Splenic Marginal Zone Lymphoma (SMZL) is an unusual, distinct lymphoma characterized by indolent clinical course and prolonged survival, and having some clinical, cellular and immunophenotypical features that differ from other indolent lymphomas. We describe a case of SMZL that presented with autoimmune hemolytic anemia.
splenic marginal zone lymphoma, autoimmune manifestation, Rituximab
SMZL was originally recognized either by histopathologic examination of splenectomised specimens [1] or by morphologic and immunophenotypical characterization of circulating neoplastic lymphocytes as splenic lymphoma with villous lymphocytes. SMZL is rare; accounting for 20% of all MZL, only 1% of non-Hodgkin’s Lymphoma in adults [2] and mainly affecting elderly or middle-aged patients, with median age of 65 years. SMZL is characterized by massive splenomegaly without significant lymphadenopathy, other than in the splenic hilum, and no other extranodal involvement other than bone marrow and liver [3], frequently with cytopenias and lymphocytosis [3]. There is no specific immunophenotypic marker for SMZL, with lymphoma cells expressing pan-B-cell antigens, usually CD20, CD45RA, CD45RB, CD79a, PAX5/BSAP, IgM, and surface IgM and/or Ig D, but negative for CD5, CD10, and CD23, CD43, bc16 and cyclin D1. B symptoms (weight loss, night sweats, fever) are rare [3-5].
Bone marrow and blood involvement are usually present in 95% in SMZL with villous lymphocytes present in blood in 15% of cases [3,6]. Different types of bone marrow infiltrations are described, namely intrasinusoidal, interstitial, nodular, and paratrabecular. Intrasinusoidal infiltration is considered highly characteristic of SMZL, and to date, apart from subtle infiltration in some extra nodal marginal lymphoma there have been no reports of intrasinusoidal infiltration in other kinds of low-grade B-cell lymphoma [7-11]. With peripheral blood involvement, numerous mature B lymphocytes with pale cytoplasm, irregular cytoplasmic borders, and villous projections can easily be recognized [12]. The co-occurrence of Hepatitis C (HCV) infection is of major importance due to possible therapeutic implications [13]. The precise pathogenesis of SMZL is unknown. In contrast to the well-established chromosomal changes associated with other B-cell NHL, few genetic alterations have been reported in SMZL [14]. In addition, SMZL may manifest autoimmune phenomena, with primary biliary cirrhosis [15], rheumatoid arthritis [16], immune thrombocytopenia, presence of lupus anticoagulant and autoimmune hemolytic anemia being the most frequent associated conditions.
There is no consensual standardized treatment for SMZL due to the lack of prospective randomized trials, the relative rarity of the disease and the prolonged survival. These include active surveillance, splenectomy, chemotherapy with rituximab alone and rituximab added to a variety of cytotoxic agents (chemo-immunotherapy) in various protocols. When concurrent HCV infection is present, this should be eradicated with newer direct acting anti-viral agents, and when chemotherapy is used, active or previous infection with Hepatitis B (HBV), should be treated with viral suppressive agents.
A 65-year-old Chinese man presented with lethargy and NYHA grade 2 dyspnea with only significant medical history of right knee joint replacement for osteoarthritis and recently diagnosed with HBV infection. He denied palpitation, central chest pain, cough, wheeze, fever and peripheral edema. He denied use of any illicit drugs, tobacco, and alcohol. Family history was noncontributory, including the absence of any malignancy.
On physical examination, the patient was lethargic, pale with tinge of jaundice, with vital signs on initial examination of tachycardia, but normal blood pressure. Abdominal examination revealed marked splenomegaly about 15 cm below the left costal margin with no abdominal tenderness. There were no other positive findings including no palpable adenopathy. Complete blood count (CBC) showed hemoglobin 5.41 g/dl (normal range: 13-18), RBC 1.28 × 109 /L (4.5-6.5), hematocrit 15% (40-54)%), MCV(Mean Corpuscular Volume 116fl (76-96), WBC 11.3 × 109/L ( 4-11), Neutrophil 2.6 × 109/L(2-7.5), Lymphocyte: 8.0 × 109/L(1.5-4), platelets 135 × 109/L(150-400), reticulocyte count: 13.6% (0.2-2), Lactate Dehydrogenase (LDH) 570 IU/L (81-234), total bilirubin 51.3 (< 22) with direct bilirubin 13.1 (0.0-5.0) and indirect bilirubin 38.1 umol/L (Table 1 and Figure 1). Peripheral blood film revealed minor agglutination of red blood cells with polychromasia without increase in spherocytes. WBC showed lymphocytosis with many lymphocytes showing villous cytoplasm and platelets were morphologically normal but slightly reduced. (Table 2) The direct antiglobulin test (Coombs direct) was strongly positive and monospecific for IgG and Anti -C3d with 3+ and 2+ reaction respectively.
Table 1: CBC, hemolytic parameters, free light chains and liver function tests.
|
Normal range |
Unit |
Admitted on
28.06.17,
Rituximub started on
30.03.17 |
5.07.17 |
20.07.17 |
31.08.17 |
Hb |
13.0-18.0 |
g/dL |
5.4 |
10.4 |
11.6 |
12.2 |
RBC |
4.5-6.5 |
× 1012/L |
1.28 |
2.34 |
3.06 |
3.86 |
PCV |
40-54 |
% |
15 |
23 |
30 |
38 |
MCV |
76-96 |
fL |
116 |
100 |
98 |
97 |
WBC |
4.0-11.0 |
× 109/L |
11.3 |
9.0 |
7.8 |
5.8 |
NEU |
2.0-7.5 |
× 109/L |
2.6 |
2.9 |
3.4 |
3.7 |
LYMPHO |
1.5-4.0 |
× 109/L |
8.0 |
6.2 |
5.9 |
1.6 |
PLATELETS |
150-400 |
× 109/L |
135 |
133 |
145 |
179 |
RETICULO |
0.2-2.0 |
% |
13.6 |
15.9 |
10.1 |
1.6 |
LDH |
81-234 |
U/L |
570 |
389 |
303 |
250 |
Coomb’s test
Monospecific |
|
|
IgG3+
AntiC3d2+ |
|
|
|
Serum Free Kappa |
3.30-19.4 |
mg/L |
82.08 |
|
|
|
Serum Free
Lambda |
5.71-26.30 |
mg/L |
32.98 |
|
|
|
ALBUMIN |
32-48 |
g/L |
40 |
- |
|
|
GLOBULIN |
20-50 |
g/L |
32 |
- |
|
|
TOTAL BILIRUBIN |
(< 22.0) |
umol/L |
51.3 |
23.4 |
16.8 |
|
DIRECT BILIRUBIN |
0.0-5.0 |
umol/L |
13.1 |
7.3 |
6.1 |
|
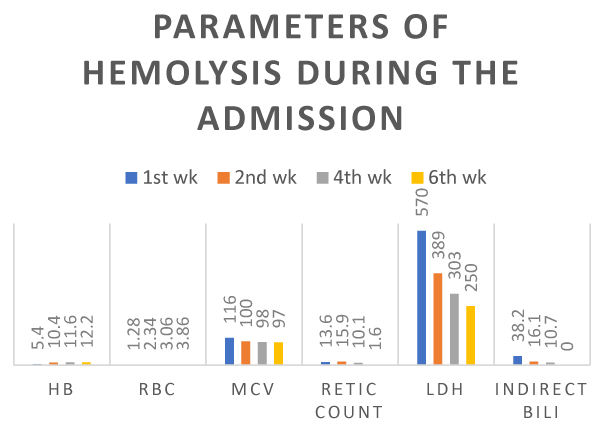
Figure 1. Hemoglobin (g/dl), Red blood cell count × 109, MCV(fl), Reticulocyte count (%), LDH(IU/L) and Indirect bilirubin (umol/L) before and after rituximab.
Bone marrow aspiration and biopsy from the posterior iliac crest demonstrated erythroid hyperplasia, normal maturation of granulocytic series with morphologically abnormal lymphocytes demonstrating cytoplasmic villous projection (Figure 2), erythroid hyperplasia with left shifted maturation, with reduced myelopoiesis and megakaryopoiesis. Bone marrow aspiration biopsy stained with CD20 demonstrated an infiltrate of small lymphoid cells positive for CD20, CD79a, kappa light chain and negative for CD3, CD5, CD10, Tdt, CD23 and CD34. Microscopically there were lymphoid cells showing interstitial and paratrabecular nodules (Figure 3).
Table 2: Report of peripheral blood, bone marrow, immunocytochemistry and flow cytometry.
|
Findings |
Comments |
Peripheral blood film |
RBC: minor agglutination, polychromasia without increase in spherocytes
WBC: Lymphocytosis with many lymphocytes showing villous cytoplasm.
Platelets: mildly reduced, but normal morphologically
|
|
Bone marrow trephine biopsy |
The marrow is patchily hypercellular, infiltrated by groups of small to medium sized lymphoid cells surrounded by other haemopoietic cells. The lymphoid cells show interstitial distribution as well as central and paratrabecular nodules. |
|
Immunocytochemistry of bone marrow |
The cells are positive for CD20, CD79a and kappa light chain. CD 20 shows that the lymphoid cells occupy more than 30% of cell volume. They are negative for CD3, CD5, CD10, CD23, CD34, cyclin D1, CD138 and TdT and CD34 |
Low grade B cell lymphoma, compatible with splenic marginal lymphoma |
Flow cytometry of bone marrow report |
Positive markers: CD19, CD 79a, HLA-DR, Bright CD20, FMC7, sIgM, sIgD and sIgM Negative markers: CD25, CD34, CD38, CD43, CD5, CD10, CD103, sIgG lambda
|
There is infiltration of the marrow by a clonal B-lymphoproliferative disorder. The morphological and immunophenotypic findings are most consistent with a Splenic Marginal Zone Lymphoma |
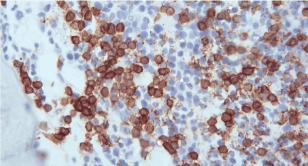
Figure 2. Lymphocytes with villous projections in bone marrow aspiration.
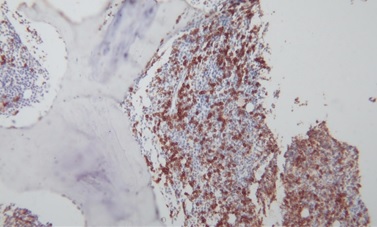
Figure 3. Bone marrow trephine biopsy with CD 20 stain.
Abdominal Ultrasonography demonstrated a markedly enlarged spleen of 22.9 cm and a small 0.5 cm gall bladder polyp. Contrast Enhanced Computed Tomography (CECT) of the neck, thorax, abdomen and pelvis, apart from massive splenomegaly, (Figure 4) revealed no adenopathy except for minimally enlarged para aortic lymph nodes with largest measuring 1.3 × 1.1 cm at the level of L3/L4 intervertebral disc and an enlarged prostate of 3.7 × 5.1 × 3.2 cm. Hepatitis B core antibody was reactive with undetectable HBS antigen and Hepatitis C antibody. Serum protein electrophoresis demonstrated reduced alpha 2 globulins, no paraproteins, and elevated free kappa light chains. Free serum Kappa was 82.08 mg/L (normal range 3.30-19.40), Free Lambda 32.98 mg/L (normal range: 5.71-26.3) and ratio of Kappa/Lambda high at 2.488. MYD88 p. Leu265Pro mutation was negative.
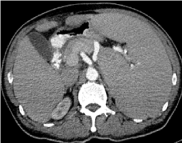
Figure 4. CT abdomen showing spleen size before rituximab.
A diagnosis of autoimmune hemolytic anemia (AIHA) secondary to SMZL was made. Corticosteroids at 1 mg/kg with folate supplements were commenced and the patient was started on 6 weekly doses of rituximab at 375 mg/m2 as the patient refused splenectomy. Because of presence of Hepatitis B core antibody and treatment with rituximab and steroids, prophylactic Tenofovir was commenced to avert a Hepatitis B flare. Splenomegaly and signs of hemolysis rapidly improved. Hemolysis abated and CBC improved rapidly (Table 1 and Figure 1) and rapid steroid taper was instituted after 2nd week of treatment. Repeat CT abdomen in follow-up clinic revealed spleen size of 9 cm after only two rituximab injections (Figure 5).
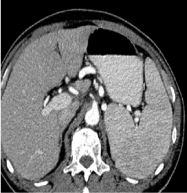
Figure 5. CT abdomen showing reduction of spleen size after two injections of rituximab.
There were no adverse reactions to rituximab and at present, with maintenance rituximab 375 mgmg/m2 every two months the patient continues in apparent complete remission. Repeat Coomb’s test (polyspecific test) was 3+ and 2+ after 4th and 5th month of rituximab and Monospecific Coomb’s test was IgG0 and anti Cd31+ after 7th month of rituximab. His Hepatitis B status was within control and there were no signs or symptoms of exacerbation.
Rituximab maintenance 500 mg every 2 months for 1 – 2 years was planned. Subsequent CBC’s and parameters of hemolysis are shown in Table 3 and Figure 6. Patient has been well since completion of 2nd dose of rituximab and after 5th month of treatment his performance status has been fully normal, and he has been playing rugby.
Table 3: Full blood count and other parameters of hemolysis after discharge or during follow up.
|
Normal range |
Unit |
2 months after
treatment
31.08.17 |
3 months after treatment
30.09.17 |
4 months after treatment
31.10.17 |
5 months after treatment
1.12.18 |
6 months after treatment |
Hb |
13.0-18.0 |
g/dL |
14.3 |
16.0 |
13.8 |
15.3 |
|
RBC |
4.5-6.5 |
× 1012/L |
4.69 |
5.27 |
4.61 |
5.12 |
|
PCV |
40-54 |
% |
43 |
47 |
41 |
44.9 |
|
MCV |
76-96 |
fL |
91 |
88 |
89 |
87.7 |
|
WBC |
4.0-11.0 |
× 109/L |
5.9 |
5.1 |
4.0 |
4.5 |
|
NEU |
2.0-7.5 |
× 109/L |
3.7 |
4.5 |
0.9 |
2.8 |
|
LYMPHO |
1.5-4.0 |
× 109/L |
1.7 |
0.5 |
0.9 |
1.3 |
|
PLATELETS |
150-400 |
× 109/L |
135 |
122 |
118 |
116 |
|
RETICULO |
0.2-2.0 |
% |
1.4 |
1.0 |
0.6 |
0.6 |
|
LDH |
81-234 |
U/L |
200 |
189 |
187 |
184 |
|
Coomb’s test
Polyspecific |
|
|
|
|
3+ |
2+ |
|
Coomb’s test Monospecific |
|
|
|
|
|
|
IgG0
AntiCd31+ |
ALBUMIN |
32-48 |
g/L |
- |
- |
|
|
|
GLOBULIN |
20-50 |
g/L |
- |
- |
|
|
|
TOTAL BILIRUBIN |
(< 22.0) |
umol/L |
- |
- |
|
10.7 |
|
DIRECT BILIRUBIN |
0.0-5.0 |
umol/L |
- |
- |
- |
5.6 |
|
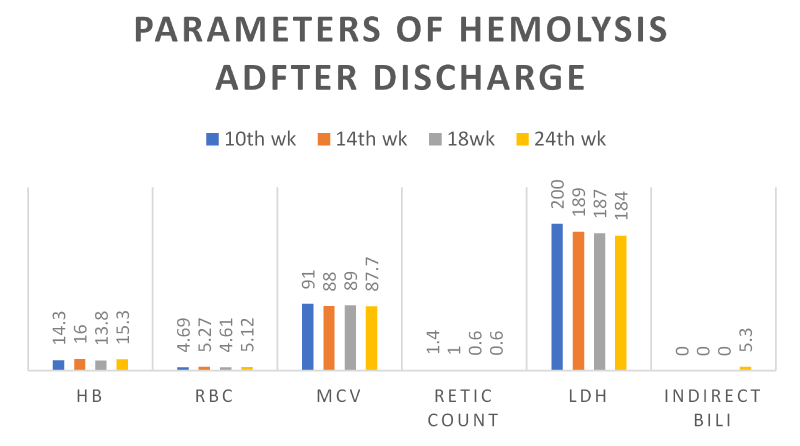
Figure 6. Hemoglobin (g/dl), Red blood cell count x 109, MCV (fl), Reticuocyte count (%), LDH (IU/L) and Indirect bilirubin (umol/L) before and after rituximab during admission.
The diagnosis of hemolysis was based on complete blood count, reticulocyte count, higher indirect than direct bilirubin, high LHD, and serological evidence of an autoantibody. The finding of a positive direct antiglobulin test (DAT), also called direct Coombs, in the setting of hemolytic anemia is diagnostic of AIHA [17]. Positive DAT for autoantibodies against IgG (warm AIHA) and anti C3d in our patient was consistent with mixed warm/cold AIHA [18]. In older patients, 90% of AIHA occurs secondary to an underlying B-cell lymphoproliferative disorder; most commonly MZL or LPL. Massive splenomegaly is not compatible with idiopathic AIHA; and presence of significant splenomegaly should raise concern of underlying lymphoma [19].
Diagnosis of SMZL is based on clinical, morphological, immunophenotying, and bone marrow biopsy, (unusually with splenic biopsy) as there are many features in common between SMZL, hairy cell lymphoma, follicular cell lymphoma, mantle cell lymphoma, B-chronic lymphatic leukemia and lymphoplasmocytic lymphoma (LPL) or Waldenstrom Macroglobulinemia. Diagnosis of SMZL here was made by presence the following features: massive splenomegaly, absence of clinical peripheral lymphadenopathy, sinusoidal pattern of infiltration coupled with an interstitial or nodular component on marrow biopsy, appropriate positive and negative immunopheotypic features (positive markers: CD19, CD 29a, HLA-DR, Bright CD20, FMC7, sIgM, sIgD and sIgM, negative markers: CD5, CD10, CD25, CD34, CD38, CD43, CD103, sIgG lambda) and finally presence of AIHA [3-12].
Because of AIHA and massive splenomegaly, active surveillance was not appropriate. Splenectomy, the oldest active therapeutic approach, with its pros (prolonged remission with one procedure) and cons (feasibility, side effects, surgical morbidity and poor choice with significant bone marrow involvement), could also be diagnostic and can be usually safely performed using laparoscopy, demonstrating high short- and long-term efficacy. In some patients, such as those with AIHA associated with splenic marginal zone lymphoma, splenectomy could provide favorable overall survival [20]. Thieblemont et al., [21] stated that splenic marginal zone lymphoma is a rare subtype of non-Hodgkin lymphoma in which autoimmune manifestations, such as autoimmune hemolytic anemia with massive splenomegaly, are relatively common, our patient being such an example. In cases of steroid refractory AIHA with massive splenomegaly and uncertain pathologic diagnosis, splenectomy is recommended since it can usually ameliorate the anemia and establish the diagnosis [22].
Rituximab monotherapy is equally effective with lesser side effects [4,23] and has dual action on both AIHA and lymphoma remission with minimal apparent toxicity [24]. However, our patient was reactive to Hepatitis B core antibody and rituximab therapy risks revitalization of hepatitis B virus. The option of splenectomy was offered but refused by patient. Therefore, rituximab monotherapy was instituted with corticosteroids and HBV prophylaxis with Tenofovir. Three weeks after admission, hemolysis abated, hemoglobin rapidly increased and bilirubin, MCV and LDH decreased toward normal ranges, allowing the rapid taper of corticosteroid therapy during the second week. Spleen size decreased remarkably after two injections of rituximab and lymphocyte counts decreased to normal.
SMZL, a relatively rare lymphoma, usually has an indolent clinical course with prolonged survival. Active surveillance can be a strategy for relatively asymptomatic patients, with splenectomy in select individuals and immunotherapy or chemo-immunotherapy for the majority. Our patient was treated with rituximab monotherapy for both lymphoma and secondary AIHA and had a rapid response.
- Tohda M, Mingmalairak S. (2013) Evidence of Antidepressive Effects of a Wakan-yaku, Hochuekkito, in Depression Model Mice with Learned-Helplessness Behavior. Evid Based Complement Alternat Med 2013: 319073. [Crossref]
- Tohda M, Abdel-Fattah AFM, Nakamura S, Watanabe H. (2000) Effects of Hochu-ekki-to (Bu-Zhong-Yi-Qi-Tang), a Kampo medicine, on serotonin 2C subtype receptor-evoked current response and the receptor mRNA expression. J. Traditional Medicines 17: 34-40.
- Tohda M, Takasu T, Nomura Y (1989) Effects of antidepressants on serotonin-evoked current in Xenopus oocytes injected with rat brain mRNA. Eur J Pharmacol 166: 57-63. [Crossref]
- Sukma M, Tohda M, Watanabe H (2003) Chronic treatment of imipramine inhibited cell growth and enhanced 5-HT2C receptor mRNA expression in NG108-15 cells. J Pharmacol Sci 92: 433-436. [Crossref]
- Tohda M, Hang PNT, Matsumoto K (2009) Developmental changes in serotonin 2C receptor mRNA editing in the rat cerebral cortex and primary cultured cortical neurons. Biol Pharm Bull 32: 289-292. [Crossref]
- Tohda M (2014) Serotonin 2C receptor as a superhero: diversities and talents in the RNA universe for editing, variant, small RNA and other expected functional RNAs. J Pharmacol Sci 126: 321-328. [Crossref]
- Tohda M (2018) Possible interaction of serotonin 2C receptor mRNA editing at C-site with expression of microtubule-associated protein 2 and neurite outgrowth in rat cultured cortical cells. MOJ Curr Res & Rev. 1(1): 00004.
- Tohda M, Hayashi H, Sukma M, Tanaka K (2008) BNIP-3: a novel candidate for an intrinsic depression-related factor found in NG108-15 cells treated with Hochu-ekki-to, a traditional oriental medicine, or typical antidepressants. Neurosci Res 62: 1-8. [Crossref]
- Tohda M (2014) Changes in the expression of BNIP-3 and other neuronal factors during the cultivation period of primary cultured rat cerebral cortical neurons and an assessment of each factor's functions. Cell signalling and Trafficking 2:1.
- Boyd JM, Malstrom S, Subramanian T, Venkatesh LK, Schaeper U, et al. (1994) Adenovirus E1B 19 kDa and Bcl-2 proteins interact with a common set of cellular proteins. Cell 79: 341–35. [Crossref]
- Matsuo K, Harada K, Fujita Y, Okamoto Y, Ota M, et al. (2016) Distinctive neuroanatomical substrates for depression in bipolar disorder versus major depressive disorder. Cereb Cortex 1-13. [Crossref]
- Kraus C, Manfred Klöbl M, Tik M, Auer B, Vanicek T, et al. (2018) The pulvinar nucleus and antidepressant treatment: dynamic modeling of antidepressant response and remission with ultra-high field functional MRI. Mol Psychiatry. [Crossref]
- Fu CH, Steiner H, Costafreda SG (2013) Predictive neural biomarkers of clinical response in depression: a meta-analysis of functional and structural neuroimaging studies of pharmacological and psychological therapies. Neurobiol Dis 52: 75-83. [Crossref]
- Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, et al. (1999) Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 402: 656-660. [Crossref]
- Takeda H, Sadakane C, Hattori T, Katsurada T, Ohkawara T, et al. (2008) Rikkunshito, an herbal medicine, suppresses cisplatin-induced anorexia in rats via 5-HT2 receptor antagonism. Gastroenterology. 134: 2004-13. [Crossref]
- García-García F, Juárez-Aguilar E, Santiago-García J, Cardinali DP (2014) Ghrelin and its interactions with growth hormone, leptin and orexins: implications for the sleep-wake cycle and metabolism. Sleep Med Rev 18(1):89-97. [Crossref]
- Date Y (2012) Ghrelin and the vagus nerve. Methods Enzymol 514: 261-269. [Crossref]
- Poher AL, Tschöp MH, Müller TD (2018) Ghrelin regulation of glucose metabolism. Peptides 100: 236-242. [Crossref]






