the novel substances Sedeprovid and AlphaH℗ was the immediate exploration of possible positive effects on COVID-19 infected patients.
We used the newly invented and described water soluble transport form of 1,25-D3 (Cholecalciferol) called Sedeprovid, also known as IL-42 (ImmunoD® CLS℗, HG Pharma, Vienna, Austria). AlphaH℗ is an oral supplement containing following natural compounds namely alpha-ketoglutarate (AKG), vitamin C, 5 - Hydroxymethylfurfural (5-HMF) and carnosine.
ImmunoD® CLS℗ and AlphaH℗ were applied to confirmed Coronavirus infected patients according to a prescribed treatment plan. The course of the disease was recorded similar to documented COVID-19 cases in literature. The outcome is reported here.
Sedeprovit (ImmunoD® CLS℗, HG Pharma, Vienna, Austria) was applied twice daily (morning and evening) by diluting the lyophilized preparation of one ampoule with 2 ml of water. The liquid was kept in the mouth for 5 minutes for a sufficient absorption via the buccal mucosa. In case 3 and 4 (7 and 18 months old breastfed baby) one ampoule of ImmunoD was diluted with 1 ml of water and provided with a spray nozzle. Two sprays were given hourly over the day.
Additionally, AlphaH℗ 20 ml concentrate (HG Pharma, Vienna, Austria) was diluted with 50 ml of water and consumed once daily in the morning. In case 3 and 4 (7 and 18-month-old breastfed baby) 10 ml AlphaH concentrate were mixed with breast milk / baby food and feed twice daily.
Ethical approval from ethical committee of Sigmund Freud University, Vienna, Austria (Reg.-Nr.: 147/2020) and informed consent prior to documentation was obtained.
Clinical course was documented for all cases as shown in Tables 1. The categorization of symptom severity was classified as described in Table 4.
Table 1. Course of disease (Case 1)
Date |
Day |
COVID-19 Test |
ImmunoD |
AlphaH |
Temperature °C |
Breathing status (0-4 worst) |
Vitality status (0-4 worst) |
Symptom status |
21.03.20 |
Day 1 |
|
|
|
39.8 |
0 |
2 |
Fever and chills in the evening |
22.03.20 |
Day 2 |
testing |
|
|
39.0 |
0 |
3 |
Persistent fever |
23.03.20 |
Day 3 |
|
|
|
37.5 |
0 |
1 |
No symptoms |
24.03.20 |
Day 4 |
positive result |
|
|
37.2 |
0 |
1 |
No symptoms |
25.03.20 |
Day 5 |
|
|
|
37.5 |
0 |
1 |
No symptoms |
26.03.20 |
Day 6 |
|
|
|
38.0 |
1 |
1 |
Start of breathing difficulties |
27.03.20 |
Day 7 |
|
|
|
39.7 |
2 |
2 |
Further vitality and symptom aggravation |
28.03.20 |
Day 8 |
|
0-1-0 |
1-0-1 |
39.5 |
3 |
3 |
Further vitality and symptom aggravation |
29.03.20 |
Day 9 |
|
1-0-1 |
1-0-1 |
39.0 |
3 |
2 |
Vitality amelioration and symptom stabilization |
30.03.20 |
Day 10 |
|
1-0-1 |
1-0-1 |
38.5 |
2 |
1 |
Vitality and symptom amelioration |
31.03.20 |
Day 11 |
|
|
1-0-1 |
37.5 |
1 |
1 |
Vitality and symptom amelioration |
01.04.20 |
Day 12 |
|
|
1-0-1 |
37.0 |
0 |
0 |
Vitality satisfactory and symptom freedom |
Table 2. Literature review on vitamin D (MASR: Meta-Analysis of SystemaEc Reviews; RCT: Randomized Controlled Trial)
Author |
Study |
Topic |
Trials: Patients |
Results |
Conclusion |
Ginde et al.
Arch Intern Med
2009 |
Cross Sectional Analysis |
URTI: Upper Respiratory Tract Infection |
1 Trial: 18 883 Pat |
VitD25OH associated with URTI: P<0.001
<10ng/ml: 24% with URTI
10-29ng/ml: 20% with URTI
≥30ng/ml: 17% with URTI |
- VitD levels are inversely associated
with recent URTI.
- RCTs are warranted |
Martineau et al.
BMJ
2017 |
MASR |
ARTI: Acute Respiratory Tract Infections |
24 Trial: 10 899 Pat |
≥1 ARTI (%):
- Overall: P=0.001
- Daily or weekly dosing
VitD<20ng/ml: 47.6% vs 43.1%: P=0.006
- Bolus dosing: P=n.s. |
- VitD supplements: safe & protective
against ARTI
- Benefit was greater when receiving
daily or weekly VitD
- protective effect was strongest in
cohort with profound VitD deficiency |
Kempker et al.
Clin Infect Dis
2019 |
Case Cohort Analysis |
Community acquired sepsis |
1 Trial: 30 239 Pat |
VitD25OH: HR (Reference Q5>33.6ng/ml)
Q1: <16.5ng/ml: HR 6.81
Q2: 16.5-22.4ng/ml: HR 3.21
Q3: 22.5-27.1ng/ml: HR 1.55
Q4: 27.2-33.6ng/ml: HR 1.06 |
Low plasma VitD25OH measured at a time of relative health was independently associated with increased risk of sepsis |
Loeb et al.
Influenza Other
Respir Viruses
2019 |
RCT |
Respiratory Infection |
1 Trial: 1300 Pat |
VitD Supplementation: 14 000 IU/week: 8mo
- Influenza Virus: P=0.64
7.7% vs 6.6%: HR: 1.18
- NonInfluenza Virus: P=0.011
22.5% vs 28.5%: HR: 0.76%
- All respiratory viruses: P<0.05
27.2% vs 32.2%: HR: 0.81 |
In healthy kids and adolescents VitD supplement significantly reduced NonInfluenza respiratory viral infections by about 25%. |
Öztekin et al.
Viral Immunol 2019 |
Case Control
Study |
Recurrent Herpes Labialis |
1 Trial: 101 Pat |
VitD Status (ng/ml): 16.8 vs. 9.5: P<0.001
Control vs Case
VitD Deficiency: 66.7% vs 92.0% P=0.003
VitD Insufficiency: 21.6% vs 8.0%
VitD Adequacy: 11.8% vs 0% |
- Adequate VitD levels contribute to
mounting a powerful immune response
against recurrent HSV1 infections
- More research is warranted for
clarifying the degree of association, |
Quraishi et al.
PLOSOne
2013 |
Cross Sectional Cohort |
CAP: Community acquired sepsis |
1 Trial: 16 975 Pat |
VitD25OH: P<0.05
<30ng/ml vs ≥30ng/ml: OR 1.56 |
25(OH)D levels were inversely associated with a history of CAP. |
Han et al.
J Clin Translat Endocrinol 2016 |
RCT |
Critical Illness
Ventilation at Intensive Care |
1 Trial: 31 Pat |
VitD: 250 000 vs 500 000IU vs Placebo
45.7 vs 55.2ng/ml vs 21ng/ml
Hospital stay (days); 25 vs 18 vs 36: P=0.03 |
- High-dose VitD increased VitD25OH
- High-dose VitD associated with
decreased hospital stay |
Han et al.
Eur J Clin Nutr 2018 |
RCT |
Critical Illness ventilation at Intensive Care: Oxidative Stress |
1 Trial: 30 Pat |
VitD: 250 000 vs 500 000IU vs Placebo
- éGSH (Glutathion): P=0.001
- êGSSG (Glutationdisulfid): P=0.009
- êehGSSG: P=0.009 |
- Oxidative stress is positively associated
with alveolar macrophage phagocytic
function
- VitD may improve oxidative stress |
Han et al.
Nutrition
2017 |
RCT |
Critical Illness ventilation at Intensive Care: Antimicrobial peptides: AMP |
1 Trial: 30 Pat |
VitD: 250 000 vs 500 000IU vs Placebo
- High-dose VitD correlates with free
VitD25OH levels: P<0.001
- Free VitD25OH levels is correlated with
hCAP18 mRNA, an AMP
- Total 25 (OH)D is not correlated with AMP |
- High-dose VitD increased plasma free
VitD25OH levels,
- Larger studies appear warranted to see
the effect of high-dose VitD on AMPs. |
Amrein et al.
JAMA 2014 |
RCT |
Critical Illness at Intensive Care |
1 Trial: 475 Pat |
VitD: 540 000IU + 90 000IU/mo for 5 months
Severe deficiency VitD≤12ng/ml: 200 Pat
ICU-Mortality: 33.3% vs 23.5% P=0.10
28d-Mortality: 36.3% vs 20.4% P=0.06
Hospital Mortality: 46.1% vs 28.6% P=0.04
6mo Mortality: 50.0% vs 34.7% P=0.12 |
- Severe VitD deficiency ≤12ng/ml is
associated with hospital mortality
- Findings require further study. |
Table 3. Literature review on vitamin C (MASR: Meta-Analysis of Systematic Reviews)
Author |
Study |
Topic |
Trials: Patients |
Results |
Conclusion |
1 Hemilä et al.
Cochrane Database Syst Review 2013 |
MASR |
Common cold |
29 Trials: 11 306 Pat |
Regular VitC supplementation
- êIncidence of cold: P=0.001
- êDuration of cold: P<0.00001 |
- VitC reduces duration of colds in regular supplementation trials
- low costs and good safety
- Further therapeutic RCTs are warranted |
2 Hemilä et al.
Cochrane Database Syst Review 2013 |
MASR |
Pneumonia |
4 prophylactic Trials: 50 Pat
2 therapeutic Trials: 197 Pat |
VitC effect:
- êmost severe ill pat with respiratory
symptom score ≥ 8/10: P=0.02
- êhospital duration: P<0.0001 |
- therapeutic VitC may be reasonable for pneumonia pat with low VitC blood levels
- low costs and risks
- More research is needed |
Hemilä et al.
Allergy, Asthma Clin Immunol 2013 |
MASR |
Common Cold induced Asthma |
3 Trials: 79 Pat |
VitC effect:
- êIncidence asthma attacks: P=0.019
- êIncidence of severe and moderate
asthma attacks: P=0.003
- êhistamine sensitivity: P=0.0005
Interaction VitC and cold: P=0.003 |
- VitC alleviates common cold symptoms
- reasonable for asthmatic patients to test VitC, if they have asthma exacerbations caused by respiratory infections.
- More research on the role of VitC on common cold-induced asthma is needed. |
Hemilä et al.
Nutrients
2017 |
MASR |
Common cold
Pneumonia |
Animal Trials: 148
Human Trials: 34
- Common cold:
31 Trials: 9745 Pat
- Pneumonia:
3 Trials: 2335 Pat |
Animal Trials: 86/148 (58.1%): VitC effect on
- êinfections of bacteria & viruses: P≤0.01
Human Trials: VitC effect:
- êCommon Cold: 9745 Pat: P<0.01
- êPneumonia: 2335 Pat: P=0.00002 |
- Negative findings of common cold studies explained by low doses of VitC.
- The effects of VitC against infections should be investigated further. |
Hemilä et al.
J Intensive Care 2020 |
MASR |
Critically Ill pat. with mechanical ventilation |
8 Trials: 685 Pat |
VitC effect:
- êoverall mechanical ventilation by 14%:
P=0.00001 (I2=83%)
- êventilation time of severely iill pat by 25%:
P<0.0001 |
- Strong evidence: VitC shortens duration of mechanical ventilation
- Level of baseline illness severity should be considered in further research |
Table 4. Explanation of applied scoring scale
Explanation |
|
|
Day of Infection |
Please write down when you noticed the first symptoms |
|
|
|
|
ImmunoD (-/+): |
Please note when you took ImmunoD and how often |
|
|
|
|
AlphaH Sport+: |
Please note when you took AlphaH Sport + and how often |
|
|
|
|
Max. Body Temperature: |
Please document the highest measured body temperature of the day |
|
|
|
|
Breathing Problems: |
Please indicate the maximum complaints of the day regarding your breathing |
|
|
0 = no problems |
|
|
1 = slight problems (occurs with physical exertion) |
|
|
2 = medium problems, (occurs with normal activities) |
|
|
3 = serious problems (occurs with light activities) |
|
|
4 = very serious problems (occurs already at rest) |
|
|
|
|
Activity Status: |
Please write the maximum complaints of the day about your activity |
|
|
0 = no problems (sport with normal load possible) |
|
|
1 = slight problems (normally active at home all day) |
|
|
2 = medium problems, (less than 50% of the day bedridden) |
|
|
3 = serious problems, (more than 50% of the day bedridden) |
|
|
4 = very serious problems (bedridden all day) |
|
|
|
|
Remarks: |
Please write down what you think is important to note that day. Include other symptoms such as cough, runny nose, chills, loss of smell and / or taste. |
|
|
|
|
Co-Morbidities: |
Diabetes |
Yes/No |
|
Chonic Obstructive Pulmonary Disease |
Yes/No |
|
Hypertension |
Yes/No |
Case 1: H.O. 54 years old male patient, Co-morbidities: none
Suspected infection in Westendorf, Tyrol, Austria on March 13th, 2020. COVID-19 testing on March 22nd with positive COVID-19 test result on March 24th, 2020. Patient was in home quarantine from day 10 to day 29.
Clinical course: Symptom start on Day 1 with fever, severe symptoms from Day 6 - Day 10. Application of ImmunoD for 3 days + AlphaH for 5 days from Day 8 - Day 12. Symptom recovery on Day 11: fever-free, active, low cough. No more complaints at the moment (Table 1) (Figure 1).
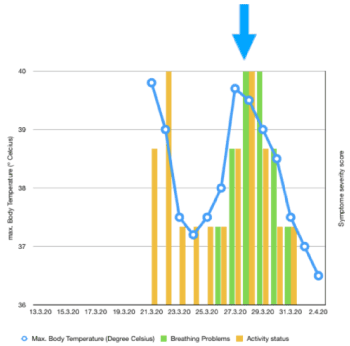
Figure 1. Course of case 1
Case 2: E.P., 28 years old female, ski racer, Co-morbidities: none
Suspected infection in Verbier, Switzerland on March 6th 2020. COVID-19 testing on March 15th, 2020. Patient was in home quarantine for 14 days. Application of ImmunoD + AlphaH: 3 days
Clinical course
- March 12th, 2020: Symptoms start: Day 1: Mild course, like the start of a cold.
- March 13th - 15th, 2020: Day 2-4: Mild course
- March 16th, 2020: Day 5: Mild course, starting to feel tired.
- March 17th, 2020: Day 6-9: Moderate disease progression: really exhausted and feverish and bedridden with loss of appetite, body and joint pain from day 10, no sense of smell or taste.
- March 21st, 2020: Day 10: Positive test result of COVID-19 infection
- March 23rd, 2020: Day 12: Severe disease status: when trying to move, breathing restricted, max. Body temperature 39ºC.
- March 23rd, 2020: Day 12-14: Application of ImmunoD 1 ampoule orally twice a day. AlphaH as described before
- March 24th, 2020: Day 13: Symptom improvement: significantly more energy, max. temperature 38ºC, but still not normal activity.
- March 25th, 2020: Symptom recovery on Day 14: fever-free (max. 37ºC), can leave the house and do light exercise without dyspnea. Currently, no symptoms. Since then has worked full time in the home office.
Case 3: K.M. 7 months old male patient. Suspected infection in London, Great Britain on March, 18th, 2020, COVID-19 testing on March 28th, 2020. Patient was in home quarantine with temporary 1-night hospitalization for 14 days. Application of ImmunoD + AlphaH: 3 days
Clinical course:
- March 23th, 2020: Day 1: initially anorexia, fatigue, fever up to 39ºC, severe cough and difficulty breathing.
- March 25th, 2020: Day 3: Presentation at hospital in London with diagnosis of severe pneumonia, no corona infection suspected. Unchanged severe pneumonia symptoms.
- March 28th, 2020: Day 6: Positive test result of COVID-19 infection. Hospitalization in London for one night, the next day discharge due to triage reasons. Further anorexia, fatigue, fever up to 39ºC, severe cough and difficulty breathing.
- March 29th, 2020: Day 7-9 Application of ImmunoD orally 1/2 amp./day (in several spray strokes). AlphaH 10 ml mixed with breast milk.
- March 30th, 2020: Day 7: Fever significantly reduced, the baby drinks well (breast feeded) and is significantly more active.
- March 31st, 2020: Complete symptom recovery on Day 9 fever-free, active, no cough. No more complaints at the moment (Figure 2).
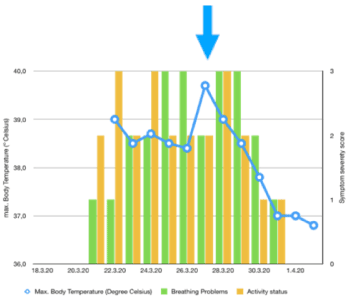
Figure 2. Course of case 3
Case 4: K.M. 18 months old male patient. Co-morbidities: none. Suspected infection in Kirchberg, Tyrol, Austria on April, 3rd, 2020, COVID-19 testing on April 10th, 2020. Patient is in home quarantine since April 10th. Application of ImmunoD + AlphaH: 3 days
Clinical course:
- April 8th, 2020: Symptom start: Day 1: initially anorexia, fatigue, fever up to 40.8ºC, severe cough and difficulty breathing.
- April 10th, 2020: Day 3: Presentation at hospital in Sankt Johann in Tyrol, Austria with diagnosis of severe pneumonia, no corona infection suspected. Unchanged severe fever up to 41.4ºC.
- April 11th, 2020: Day 4: Positive test result of COVID-19 infection. Further anorexia, fatigue, fever up to 41.4ºC, light cough.
- April 11th, 2020: Day 4-6: Application of ImmunoD orally 1/2 amp./day (in several spray strokes). AlphaH 10 ml mixed with breast milk.
- April 11th, 2020: Day 4: Fever significantly reduced after 7 hours, the baby drinks well and is significantly more active.
- April 13st, 2020: Symptom recovery on Day 6: fever-free, active, no cough. No more complaints at the moment.
Case 5: K.S. 67 year old male patient. Co-morbidities: hypertension. Suspected infection in Vienna, Austria on March, 18t, 2020, COVID-19 IgG testing on April 10th, 2020 after infection. Patient was in home quarantine for 14 days. Application of ImmunoD + AlphaH: 3 days.
Clinical course:
- March 28th, 2020: Symptom start: Day 1: sudden onset with initial fatigue, fever up to 39ºC, no cough, dyspnea (Grade 3).
- March 29th, 2020: Day 2: Severe dyspnea (Grade 4), bedridden all day (Grade 4), no corona infection suspected.
- March 30th, 2020: Day 3-5: Application of ImmunoD for 3 days + AlphaH for 5 days
- March 30th, 2020: Day 3: Fever significantly reduced (37.8ºC) in the evening, significantly more energy, dyspnea reduced (Grade 3), but still not normal activity (Grade 2).
- April 1st, 2020: Symptom recovery on Day 5: fever-free (36.5 ºC), active (Grade 0-1), no cough, significantly less dyspnea (Grade 2-1).
- April 2nd, 2020: Complete recovery. No more complaints at the moment.
- April 14th, 2020: positive Test result COVID-19 IgG.
Recent literature reviews report about the evidence that Vitamin D supplementation could reduce risk of influenza and COVID-19 infections and deaths [8].
Vitamin D may reduce the risk of infection through multiple mechanisms. Vitamin D boosts innate immunity by modulating production of anti-microbial peptides (AMPs) and cytokine response.
Moreover, B and T cell activation as well as boosting the activity of monocytes and macrophages also contribute to a potent systemic anti-microbial effect [4,18].
The direct invasion by pathogenic organisms may be minimized at sites such as the respiratory tract [19] by enhancing clearance of invading organisms. A vitamin D replete state appears to benefit most infections, with the possible noteworthy exception of Leishmaniasis. Vitamin D constitutes a prophylactic option and possibly therapeutic product either by itself or as a synergistic agent to traditional antimicrobial agents [4].
Vitamin (1,25-D) acts as an immune system modulator [20]. Nearly all cells display a specific vitamin D receptor (VDR), including B and T lymphocytes (both resting and activated), monocytes [10] and dendritic cells [20]. The deficiency impairs significantly regulatory T-cells [21]. Vitamin D exerts its immunomodulatory activity on both mononuclear and polynuclear cell lines through its effects on the VDR22. Vitamin D tends to favor a mononuclear phenotype, increasing VDR expression on monocytes and macrophages [22,23]. Circulating vitamin D levels have a direct influence on macrophages, increasing their “oxidative burst” potential (maturation and production of cytokines, acid phosphatase and hydrogen peroxide) [6,24], and prevent excessive expression of inflammatory cytokines. Vitamin D also facilitates neutrophil motility and phagocytic function [25].
After the outbreaks of H1N1 influenza in 2009, Edlich et al. [26] strongly recommended that all health care workers and patients be tested and treated for vitamin D deficiency to prevent exacerbation of respiratory infections. Vitamin D also reduces the production of proinflammatory cytokines, which may reduce the risk of cytokine storm in H1N1 infection [27].
Each of the forms of vitamin D is hydrophobic and is transported in blood bound to carrier proteins. The major carrier is called, appropriately, vitamin D-binding protein (VDBP). The half-life of 25-hydroxycholecalciferol is several weeks, while that of 1,25-dihydroxycholecalciferol is only a few hours. Therefore, the urgent supplementation to achieve this prophylactic and therapeutic effect of 1,25-D3 (Cholecalciferol) is complicated due to its natural lipophilic structure.
Factors that regulate vitamin D metabolism are of main importance. The hormonal form and most active metabolite of vitamin D is calcitriol. This hormone mediates its biological effects through a specific nuclear receptor, which is found in many tissues. Calcitriol synthesis and degradation depend on the expression and activity of CYP27B1 and CYP24A1 cytochromes, respectively, for which regulation is tissue specific. Among the factors that modify these cytochromes expression and/or activity are calcitriol itself, parathyroid hormone, fibroblast growth factor 23, cytokines, calcium and phosphate [28].
Pro-hormone 25OHD is a lipophilic molecule that is transported in the circulation bound primarily to vitamin D binding protein (DBP). While the association between 25OHD and DBP is pivotal for renal handling of 25OHD and endocrine synthesis of 1,25(OH)2D, what is the role of DBP for extra-renal synthesis of 1,25(OH)2D? [29].
It now seems likely that localized, tissue-specific, conversion of 25-hydroxyvitamin D (25OHD) to 1,25-dihydroxyvitamin D (1,25(OH)2D) drives many of the newly recognized effects of vitamin D on human health [29]?
The question of utmost importance in this context is, how the VDBP-bound Vitamin D in the blood stream tissue-specifically reaches the intracellular VDR in the effector cell in need of Vitamin D reaction? What is the key receptor for distribution into the different tissues?
The answer might be, that Vitamin D binding protein is a sparsely glycosylated serum protein responsible for highly specific binding and tissue-specific delivery of vitamin D and its metabolites. In addition, it is also an actin scavenger, and the precursor to the immunomodulatory protein. Vitamin D binding protein has been proposed to have significant roles in C5a chemotaxis [30], osteoclast development and possibly in macrophage activation/recruitment [31].
DBP is internalized by megalin-mediated endocytosis in kidney and mammary cells that express megalin and cubilin. Megalin-mediated endocytosis of DBP facilitates uptake and conversion of 25(OH)D3 to 1,25(OH)2D3 in these types of cells [32-34].
Whereby, DBP enters the renal proximal tubules by receptor-mediated endocytosis 33.
In addition, a significant increase in VDBP and 25(OH)D(3) in human BALF 24 h after allergen challenge, suggests a role for these factors in the asthmatic late-phase reaction [35,36].
Concerning the immune system, it could be demonstrated, that the membrane immunoglobulin (MIg) of B lymphocytes displays physicochemical and immunological properties indistinguishable from those of Gc (group-specific component). In addition, evidence suggests that this vitamin D3-binding protein is involved in the linkage between MIg and actin and may therefore be important in signal transduction [37].
More recently, it could be shown that activated T cells express CYP27B1 and convert 25(OH)D3 to 1,25(OH)2D3 in sufficiently high concentrations to affect vitamin D-responsive genes when cultured in serum-free medium [38]. Furthermore, flow cytometry and representative confocal microscopy images revealed increased DBP-uptake of activated T cells compared to naïve T cells [38].
Furthermore, an inhibitory effect of 25(OH)D3 on the Th17 response is mediated via both T cells and DCs. DCs pathway is involved in the direct inhibition of 25(OH)D3 on Th17 cell differentiation in young asthmatics [39].
Unfortunately, VDBP has a broad genotypic and phenotypic variety. Therefore, standardized scientific research is complex.
The polymorphisms of DBP have been associated with susceptibility or resistance to a large number of chronic conditions, such as osteoporosis [40-42], type 1 and type 2 diabetes [43], thyroid autoimmunity [44], inflammatory bowel disease [45], and chronic obstructive lung disease [46]. Furthermore, it plays a role in infectious diseases [47-49]. It was also reported that the polymorphisms in the vitamin D receptor (VDR) and VDBP genes appeared to be responsible for host susceptibility to human tuberculosis [50].
Therefore, it is crucial to choose the right transporter to get the best effectiveness of Vitamin D related response in infectious disease.
We recently reported a newly developed dimeric compound, namely cholecalciferol-N-acetyl-galactosamine-Vitamin D binding protein (VitD~dgVDBP) and its positive and non-toxic effects in mice after intravenous injection compared to a sham group [9], effectively increasing the vitamin D level.
In addition, VitD~dgVDBP (Il-42) showed a higher macrophage activation and lower oxidative burst than VitD free dgVDBP and VDBP. This may result from a synergistic effect through a better presentation / exposition of protein bound Vitamin D to macrophages [10]. This form has shown to have no side effects, even in high doses and neither as oral or iv.-application form, in animals [9] and humans [11,12].
Furthermore, investigations demonstrated a significant improvement in macrophage function [10]. Preliminary data confirmed attenuated clinical courses of sepis [51], critical illness [52-54], respiratory tract infections [55-58], asthma exacerbations [59] and infectious diseases like influenza [27,60], HIV [60] and Herpes [60,61].
The beneficial effect of Vitamin C in infective disease is widely known [62-65].
There is little awareness, that Vitamin C can have an adverse effect on infections, if not combined with the recoverage substance Alpha-ketoglutarate (AKG) [66].
Alpha-Ketoglutaric acid is the is one of two ketone derivatives of glutaric acid. Its anion, α-ketoglutarate also called 2-oxoglutarate, is an important biological compound. It is the keto acid produced by deamination of glutamate and is widely known as an intermediate in the Krebs cycle. But, this is only a small range of its function. AKG, an endogenous intermediary metabolite in the Krebs cycle, is a molecule involved in multiple metabolic and cellular pathways. It functions as an energy donor, a precursor in the amino acid biosynthesis, a signaling molecule, as well as a regulator of epigenetic processes and cellular signaling via protein binding. ΑKG is an obligatory co-substrate for 2-oxoglutarate-dependent dioxygenases (OGDD), which catalyze hydroxylation reactions on various types of substrates [67]. It regulates the activity of prolyl-4 hydroxylase, which controls the biosynthesis of collagen, a component of bone tissue. ΑKG also affects the functioning of prolylhydroxylases, which, in turn, influences the function of the hypoxia-inducible factor [68,69], an important transcription factor in cancer development and progression. Additionally, it affects the functioning of enzymes that influence epigenetic modifications of chromatin: ten–eleven translocation hydroxylases involved in DNA demethylation and the Jumonji C domain containing lysine demethylases, which are the major histone demethylases. Thus, it regulates gene expression [70].
Most important, AKG is the obligate co-substrate of Fe(II)/2-oxoglutarate-dependent dioxygenases (OGDD), a superfamily of enzymes that catalyze the oxidative decarboxylation of AKG producing succinate and CO2 from O2 [70].
We first described the beneficial and additive effect of this combination. Recent confirming findings have been released and are under review [71].
This oral supplement is able to decrease cellular stress situations caused by an overload of reactive oxygen and nitrogen substances in pathogenic diseases, as frequently found in viral infections [17,64,72-81]. Vitamin C and AKG supplementation was performed using AlphaH℗ (HG Pharma, Vienna, Austria).
We used the newly invented and described water soluble transport immune-competent form of 1,25-D3 (Cholecalciferol) called Sedeprovid, also known as IL-42 (ImmunoD® CLS℗, HG Pharma, Vienna, Austria) for the treatment of five conclusive patients. Additionally, we also added the AlphaH concentrate to reduce known oxidative stress side effects of infections.
Prior to treatment the patients suffered from severe symptoms of confirmed Covid-19 infections, like cough, inappetence, tiredness, bone and body pain and body temperature of 39ºC or above for several days.
Within 24 hours after application of ImmunoD and AlphaH, patients and parents reported a significant reduction of symptoms and a drop down of maximal body temperature. After 48 hours the patients and parents reported a progressive recovery with regained energy and normal or close to normal activity over the day.
No side effects were reported from the patients or parents.
This novel treatment regimen might offer a good opportunity for the treatment of patients with moderate to severe symptoms before entering or after leaving the ICU ward. This protocol might also be used for critically ill patients during ICU stay and even be implemented as prophylactic program for healthcare providers.
Therefore, we strongly recommend the instant confirmation of these results in a controlled randomized trial for possible rapid benefit of Covid-19 infected patients and simultaneously a prophylactic program for healthcare providers.
We have to thank Mrs. Karen Pocock and Mrs. Margret Herwig for her input, patient acquisition and revising this article.
The Authors received the study medications from HG Pharma GmbH, Vienna, Austria. The authors received no financial support for the research, authorship, and/or publication of this article.
None.
- Organization (2020) WH. Novel coronavirus - China.
- Yang X, Yu Y, Xu J (2020) Clinical course and outcomes of critically ill patients with SARS-CoV-2 pneumonia in Wuhan, China: a single-centered, retrospective, observational study. Lancet Respir Med.
- Grasselli G, Pesenti A, Cecconi M (2020) Critical Care Utilization for the COVID-19 Outbreak in Lombardy, Italy: Early Experience and Forecast During an Emergency Response. JAMA.
- Youssef DA, Miller CW, El-Abbassi AM (2011) Antimicrobial implications of vitamin D. Dermatoendocrinol 3: 220-229.
- Holick MF (2004) Vitamin D: importance in the prevention of cancers, type 1 diabetes, heart disease, and osteoporosis. Am J Clin Nutr 79: 362-371.
- Cannell JJ, Vieth R, Umhau JC (2006) Epidemic influenza and vitamin D. Epidemiol Infect 134: 1129-1140.
- Chao CT, Lee SY, Yang WS (2014) Serum vitamin D levels are positively associated with varicella zoster immunity in chronic dialysis patients. Sci Rep 4: 7371.
- Grant WB, Lahore H, McDonnell SL (2020) Evidence that Vitamin D Supplementation Could Reduce Risk of Influenza and COVID-19 Infections and Deaths. Nutrients 12.
- Greilberger J, Greilberger M, Petek T (2019) Effective Increase of Serum Vitamin D3 by IV Application of a Cholecalciferol-N-Acetyl-Galactosamine-Stabilized Dimer: a Clinical Murine Trial Study. Clin Lab 65.
- Greilberger J, Herwig R (2020) Vitamin D - Deglycosylated Vitamin D Binding Protein Dimer: Positive Synergistic Effects on Recognition, Activation, Phagocytosis and Oxidative Stress on Macrophages. Clin Lab 66: 1.
- Greilberger J, Greilberger M, Herwig R (2017) Measurement of oxidative stress parameters, vitamin D and vitamin D binding protein during vitamin D treatment in a patient with amyotrophic lateral sclerosis. Integr Mol Med 4: 1-5.
- Greilberger J, Greilberger M, Herwig R (2018) Positive Effect on Behaviour of Autistic Children by Supplementation of New Complexed Cholecalciferol is Combined With Reduction of Lipid Peroxidation: A Pilot Study. Curr Trends Biomedical Eng & Biosci 14: 555893.
- Wang MM, Lu M, Zhang CL (2018) Oxidative stress modulates the expression of toll‑like receptor 3 during respiratory syncytial virus infection in human lung epithelial A549 cells. Mol Med Rep 18: 1867-1877.
- Qin T, Ma R, Yin Y (2019) Catalytic inactivation of influenza virus by iron oxide nanozyme. Theranostics 9: 6920-6935.
- Hosakote YM, Jantzi PD, Esham DL (2011) Viral-mediated inhibition of antioxidant enzymes contributes to the pathogenesis of severe respiratory syncytial virus bronchiolitis. Am J Respir Crit Care Med 183: 1550-1560.
- Greilberger JF (2018) Combination of 2-oxoglutarate/ascorbic acid/5-hydroxy- methyl-furfur-aldehyde/carnosine inhibits protein oxidation during radical exposure of cigarette smoke. Integr Mol Med 5: 1.
- Matzi V, Lindenmann J, Muench A (2007) The impact of preoperative micronutrient supplementation in lung surgery. A prospective randomized trial of oral supplementation of combined alpha-ketoglutaric acid and 5-hydroxymethylfurfural. Eur J Cardiothorac Surg 32: 776-782.
- Jeffery LE, Wood AM, Qureshi OS (2012) Availability of 25-hydroxyvitamin D(3) to APCs controls the balance between regulatory and inflammatory T cell responses. J Immunol 189: 5155-5164.
- Hasegawa K, Stewart CJ, Celedón JC, Mansbach JM, Tierney C, et al. (2018) Circulating 25-hydroxyvitamin D, nasopharyngeal airway metabolome, and bronchiolitis severity. Allergy 73: 1135-1140.
- Toubi E, Shoenfeld Y (2010) The role of vitamin D in regulating immune responses. Isr Med Assoc J 12: 174-175.
- Vijayendra Chary A, Hemalatha R, Seshacharyulu M, Vasudeva Murali M, Jayaprakash D, et al. (2015) Reprint of “Vitamin D deficiency in pregnant women impairs regulatory T cell function”. J Steroid Biochem Mol Biol 148: 194-201.
- Veldman CM, Cantorna MT, DeLuca HF (2000) Expression of 1,25-dihydroxyvitamin D(3) receptor in the immune system. Arch Biochem Biophys 374: 334-338.
- Rockett KA, Brookes R, Udalova I, Vidal V, Hill AV, et al. (1998) 1,25-Dihydroxyvitamin D3 induces nitric oxide synthase and suppresses growth of Mycobacterium tuberculosis in a human macrophage-like cell line. Infect Immun 66: 5314-5321.
- Cannell JJ, Zasloff M, Garland CF, Scragg R, Giovannucci E, et al. (2008) On the epidemiology of influenza. Virol J 5: 29.
- Lorente F, Fontan G, Jara P, Casas C, Garcia-Rodriguez MC, et al. (1976) Defective neutrophil motility in hypovitaminosis D rickets. Acta Paediatr Scand 65: 695-699.
- Edlich RF, Mason SS, Dahlstrom JJ, Swainston E, Long WB, et al. (2009) Pandemic preparedness for swine flu influenza in the United States. J Environ Pathol Toxicol Oncol 28: 261-264.
- Grant WB, Giovannucci E (2009) The possible roles of solar ultraviolet-B radiation and vitamin D in reducing case-fatality rates from the 1918-1919 influenza pandemic in the United States. Dermatoendocrinol 1: 215-219.
- Olmos-Ortiz A, Avila E, Durand-Carbajal M, Díaz L (2015) Regulation of calcitriol biosynthesis and activity: focus on gestational vitamin D deficiency and adverse pregnancy outcomes. Nutrients 7: 443-480.
- Chun RF, Peercy BE, Orwoll ES, Nielson CM, Adams JS, et al. (2014) Vitamin D and DBP: the free hormone hypothesis revisited. J Steroid Biochem Mol Biol 144 Pt A: 132-137.
- Kew RR, Fisher JA, Webster RO (1995) Co-chemotactic effect of Gc-globulin (vitamin D binding protein) for C5a. Transient conversion into an active co-chemotaxin by neutrophils. J Immunol 155: 5369-5374.
- Haddad JG (1995) Plasma vitamin D-binding protein (Gc-globulin): multiple tasks. J Steroid Biochem Mol Biol 53: 579-582.
- Nykjaer A, Dragun D, Walther D (1999) An endocytic pathway essential for renal uptake and activation of the steroid 25-(OH) vitamin D3. Cell 96: 507-515.
- Nykjaer A, Fyfe JC, Kozyraki R (2001) Cubilin dysfunction causes abnormal metabolism of the steroid hormone 25(OH) vitamin D(3). Proc Natl Acad Sci U S A 98: 13895-13900.
- Rowling MJ, Kemmis CM, Taffany DA, Welsh J (2006) Megalin-mediated endocytosis of vitamin D binding protein correlates with 25-hydroxycholecalciferol actions in human mammary cells. J Nutr 136: 2754-2759.
- Bratke K, Wendt A, Garbe K (2014) Vitamin D binding protein and vitamin D in human allergen-induced endobronchial inflammation. Clin Exp Immunol 177: 366-372.
- Ali NS, Nanji K (2017) A Review on the Role of Vitamin D in Asthma. Cureus 9: e1288.
- Petrini M, Emerson DL, Galbraith RM (1983) Linkage between surface immunoglobulin and cytoskeleton of B lymphocytes may involve Gc protein. Nature 306: 73-74.
- Kongsbak M, von Essen MR, Levring TB (2014) Vitamin D-binding protein controls T cell responses to vitamin D. BMC Immunol 15: 35.
- Hamzaoui A, Berraïes A, Hamdi B, Kaabachi W, Ammar J, et al. (2014) Vitamin D reduces the differentiation and expansion of Th17 cells in young asthmatic children. Immunobiology 219: 873-879.
- Ezura Y, Nakajima T, Kajita M (2003) Association of molecular variants, haplotypes, and linkage disequilibrium within the human vitamin D-binding protein (DBP) gene with postmenopausal bone mineral density. J Bone Miner Res 18: 1642-1649.
- Lauridsen AL, Vestergaard P, Hermann AP, Moller HJ, Mosekilde L, et al. (2004) Female premenopausal fracture risk is associated with gc phenotype. J Bone Miner Res 19: 875-881.
- Papiha SS, Allcroft LC, Kanan RM, Francis RM, Datta HK, et al. (1999) Vitamin D binding protein gene in male osteoporosis: association of plasma DBP and bone mineral density with (TAAA)(n)-Alu polymorphism in DBP. Calcif Tissue Int 65: 262-266.
- Jorde R (2019) The Role of Vitamin D Binding Protein, Total and Free 25-Hydroxyvitamin D in Diabetes. Front Endocrinol (Lausanne) 10: 79.
- Kurylowicz A, Ramos-Lopez E, Bednarczuk T, Badenhoop K (2006) Vitamin D-binding protein (DBP) gene polymorphism is associated with Graves’ disease and the vitamin D status in a Polish population study. Exp Clin Endocrinol Diabetes 114: 329-335.
- Eloranta JJ, Wenger C, Mwinyi J (2011) Association of a common vitamin D-binding protein polymorphism with inflammatory bowel disease. Pharmacogenet Genomics 21: 559-564.
- Chishimba L, Thickett DR, Stockley RA, Wood AM (2010) The vitamin D axis in the lung: a key role for vitamin D-binding protein. Thorax 65: 456-462.
- Junaid K, Rehman A, Jolliffe DA, Saeed T, Wood K, et al. (2016) Vitamin D deficiency associates with susceptibility to tuberculosis in Pakistan, but polymorphisms in VDR, DBP and CYP2R1 do not. BMC Pulm Med 16: 73.
- Petta S, Grimaudo S, Marco VD (2013) Association of vitamin D serum levels and its common genetic determinants, with severity of liver fibrosis in genotype 1 chronic hepatitis C patients. J Viral Hepat 20: 486-493.
- Putkonen P, Albert J, Karlsson A (1988) Group specific component and susceptibility to HIV infection and progression to AIDS. Scand J Infect Dis 20: 11-14.
- Lee SW, Chuang TY, Huang HH, Liu CW, Kao YH, et al. (2016) VDR and VDBP genes polymorphisms associated with susceptibility to tuberculosis in a Han Taiwanese population. J Microbiol Immunol Infect 49: 783-787.
- Kempker JA, Panwar B, Judd SE, Jenny NS, Wang HE, et al. (2019) Plasma 25-Hydroxyvitamin D and the Longitudinal Risk of Sepsis in the REGARDS Cohort. Clin Infect Dis 68: 1926-1931.
- Amrein K, Schnedl C, Holl A (2014) Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA 312: 1520-1530.
- Han JE, Jones JL, Tangpricha V (2018) High Dose Vitamin D Administration in Ventilated Intensive Care Unit Patients: A Pilot Double Blind Randomized Controlled Trial. J Clin Transl Endocrinol 4: 59-65.
- Han JE, Alvarez JA, Staitieh B (2018) Oxidative stress in critically ill ventilated adults: effects of vitamin D. Eur J Clin Nutr 72: 744-751.
- Ginde AA, Mansbach JM, Camargo CA (2009) Association between serum 25-hydroxyvitamin D level and upper respiratory tract infection in the Third National Health and Nutrition Examination Survey. Arch Intern Med 169: 384-390.
- Loeb M, Dang AD, Thiem VD (2019) Effect of Vitamin D supplementation to reduce respiratory infections in children and adolescents in Vietnam: A randomized controlled trial. Influenza Other Respir Viruses 13: 176-183.
- Martineau AR, Jolliffe DA, Hooper RL (2017) Vitamin D supplementation to prevent acute respiratory tract infections: systematic review and meta-analysis of individual participant data. BMJ 356: i6583.
- Quraishi SA, Bittner EA, Christopher KB, Camargo CA (2013) Vitamin D status and community-acquired pneumonia: results from the third National Health and Nutrition Examination Survey. PLoS One 8: e81120.
- Castro M, King TS, Kunselman SJ (2014) Effect of vitamin D3 on asthma treatment failures in adults with symptomatic asthma and lower vitamin D levels: the VIDA randomized clinical trial. JAMA 311: 2083-2091.
- Teymoori-Rad M, Shokri F, Salimi V, Marashi SM (2019) The interplay between vitamin D and viral infections. Rev Med Virol 29: e2032.
- Oztekin A, Öztekin C (2019) Vitamin D Levels in Patients with Recurrent Herpes Labialis. Viral Immunol 32: 258-262.
- Hemilä H, Louhiala P (2007) Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev 1: CD005532.
- Hemilä H, Louhiala P (2013) Vitamin C for preventing and treating pneumonia. Cochrane Database Syst Rev 8: CD005532.
- Hemilä H (2017) Vitamin C and Infections. Nutrients 9: 4
- Hemilä H, Chalker E (2020) Vitamin C may reduce the duration of mechanical ventilation in critically ill patients: a meta-regression analysis. J Intensive Care 8: 15.
- Greilberger J, Wintersteiger R, Ortner A, Greilberger M, Herwig R, et al. (2018) Combination of 2-oxoglutarate/ascorbic acid/5-hydroxy-methyl-furfur-aldehyde/carnosine inhibits protein oxidation during radical exposure of cigarette smoke. Integr Mol Med 5: 1-5.
- Saladi S, Boos F, Poglitsch M (2020) The NADH Dehydrogenase Nde1 Executes Cell Death after Integrating Signals from Metabolism and Proteostasis on the Mitochondrial Surface. Mol Cell 77: 189-202.e6.
- Liu Y, Borchert GL, Donald SP, Diwan BA, Anver M, et al. (2009) Proline oxidase functions as a mitochondrial tumor suppressor in human cancers. Cancer Res 69: 6414-6422.
- Tennant DA, Gottlieb E (2010) HIF prolyl hydroxylase-3 mediates alpha-ketoglutarate-induced apoptosis and tumor suppression. J Mol Med (Berl) 88: 839-849.
- Zdzisińska B, Żurek A, Kandefer-Szerszeń M (2017) Alpha-Ketoglutarate as a Molecule with Pleiotropic Activity: Well-Known and Novel Possibilities of Therapeutic Use. Arch Immunol Ther Exp (Warsz) 65: 21-36.
- Greilberger J, Wintersteiger R, Greilberger M, Zangger K, Herwig R, et al. (2020) Alpha-ketoglutarate prevents nitration of protein tyrosine residues as effective peroxynitrite scavenger. Free Rad Biol Med.
- Fowler Iii AA, Kim C, Lepler L (2017) Intravenous vitamin C as adjunctive therapy for enterovirus/rhinovirus induced acute respiratory distress syndrome. World J Crit Care Med 6: 85-90.
- Leal E, Ordás MC, Soleto I, Zarza C, McGurk C, et al. (2019) Functional nutrition modulates the early immune response against viral haemorrhagic septicaemia virus (VHSV) in rainbow trout. Fish Shellfish Immunol 94: 769-779.
- Wu J, Yang R, Yang Z (2017) ROS accumulation and antiviral defence control by microRNA528 in rice. Nat Plants 3: 16203.
- Gatterer H, Greilberger J, Philippe M, Faulhaber M, Djukic R, et al. (2013) Short-term supplementation with alpha-ketoglutaric acid and 5-hydroxymethylfurfural does not prevent the hypoxia induced decrease of exercise performance despite attenuation of oxidative stress. Int J Sports Med 34: 1-7.
- Greilberger J (2013) Preclinical and clinical testing of a alpha-ketoglutarate/5-HMF/N-actyl-selenomethionine and N-acteyl-methionine for treating tumors. Journal of Cancer Science and Therapy 5: 76-76.
- Groke K, Herwig R (2002) Agent having a destructive effect on malignant tumors and method for the production thereof. C Y L Handelsgersellschaft GmbH Austria 3: 1-28.
- Herwig R, Horninger W, Pinggera GM (2005) Metabolomics therapy with 2-oxo-glutaric acid solution (Karal solution) in patients with hormone and chemotherapy insensitive metastatic prostate cancer leads to an increase of PSA doubling time and decrease of blood supply in tumour lesions. European Urology Supplements 4: 146.
- Herwig R, Knoll C, Planyavsky M, Pourbiabany A, Greilberger J, et al. (2013) Proteomic analysis of seminal plasma from infertile patients with oligoasthenoteratozoospermia due to oxidative stress and comparison with fertile volunteers. Fertil Steril 100: 355-366.
- Herwig R, Greilberger J (2016) Medication against male infertility (Arzneimittel gegen männliche Sterilität). HG Pharma Austria 3: 1-8.
- Matzi V, Greilberger JF, Lindenmann J (2015) Application of hyperbaric oxygen reduce oxidative damage of plasmatic carbonyl proteins and 8-ohdg by activating glutathion peroxidase. Clin Lab 61: 587-593.


