A 61-year-old Han Chinese male presented with 4-5 weeks of failure to thrive, hiccups, malaise, and fevers up to 101.5 at home. The patient had been admitted one week prior due to similar symptoms. His primary care physician recently (within 3 months) started gabapentin and chlorpromazine for hiccups, and allopurinol for gout. Drug fever was suspected, and the patient was asked to discontinue gabapentin, allopurinol and chlorpromazine. A short infectious workup for eosinophilia was negative for Herpes Simplex, Strongyloides, and Mycoplasma Pneumoniae, and he was discharged.
On repeat admission he admitted to occasional use of allopurinol since his discharge home. Blood pressure was 90/50, temperature was 100.5 Degrees Fahrenheit, and heart rate was 100. Physical exam showed facial plethora, dry mucus membranes, and a diffuse morbiliform rash covering 70 percent of his body. Labs were notable for white blood cell count of 20,000 per microliter, with 6,200 eosinophils per microliter, and 31% eosinophils. Peripheral blood smear confirmed eosinophilia. A skin biopsy showed combined spongiotic and perivascular dermatitis, with eosinophils and neutrophils, all of which was consistent with Severe Cutaneous Adverse Reaction (SCAR). His symptoms quickly improved with oral prednisone. HLA B58:01 allele was positive, which is associated with allopurinol hypersensitivity. The patient was warned to avoid use of allopurinol in the future. HLA B58:01 allele testing in the Han Chinese population is routine in East Asian countries prior to treatment with allopurinol. Despite recommendations by the American College of Rheumatology, testing is often overlooked.
Gout affects 1% of the Unites States population annually, representing about 3 million cases per year. 16 million prescriptions of allopurinol are written annually, and it is associated with a severe, life threatening side effect of SCAR, which may be underappreciated by Internists and Rheumatologists. In general, Type A adverse drug reactions account for 85% of drug side effects. These are dose dependent and related to the primary effect of a drug, an example being hypotension after taking an increased dose of blood pressure medication. Type B adverse drug reactions account for 15% of drug side effects. These are less predictable and typically involve hypersensitivity in unpredictable drug doses. Allopurinol is commonly implicated in Type B reactions – especially cutaneous. Stevens Johnson, Toxic epidermal necrolysis, and DRESS have all been associated with allopurinol.
A 61-year-old Han Chinese male presented with 4-5 weeks of failure to thrive, hiccups, malaise, and fevers up to 101.5 at home. His primary care physician recently (within 3 months) started gabapentin and chlorpromazine for hiccups, and allopurinol for gout. The patient had been admitted one week prior due to similar symptoms. During that admission, drug fever was suspected, and the patient was asked to discontinue gabapentin, allopurinol and chlorpromazine. A short infectious workup for eosinophilia was negative for Herpes Simplex, Strongyloides, and Mycoplasma Pneumoniae, and an EGD revealed gastritis due to H. Pylori infection. He was discharged with instructions to discontinue all his new medications.
On repeat admission one week later, he admitted to occasional use of allopurinol since his discharge home. His symptoms of lethargy, malaise, and loss of appetite persisted. He also noticed a worsening rash in his abdominal area, which had begun spreading to his entire trunk and arms over the course of three days.
Physical exam
Vital signs: BP 92/63, pulse 94, respirations 18 breaths per minute, Temperature 100.0
Physical Examination
General: Awake, cooperative, appears sad.
HEENT: slightly swollen face, no lymphadenopathy.
Chest Wall: no tenderness, no deformities.
Heart: Regular rate and rhythm, S1 and S2 present, no murmur.
Abdomen: soft, nontender, nondistended, no masses, rash present as described below.
Extremities: 2+ pulses, symmetric, rash as described below.
Skin: Erythematous, patchy, morbilliform rash covering trunk, arms, legs, and sparing face and hands.
Neurologic: Alert and oriented x3, CN II-XII intact, normal reflexes throughout.
Labs were notable for an acute kidney injury and slight elevation in alkaline phosphatase to 158. White blood cell count was elevated at 20,000 per microliter (normal 12,000 per microliter), with 6,200 eosinophils per microliter (normal 600 per microliter), and 31% eosinophils. Peripheral blood smear confirmed eosinophilia. Dermatology and Infectious disease were consulted. A skin biopsy revealed combined spongiotic and perivascular dermatitis, with eosinophils and neutrophils, all of which was consistent with Severe Cutaneous Adverse Reaction (SCAR), likely DRESS syndrome. Laboratory workup is linked in the attached figures (Figure 1 and 2).
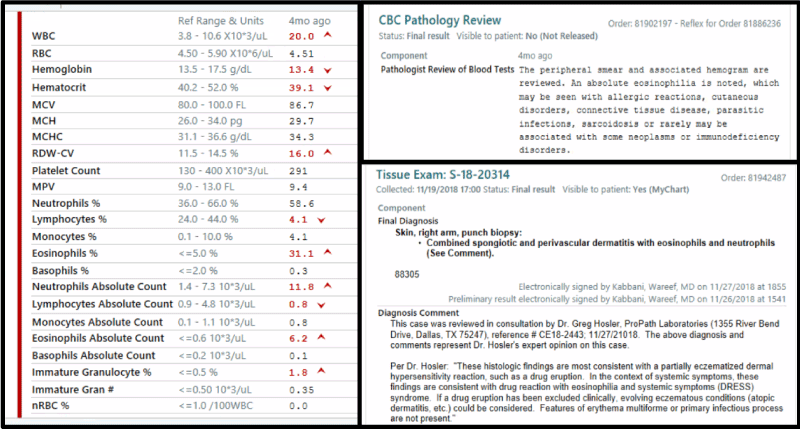
Figure 1. Laboratory workout
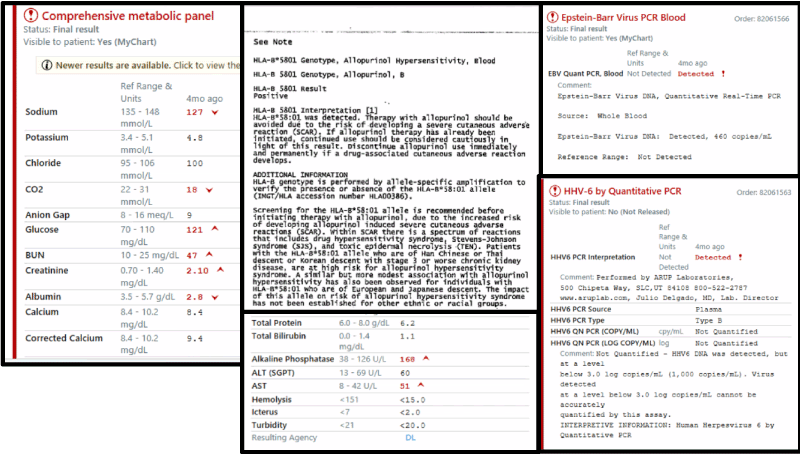
Figure 2. Laboratory workout
Based on the above workup, the patient was diagnosed with a severe cutaneous adverse reaction. He was started on 40 mg intravenous methylprednisolone and showed rapid improvement. By day 3 of hospitalization his rash had significantly improved, and his PO intake was back to normal. Blood pressure improved and there were no further fevers. His white blood cell count and eosinophilia also resolved. He was not started on any antibiotics. His liver function tests and kidney function tests returned to baseline. He was discharged with a steroid taper and was given instructions to avoid all future allopurinol use based on his HLA-B*5801 phenotype.
Allopurinol is FDA approved to treat gout. The FDA drug label does not discuss HLA genotype testing. The American College of Rheumatology (ACR) and the Clinical Pharmacogenetics Implementation Consortium (CPIC), however, both recommend HLA testing in certain high-risk groups prior to starting allopurinol and avoiding allopurinol in anyone who tests positive. Their statements are below:
2015 Statement from the Clinical Pharmacogenetics Implementation Consortium (CPIC): Given the high specificity for allopurinol-induced SCAR, allopurinol should not be prescribed to patients who have tested positive for HLA-B*58:01. Alternative medication should be considered for these patients to avoid the risk of developing SCAR. For patients who have tested negative, allopurinol may be prescribed as usual. However, testing negative for HLA-B*58:01 does not eliminate the possibility of developing SCAR, especially in the European population.
2012 Statement from the American College of Rheumatology (ACR):Prior to initiation of allopurinol, rapid polymerase chain reaction-based HLA-B*5801 screening should be considered as a risk management component in subpopulations where both the HLA-B*5801 allele frequency is elevated and the HLA-B*5801-positive subjects have a very high hazard ratio ("high risk") for severe allopurinol hypersensitivity reaction (e.g., Koreans with stage 3 or worse chronic kidney disease and all those of Han Chinese and Thai descent).
Type B hypersensitivity reactions can occur at any drug dosage and are generally unpredictable. Allopurinol induced drug reaction is particularly difficult to diagnose because of heterogenous presentations, and a long latency period (8-10 weeks) between drug administration and systemic reaction. One theory about the pathogenesis of allopurinol induced SCAR is the p-I concept. It posits that cytotoxic T cell (CD8+) induction and widespread activation leads to a hypersensitivity reaction. The signal may be either triggered or amplified by presence of HLA-B*58:01 allele.
The HLA genes encode proteins which interact with the immune system to recognize self and non-self. The HLA group consists of over 200 genes, and is divided into the classes: Class I, Class II, and Class III. In general HLA class I proteins present immune cells with proteins from cellular breakdown, whereas class II proteins carry protein fragments from outside the body. If the HLA system presents a foreign particle, CD8+ cells will release inflammatory cytokines and commence an immunologic response.
One study conducted in a Han Chinese population revealed all patients with allopurinol-induced SJS/TEN (51/51) carried HLA B*58:01, while only 15% allopurinol-tolerant patients (20/135) carried the allele. Serious drug reactions have also been described in non-carriers of HLA B*58:01, so our knowledge the pathophysiology remains incomplete.
HLA B*58:01 is a codominant gene, therefore carriers of even a single allele pose risk of allopurinol-induced hypersensitivity. Cost effectiveness of testing is still being studied, with mixed results. One study found a net benefit to testing those of Korean descent with kidney disease, however another study conducted in Singapore and Portugal found no benefit.
Maintaining a high index of suspicion for allopurinol hypersensitivity in patients of Asian descent and performing HLA B58:01 is of paramount importance.


