Objective: Assessment of serum 1, 25-dihydroxy vitamin D in obese Egyptian women with polycystic ovary syndrome.
Methods: This was a cross- sectional analytic study conducted at Suez Canal University Hospital outpatient clinics. We recruited eighty patients with PCOS divided into two groups according to their BMI and another 80 women without PCOS. The study group included 40 obese PCOS women, and control one included 40 non- obese PCOS women. Another two groups (obese and non-obese) were recruited, including women without evidence of PCOS. Total testosterone, free testosterone, androstenedione, and DHEAS level were measured for all women. Serum 25-OHD was assessed using enzyme-linked immunoassay ELISA.
Results: Clinical and laboratory parameters showed a significant difference between the study and control groups regarding total testosterone, free testosterone, and DHEA-S (p value=0.001). Serum 25-OH vitamin D was lower in the PCOS group (19.5 ± 6.5) versus (24.8 ± 9.6) in the control group (p value =0.001). Serum androgen levels did not differ significantly. Surprisingly, serum 25-OH vitamin D was significantly lower in obese PCOS than non-obese PCOS (11.3 ± 5.9 vs 22.1 ± 8.4 ng/ml; p value=0.001)
Conclusion: An association was found between vitamin D deficiency and obese patients with PCOS.
25(OH) D, PCOS, obese, BMI, endocrinology
Polycystic ovary syndrome (PCOS) is the most common female endocrine disorder, affecting approximately 4%–18% women of reproductive age [1]. It is a heterogeneous androgen-excess disorder causing multiple reproductive and metabolic dysfunctions with variable degrees. Metabolic disturbances, including insulin resistance, hyper-insulinemia, and dyslipidemia, are standard features in the majority of women with PCOS [2].
The diagnostic criteria for PCOs in adolescence are controversial, mainly because the pathological diagnostic features used in adult women, such as acne, irregular menses, and PCOs, may be regular pubertal physiological events [3]. The diagnostic criteria in adult women include chronic anovulation manifested as amenorrhea or oligomenorrhea, polycystic ovarian morphology, and hirsutism or acne as evidences of hyper-androgenism. Intrinsic abnormalities in ovarian steroidogenesis may underlie this ovarian dysfunction in some patients [4]. However, additional factors, such as insulin resistance and/or hyperinsulinemia, may play a significant role. Hence, similar to diabetes, PCOS is not one disorder/disease [5].
Vitamin D is a steroid hormone with its precursor; 7-dehydrocholesterol, which is an intermediary in the cholesterol pathway, present in the skin. Accumulating evidence suggests that vitamin D deficiency might be a causal factor in the pathogenesis of metabolic syndrome in PCOS. Obesity is also linked with low 25(OH) D levels in PCOS cohorts. There is some evidence; vitamin D modulates the reproductive process both in women and men together with the conventional regulators, sex steroid hormones [6].
A published review performed by Thomson, et al. about the role of vitamin D in the etiology and management of PCOS suggests that- there is an association between vitamin D status and hormonal and metabolic dysfunctions in PCOS [7].
Eighty women were included in the study and classified equally into two groups; study and control groups. All patients were diagnosed as having PCOS and were allocated to either group according to their BMI. Patients with BMI ≥ 30 were assigned as a study group while patients with BML <30 as a control group. They were recruited from the general population attending outpatients' clinics at Suez Canal university hospitals. All patients fulfilled 2003 revised Rotterdam diagnostic criteria based on the association of at least two of the three following criteria: (a) Oligomenorrhea defined as cycles at intervals > 45 days or amenorrhea defined as absent cycles more than three months. (b) Clinical Hyperandrogenism (the presence of hirsutism (modified Ferriman and Gallwey score>8), acne, or androgenic alopecia.) or hyperandrogenemia (total testosterone>0.7 ng/ml. (c) Ultrasound criterion of polycystic ovary syndrome, either; 1) Presence of 12 or more follicles in each ovary measuring 2-9 mm in diameter, 2) And/or increased ovarian volume (>10 mL), 3) And/or an ovarian area more than 5.5 cm2 unilaterally or bilaterally. An Informed written consent was obtained from all participants; they were subjected to thorough medical history and anthropometric measures. Hormonal assay of total testosterone, free testosterone, androstenedione, and DHEAS was done. Serum 25 OH vitamin D was assessed using ELISA. Another group of women without evidence of PCOS was recruited for assessment of serum vitamin D levels only. The required sample size was calculated using a-error of 0.05 [8].
Demographic characteristics among the studied women in both groups showed a non-significant difference between the control group and study group regarding age and parity but significantly differed regarding BMI. The mean age of the study and control groups is 31.5 ± 8.2 and 32.7 ± 10.9 years, respectively. BMI was 36.1 ± 5.6 and 23.7 ± 2.4 for the study group and control one, respectively, with a p-value of 0.005 (Table 1). Serum levels of total and free testosterone, as well as DHEA-S, did not differ significantly. Serum 25-OH vitamin D was significantly lower in obese PCOS than non-obese PCOS (11.3 ± 5.9 vs. 22.1 ± 8.4 ng/ml; p value<0.0001) (Table 2). There was a significant negative correlation between 25-OH vitamin D and body mass index (r= -0.4, P value=0.001) (Figure 1).
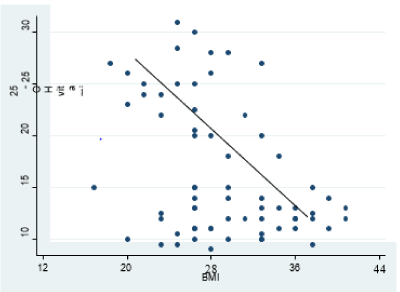
Figure 1. Correlation between Vitamin D and BMI (r= - 0.4; p-value = 0.001*)
Table 1. Demographic characteristics among the studied women in both groups
|
Study group
(obese PCOS n=40) |
Control group
(non-obese PCOS n=40) |
p-value |
Age |
Mean ± SD |
31.5 ± 8.2 |
32.7 ± 10.9 |
0.6 (NS) |
Range |
19 – 42 |
20 – 43 |
Parity |
NP |
15 |
37.5% |
10 |
25% |
0.3 (NS) |
P1 |
12 |
30% |
18 |
45% |
≥ P2 |
13 |
32.5% |
12 |
30% |
BMI (kg/m2) |
Mean ± SD |
36.1 ± 5.6 |
23.7 ± 2.4 |
0.005* |
(NS) not statistically significant difference (p-value > 0.05). *Statistically significant
Table 2. Vitamin D levels among both groups
|
Non obese control
(n=40) |
Non obese
PCOs
(n=40) |
p-value |
Obese control
(n=40) |
Obese PCOS
(n=40) |
p-value |
BMI (kg/m2)
Mean ± SD |
22.4 ± 2.2
|
23.7 ± 2.4 |
0.09 |
35.2 ± 5.1 |
36.1 ± 5.6 |
0.59 |
25-OH vitamin D
Mean ± SD |
26.9 ± 7.1 |
22.1 ± 8.4 |
0.06 |
21.7 ± 6.5 |
11.3 ± 5.9 |
<0.0001* |
*Statistically significant difference (p-value < 0.05)
Our data showed that the mean age of patients with PCOS fell in the third decade of life, disagreeing with the finding of Esmaeilzadeh, et al. who reported that, PCOS is more common in mean age of 23.5 years, proposing that due to a physiological decline of the follicular cohort leading to a normalized ovarian ultra-sonographic appearance with advancing age [9]. In our study, about one third of the patients were nulliparous, at the same time in another one, the majority (>70%) of the women recruited were nulliparous hence revealing the strong association of the syndrome with infertility [10].
Non- significant differences in the levels of total and free testosterone among our patients were reported, which was also affirmed by others [11,12].
Regarding serum levels of vitamin D, conflicting results exist between those who found that its concentration was decreased in patients with PCOS [13] and those who reported no relation between these parameters [14] with our study confirming its inverse relationship with patients' diagnosed with PCOS.
Vitamin D levels were significantly lower in obese women with PCOS than their counterparts. This was also confirmed by Hassan, et al. [15]. Also, researchers who studied vitamin D levels in women with PCOS with metabolic derangements found decreased levels in those with metabolic disorders than others without [14].
It is not clear whether vitamin D insufficiency results from obesity and/or whether obesity is a consequence of vitamin D insufficiency. Obesity may lead to low circulating vitamin D levels by trapping vitamin D in fat tissues [15,16], which needs further research.
Serum levels of vitamin D need to be evaluated in patients with PCOS with diagnosed metabolic consequences and whether this plays a significant role in its development.
Recruitment of study and control groups, both diagnosed with PCOS, empowers the results; however, a larger sample would be recommended. Our study included young participants in the childbearing period only while the recruitment of older study population with PCOS would reveal some facts about vitamin D concerning long term consequences of PCOS.
Serum 25-OH vitamin D levels decreased in women with PCOS, and the deficiency was marked in obese PCOs. Large studies are needed to assess vitamin D with biochemical and hormonal changes in obese PCOs.
ZM Ibrahim: Manuscript writing/editing.
OT Taha: Data collection and management, Data analysis.
EA Kishk: Project preparation, protocol writing.
SM Fahmy: Data collection and management, Data analysis.
Self-funded research.
None
All procedures performed study were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Informed consent was obtained from all participants before enrollment in the study.
This cross-sectional analytic study was conducted in Suez Canal University Hospita after approval by the ethical committee of faculty of medicine, Suez Canal University July 2018, number 1339.
- March WA, Moore VM, Willson KJ, Phillips DI, Norman RJ, et al. (2010) The prevalence of polycystic ovary syndrome in a community sample assessed under contrasting diagnostic criteria. Hum Reprod 25: 544-551.
- Wild RA (2012) Dyslipidemia in PCOS. Steroids 77: 295-299.
- Carmina E, Oberfield SE, Lobo RA (2010) The diagnosis of polycystic ovary syndrome in adolescents. Am J Obstet Gynecol 203: 201.e1-e5.
- McAllister JM, Modi B, Miller BA, Biegler J, Bruggeman R, et al. (2014) Overexpression of a DENND1A isoform produces a polycystic ovary syndrome theca phenotype. Proc Natl Acad Sci USA 111: E1519-E1527.
- Ibanez L, Lopez-Bermejo A, Callejo J, Torres A, Cabre S, et al. (2008) Polycystic ovaries in non-obese adolescents and young women with ovarian androgen excess: relation to prenatal growth. J Clin Endocrinol Metab 93: 196-199.
- Lerchbaum E, Obermayer-Pietsch B (2012) Vitamin D and fertility: a systematic review. Eur J Endocrinol 166: 765-778.
- Thomson RL, Spedding S, Buckley JD (2012) Vitamin D in the aetiology and management of polycystic ovary syndrome. Clin Endocrinol (Oxf) 77: 343-350.
- Fleiss JL (1981) Statistical methods for rates and proportions. New York: John Wiley & Sons, Inc.
- Esmaeilzadeh S, Andarieh MG, Ghadimi R, Delavar MA (2014) Body mass index and gonadotropin hormones (LH & FSH) associate with clinical symptoms among women with polycystic ovary syndrome. Glob J Health Sci 7: 101-106.
- Igwegbe AO, Eleje GU, Enechukwu CI (2013) Polycystic ovary syndrome: A review of management outcomes in a low resource setting. J Womens Health Issues Care 2: 1-6.
- Arentz S, Abbott JA, Smith AC, Bensoussan A (2014) Herbal medicine for the management of polycystic ovary syndrome (PCOS) and associated oligo/amenorrhoea and hyperandrogenism; a review of the laboratory evidence for effects with corroborative clinical findings. BMC Complement Altern Med 214: 511.
- Faraj RM, Jawad AH, Badr AH (2019) Effect of body mass index on abnormal ovarian secretion hormones among Iraqi women with polycystic ovarian syndrome (PCOS). Al Nahrain Journal of Science 22: 40-45.
- Chen MJ, Chen CD, Yang JH, Chen CL, Ho HN, et al. (2011) High serum dehydroepiandrosterone sulfate is associated with phenotypic acne and a reduced risk of abdominal obesity in women with polycystic ovary syndrome. Hum Reprod 26: 227-234.
- Savastano S, Valentino R, Di Somma C, Orio F, Pivonello C, et al. (2011) Serum 25-hydroxyvitamin D Levels, phosphoprotein enriched in diabetes gene product (PED/PEA-15) and leptin-to-adiponectin ratio in women with PCOS. Nutrition and Metabolism 8: 84.
- Figoruva J, Javorsky M, Dravicka I, Lazurova I (2015) Prevalence of vitamin D deficiency in Slovak women with polycystic ovary syndrome and its relation to metabolic and reproductive abnormalities. Wiener klinische Wochenschrift 128: 17-18.
- Hassan NE, El Orabi HA, Eid YM, Mohammed NR (2012) Effect of 25-hydroxyvitamin D on metabolic parameters and insulin resistance in patients with polycystic ovarian syndrome. Middle East Fertility Society Journal 17: 176-180.

