Spontaneous regression of angiosarcoma is a rare and poorly understood phenomenon. We provide a review of seven known cases and describe an eighth case involving a 76-year-old male. This case illustrates two intriguing issues. First, the onset of this cancer occurred in the setting of tissue injury and regenerative wound healing with angiogenesis apparently gone awry. Second, the malignancy underwent a 2 year long spontaneous regression. An abscopal response was a possibility. An anti-tumor immune response was suggested by a CD8 immune infiltrate of the tumor. The results of full exome sequencing of the tumor are presented.
Spontaneous regression of cancer was first noted in 1899 in melanoma and in 1901 in breast cancer by William Osler [1]. In a review of 176 well-documented cases [1,2] and another of 504 cases [3], many different tumor types were represented, but angiosarcoma was not among them. In melanoma the immune response to melanocyte antigens is thought to contribute. “Abscopal” effects are presumably due to systemic immune responses leading to regression of distant metastases that are initiated by local treatment of a primary mass, such as with irradiation or surgery.
Angiosarcomas are rare aggressive tumors of skin or deep tissues, with a 5-year survival rate of around 30% [4]. Patients are usually over 50 with male predominance [5]. A subset of angiosarcomas arises in a prior field of irradiation, such as after adjuvant radiotherapy for breast cancer. Here we present a case of a clinically aggressive angiosarcoma, with onset linked to traumatic tissue injury of 2 toes and a spontaneous remission lasting 2 years. Seven published cases of spontaneous remission in angiosarcoma are reviewed.
Immunohistochemistry
Immunoperoxidase staining was performed on a Leica Bond III automated immunostainer, with CD3, CD4, CD8, PD1, PAX5 and CD138 as well as in situ hybridization for Ig-kappa and Ig-lambda light chains according to the manufacturer’s instructions (Leica Microsystems, Bannockburn, IL).
Case presentation
A 76-year-old Asian man dropped a suitcase on his left foot sustaining a hairline fracture and hematomas of his 4th and 5th toes resulting in tissue necrosis. Amputation of both toes was performed one month later. Surprisingly, pathology revealed a high-grade epithelioid angiocarcinoma positive for typical markers: CD31 (>90%), CD34 (40-100%), vimentin, factor VIII, KI67 (72%), FLi-1, and thrombomodulin.
Margins were clear of malignant cells. Prophylactic below-the-knee amputation (BKA) was performed 3 months after the injury. Sixteen random sections from this amputation showed no evidence of malignant cells. Chemotherapy was refused. A prosthetic leg was ill tolerated, causing further injury to the stump. Recurrence of the tumor within the stump occurred nine months post injury. Five small tumors with blue skin discoloration were noted in the lower left abdominal wall, measuring 5-10 mm in diameter. A biopsy of 2 of these nodules revealed embolic angiosarcoma (Figure 1a).
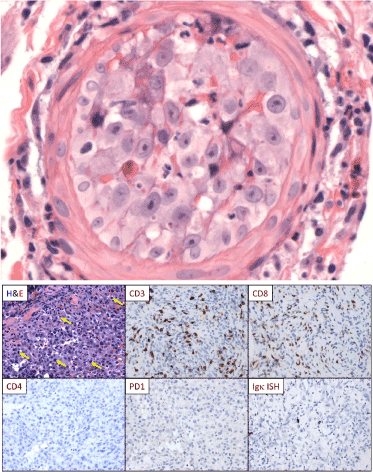
Figure 1. Immunopathology. a) H & E stain of an abdominal wall nodule showing embolic angiosarcoma completely obstructing a small arteriole. b) Immunohistochemistry (IHC), all images 200×. The angiosarcoma (H&E, upper left) was infiltrated by lymphoid cells (yellow arrows). IHC showed that the infiltrate was composed almost exclusively of CD3+/CD8+ T cells (negative for CD4 [lower left]. The T cells did not express PD-1 (lower middle) and the tumor cells did not express PD-L1 (not shown). The infiltrate contained only rare B cells (PAX5+, not shown) and rare plasma cells (Igk ISH, lower right; Igl ISH was similar [not shown]). Images are from the toe amputations, obtained one-month post injury (the earliest tissue samples).
MRI 12 months after the injury showed 2 masses in the stump area, the larger measuring 14 × 6 cm. Radiotherapy to the stump (57 Gy over 3 weeks) was without response. Rather, the stump ulcerated, exposing the underlying bone. At 15 months, the patient sustained an acute pathological fracture of the femur above the knee. X-rays, MRI and angiogram revealed extensive soft tissue and bone involvement. Above-the- knee amputation (AKA) was performed. Residual tumor was present, as assessed by MRI, in a) bone, b) 2 large soft tissue masses in the thigh (Figure 2A), c) 5 small 1 cm nodules of the abdominal wall skin (biopsy proven embolic angiosarcoma). The patient requested comfort care. He took only NSAIDs for pain and Ganoderma lucidum spores once daily, which were started about 9 months after the injury.
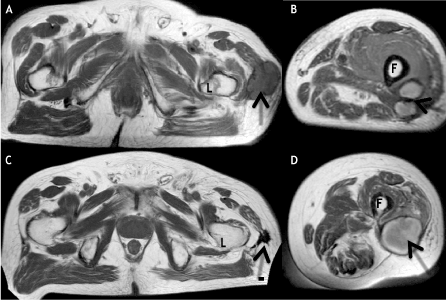
Figure 2. MRI evidence of tumor regression. 2A and 2B, Axial T1-weighted images from initial MRI reveal subcutaneous hypointense mass (arrow, 2A) posterolateral to the left femoral lesser trochanter (L), and two adjacent hyperintense masses (arrow, 2B) posterolateral to the left femoral diaphysis (F). 2C and 2D, Axial T1-weighted images from follow-up MRI demonstrate marked size/signal decrease of the mass (arrow, 2C) posterolateral to the left femoral lesser trochanter (L) as well as coalescence of the masses (arrow, 2D) posterolateral to the left femoral diaphysis (F).
Over the next 6 months there was gradual disappearance of the abdominal skin nodules and the 2 large palpable thigh masses, along with radiographic improvement of metastases in the femur and deep thigh tissues. On physical exam there were no palpable thigh masses and no abdominal wall tumors. A follow-up MRI 41 months post injury showed a) complete resolution of a large soft tissue thigh mass and fusion of 2 smaller prior masses (Figure 2). The latter mass corresponded in location to a newly palpable tender mass in the lower thigh, suspicious for recurrence. By 45 months post injury there was rapidly progressing lymphedema of all 4 limbs along with severe anemia unresponsive to steroids and the patient expired 49 months post injury.
Radiology
The initial MRI obtained 15 months post injury, at the time of pathological fracture of the femur, showed several large masses in the left thigh (Figure 2A and 2B). One of these masses underwent complete spontaneous resolution (Figure 2C). The two smaller masses were replaced by a new mass at follow-up imaging obtained 41 months post injury (Figure 2D), suspicious for possible recurrence.
Pathology
The original biopsy showed epithelioid angiosarcoma with focally prominent vascular differentiation, including focal, freely anastomosing vascular channels but also including solid areas. There were focal accumulations of hemosiderin-laden macrophages. The tumor cells showed the typical expression of CD31 and CD34 and were negative for cytokeratin and S-100. The tumor contained a focally dense infiltrate composed mainly of CD8+ T cells. These CD8 cells lacked expression of PD-1 (Figure 1b) and the tumor itself was negative for the ligand PD-L1 (data not shown). The tumor infiltrate also contained rare B cells and plasma cells, but plasma cells were focally numerous in the superficial perivascular areas remote from the tumor. Infiltrates of CD8 T cells in angiosarcoma have been previously described and are considered a favorable prognostic factor [6]. A subsequent biopsy of the 2 subcutaneous abdominal wall nodules showed focal intravascular accumulations of angiosarcoma cells (Figure 1a), while tissue from the distal aspect of the amputated leg showed diffusely infiltrative angiosarcoma cells. The cytologic features of the angiosarcoma were similar in both the original tumor and later metastases.
Exome sequencing
Pathology blocks, representing the initial toe amputation and 2 separate samples from the AKA amputation, had small regions of tumor rich areas, which were micro- dissected and genomic DNA was extracted. These 3 tumor samples were submitted along with the matched normal saliva sample for high depth whole exome sequencing. This analysis revealed few non-synonymous mutations with an overall mutation frequency in the tumor samples of 3.6 mutations/sequenced Mb (pre-therapy toe amputation sample; 28.4 Mb sequenced), 2.3 mutations/sequenced Mb (sample from AKA amputation; 14 months post injury; 32.7 Mb sequenced); 2.4 mutations/sequenced Mb (separate sample from AKA amputation at 14 months post injury; 32.2 Mb sequenced). There were 5 mutations that were present in each of the 3 analyzed tumor samples: KANSL1 Q941fs, RANBP2 K708R, ZNF208 S1153P, PRAMEF11 R135K and OGDHL D810G (Table 1). It is unclear whether these somatic variants contributed to the pathogenesis of angiosarcoma in this patient. We did not detect the mutations in the angiogenesis signaling pathway molecules PTPRB or PLCG1, which were reported in angiosarcomas at a frequency of 26% and 9%, respectively [7]. Analysis of rearrangements and copy number changes did not reveal tumor specific changes. Finally, the overall mutation frequency was low compared with other studies [8,9].
Table 1. Epidemiology
|
Case
|
Year
|
Age/Sex
|
Duration of remission
|
Location of Angiosarcoma
|
Type of angiosarcoma
|
Reference
|
|
1
|
1976
|
79M
|
2.5 years
|
Left cheek between lower eyelid and nose
|
Epithelioid angiosarcoma
|
Jones [28]
|
|
2
|
1991
|
75F
|
3 years
|
Face and scalp
|
Epithelioid angiosarcoma
|
Cerroni [29]
|
|
3
|
1995
|
63F
|
6 weeks
|
Forehead
|
Epithelioid angiosarcoma
|
Brandes [30]
|
|
4
|
2007
|
92M
|
3 years
|
Central facial cutaneous angiosarcoma
|
Epithelioid angiosarcoma
|
Sluzevich [31]
|
|
5
|
2008
|
72F
|
7 months
|
Breast angiosarcoma with metastases to lung and scalp
|
Epithelioid angiosarcoma
|
Kim [23]
|
|
6
|
2007
|
64M
|
6 months
|
Head and neck
|
Epithelioid angiosarcoma
|
Thong [32]
|
|
7
|
2013
|
73F
|
7 months
|
Subcutaneous nodule on chest
|
Epithelioid angiosarcoma
|
Tanaka [4]
|
|
8
|
2015
|
76M
|
3.4 years
|
Toes
|
Epithelioid angiosarcoma
|
Current Paper
|
Table 2. Mutated genes in angiosarcoma, Case 8
|
Gene
|
Gene name
|
Chrom. Freq.
|
Protein Change
|
Role
|
|
References
|
|
PRAMEF1
|
PRAME family member 11
|
1
|
3
|
R135K
|
Retinoic acid receptor binding
|
NCBI
|
|
RANBP2
|
RAN binding protein 2
|
2
|
3
|
K708R
|
GTP-binding protein of RAS superfamily
|
NCBI
|
|
OGDHL
|
oxoglutarate dehydrogenase- like
|
10
|
3
|
D810G
|
Growth Modulation, activation of AKT signaling pathway
|
[33]
|
|
KANSL1
|
KAT8 regulatory NSL complex subunit 1
|
17
|
3
|
Q941fs (frameshift)
|
Prominent epigenetic regulator; stabilizes microtubule minus ends in a RanGTP-dependent manner. Essential for spindle assembly and chromosome segregation.
|
[34]
|
|
ZNF208
|
zinc finger protein 208
|
19
|
3
|
S1153P
|
Zinc finger protein, regulates gene transcription
|
NCBI
|
|
P2RX1
|
purinergic receptor P2X,
ligand gated ion channel, 1
|
17
|
2
|
N204K
|
Function as ATP-gated ion channels
|
NCBI
|
|
GPS1
|
G protein pathway suppressor 1
|
17
|
2
|
H253R
|
Suppress G-protein and mitogen-activated signal transduction
|
NCBI
|
|
GAGE2A
|
G antigen 2A
|
X
|
2
|
Q59E
|
Unknown
|
NCBI
|
|
TCHH
|
trichohyaline
|
1
|
1
|
326_327EA>ERA
(insertion)
|
Involved in skin morphogenesis
|
[35]
|
|
POTEE
|
POTE ankyrin domain family,
member E
|
2
|
1
|
R910C
|
Unknown
|
NCBI
|
|
SLC4A3
|
solute carrier family 4 (anion exchanger), member 3
|
2
|
1
|
R1104Q
|
Inorganic anion exchange
|
NCBI
|
|
DCLK2
|
doublecortin-like kinase 2
|
4
|
1
|
E594_splice (splice site mutation)
|
Regulates microtubule polymerization and protein interaction
|
NCBI
|
|
LHFPL3
|
lipoma HMGIC fusion partner-
like 3
|
7
|
1
|
N226Y
|
transmembrane protein
|
NCBI
|
|
FBXW5
|
F-box and WD repeat domain containing 5
|
9
|
1
|
S558del (deletion)
|
Regulates DLC1, a Rho-GTPase tumor suppressor
|
[36]
|
|
PRB2
|
proline-rich protein
subfamily 2
|
12
|
1
|
S52del (deletion)
|
Abundant protein family in saliva
|
[37]
|
|
ZFHX3
|
Zinc Finger Homeobox 3
|
16
|
1
|
R3526del (deletion)
|
TF regulating myogenic and neuronal differentiation
|
NCBI
|
|
KRTAP1-5
|
keratin associated protein 1- 5
|
17
|
1
|
I88T
|
Keratin-associated protein family, forms matrix of keratin
intermediate filaments
|
NCBI
|
|
POTEC
|
POTE ankyrin domain family, member C
|
18
|
1
|
R477Q
|
Possible involvement in reproductive processes
|
[38]
|
|
ZNF563
|
zinc finger protein 563
|
19
|
1
|
W208fs (frameshift)
|
Possible involvement in transcriptional regulation
|
NCBI
|
|
FRG1B
|
FSHD region gene 1 family
member B, pseudogene
|
20
|
1
|
A41T
|
Unknown
|
NCBI
|
Note: Freq. represents whether the mutation was found in 1/3, 2/3 or 3/3 samples submitted for exome sequencing.
This case of angiosarcoma is unique for two reasons: a) onset of angiosarcoma in the setting of traumatic tissue injury and repair; b) dramatic spontaneous regression of angiosarcoma.
Trauma as a cause of cancer
Physiological wound healing can be followed by benign or malignant local tumors [10-14]. Angiosarcomas have been reported to arise in sites of “trauma,” including natural and surgically constructed arteriovenous fistulae, and sites of foreign bodies [15-18]. Angiogenesis regulation may be disturbed in such cases. Regulation of angiogenesis and neo-angiogenesis in tumor tissues involves many molecular mechanisms [19].
The malignant angiosarcoma cells were confined to the same 2 toes that were injured and not present anywhere else. Also, the timing of malignant transformation just 1 month after injury suggests a causal relationship.
Abscopal effect
The term “abscopal effect” refers to systemic anti-tumor effects after local radiation therapy [20,21]. The updated definition includes any type of local therapy leading to systemic effects on metastatic lesions. Abscopal effects can occur following cytoreductive surgery such as orchiectomy for prostate cancer, mastectomy for breast cancer, or nephrectomy for renal cell carcinoma [20,21]. Both abscopal effects post irradiation and post-surgical cytoreduction are likely due to immunological effects [21,22]. In the present case, systemic regression of massive residual tumors was observed only months after AKA. Prior radiotherapy to the BKA stump was probably non- contributory as there was tumor progression.
Spontaneous regression
Spontaneous regression is defined as complete or partial reduction of a malignant neoplasm without treatment or with treatment deemed insufficient. Spontaneous regressions have been described in many tumor types, but angiosarcoma is conspicuously absent in several reviews [3]. The proposed mechanisms include immune responses, elimination of carcinogens, increased level of cancer cell apoptosis, hormonal influences, and epigenetics [23].
We summarize seven additional cases of spontaneous regression of angiosarcoma from the literature (Table 1). The 8 cases are all epithelioid angiosarcomas, include 4 males and 4 females, spanning ages 63-92, consistent with known epidemiology. The organ distribution is also consistent with disease characteristics [5]. Spontaneous regression lasted between 7-41 months.
Ganoderma lucidum
The Chinese herbal medicine, Ganoderma lucidum, also known as Lingzhi or Reishi has been reported to have anti-metastatic, anti-inflammatory, cytotoxic, cytostatic, and immunomodulatory properties [24-26]. However, there have been few randomized controlled trials studying the fungus [26]. A meta-analysis of 5 randomized controlled trials comparing G. lucidum against placebo for various cancers showed greater responsiveness to chemo/radiotherapy in the G. lucidum group without reaching statistical significance. However, G. lucidum led to a statistically significant increase in percentages of peripheral blood CD3, CD4, and CD8 cells in vivo [26]. If G. lucidum can boost immune responses, its use may have enhanced the abscopal effect seen in this case leading to spontaneous regression. Indeed, combinations of radiotherapy and immunotherapy have been of special interest recently [27,28].
The CD8 cells that infiltrated the initial tumor tissue were PD-1 negative (Figure 1b) and the tumor itself was PD-L1 negative. Thus, this immune checkpoint would have been non-functional, possibly leading to unfettered CD8 T cell expansion, similar to what occurs when checkpoint inhibitors are used.
Angiosarcoma should be added to the list of cancers that can undergo spontaneous regression. An immunologic mechanism seems likely, implying that immune therapies might be effective in some patients with angiosarcoma.
Matthew Meyerson MD PhD, Broad Institute, MIT and Harvard University, Cambridge, MA 02142, provided exome sequencing of the tumor in his laboratory. Ralph Nachman MD, Weill Cornell Medicine, was a source of great ideas and encouragement.
- Osler W (1901) The medical aspects of carcinoma of the breast, with a note on the spontaneous disappearance of secondary growths. Am J Med 17: 63-66.
- Everson TC (1967) Spontaneous regression of cancer.Prog Clin Cancer3: 79-95. [Crossref]
- Challis GB, Stam HJ (1990) The spontaneous regression of cancer. A review of cases from 1900 to 1987.Acta Oncol29: 545-550.[Crossref]
- Tanaka A, Tanemura A, Tsuji C, Katayama I, Masuzawa M, et al. (2013) Epithelioid angiosarcoma of the skin with spontaneous regression. J Dermatol 40: 215-217. [Crossref]
- Conley AM, Trent JC, Patel S (2011) Soft Tissue and Bone Sarcomas. In: Kantarjian HM WR, Koller CA, ed. The MD Anderson Manual of Medical Oncology. 2nd Edition ed. New York: McGraw-Hill Co, USA.
- Fujii H, Arakawa A, Utsumi D, Sumiyoshi S, Yamamoto Y, et al. (2014) CD8+ tumor-infiltrating lymphocytes at primary sites as a possible prognostic factor of cutaneous angiosarcoma. Int J Cancer 134: 2393-2402. [Crossref]
- Behjati S, Tarpey PS, Sheldon H, Martincorena I, Van Loo P, et al. (2014) Recurrent PTPRB and PLCG1 mutations in angiosarcoma. Nat Genet 46: 376-379. [Crossref]
- Snyder A, Makarov V, Merghoub T, Yuan J, Zaretsky JM, et al. (2014) Genetic basis for clinical response to CTLA-4 blockade in melanoma.N Engl J Med371: 2189-2199. [Crossref]
- Le DT, Uram JN, Wang H, Bartlett BR, Kemberling H, et al. (2015) PD-1 Blockade in Tumors with Mismatch-Repair Deficiency.N Engl J Med372: 2509-2520. [Crossref]
- Smith JC, Boone BE, Opalenik SR, Williams SM, Russell SB (2008) Gene profiling of keloid fibroblasts shows altered expression in multiple fibrosis-associated pathways.J Invest Dermatol128: 1298-1310. [Crossref]
- Halim AS, Emami A, Salahshourifar I (2012) Keloid scarring: understanding the genetic basis, advances, and prospects. Arch Plast Surg 39: 184-189. [Crossref]
- Russell SB, Russell JD, Trupin KM, Gayden AE, Opalenik SR, et al. (2010) Epigenetically altered wound healing in keloid fibroblasts. J Invest Dermatol 130: 2489-2496. [Crossref]
- Saaiq M, Ashraf B (2014) Marjolin's ulcers in the post-burned lesions and scars.World J Clin Cases2: 507-514. [Crossref]
- Pekarek B, Buck S, Osher L (2011) A Comprehensive Review on Marjolin's Ulcers: Diagnosis and Treatment. J Am Col Certif Wound Spec 3: 60-64. [Crossref]
- Byers RJ, McMahon RF, Freemont AJ (1994) Angiosarcoma at the site of a ligated arteriovenous fistula in a renal transplant recipient. Nephrology Dialysis Transplantation 9: 112.
- Wehrli BM, Janzen DL, Shokeir O, Masri BA, Byrne SK, et al. (1998) Epithelioid angiosarcoma arising in a surgically constructed arteriovenous fistula: a rare complication of chronic immunosuppression in the setting of renal transplantation. Am J Surg Pathol 22: 1154-1159. [Crossref]
- Hayman J, Huygens H (1983) Angiosarcoma developing around a foreign body.J Clin Pathol36: 515-518. [Crossref]
- Jennings TA, Peterson L, Axiotis CA, Friedlaender GE, Cooke RA, et al. (1988) Angiosarcoma associated with foreign body material. A report of three cases.Cancer62: 2436-2444. [Crossref]
- Lee SH JD, Han YS, Baek MJ (2015) Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann Surg Treat Res 89: 1-8. [Crossref]
- Kaminski JM, Shinohara E, Summers JB, Niermann KJ, Morimoto A, et al. (2005) The controversial abscopal effect. Cancer Treat Rev 31: 159-172. [Crossref]
- Vatner RE, Cooper BT, Vanpouille-Box C, Demaria S, Formenti SC (2014) Combinations of immunotherapy and radiation in cancer therapy.Front Oncol4: 325. [crossref]
- Predina JD, Kapoor V, Judy BF, Cheng G, Fridlender ZG, et al. (2012) Cytoreduction surgery reduces systemic myeloid suppressor cell populations and restores intratumoral immunotherapy effectiveness. J Hematol Oncol 5: 34. [Crossref]
- Kim SW, Wylie J (2008) Spontaneous regression of pulmonary metastases from breast angiosarcoma. Sarcoma 2008: 940656. [Crossref]
- Gao Y, Tang W, Dai X, Gao H, Chen G, et al. (2005) Effects of water-soluble Ganoderma lucidum polysaccharides on the immune functions of patients with advanced lung cancer. J Med Food 8: 159-168. [Crossref]
- Cheng S, Sliva D (2015) Ganoderma lucidum for cancer treatment: we are close but still not there.Integr Cancer Ther14: 249-257. [Crossref]
- Golden EB, Chhabra A, Chachoua A, Adams S, Donach M, et al. Local radiotherapy and granulocyte- macrophage colony-stimulating factor to generate abscopal responses in patients with metastatic solid tumours: a proof-of-principle trial. Lancet Oncol 16: 795-803. [Crossref]
- Golden EB, Demaria S, Schiff PB, Chachoua A, Formenti SC (2013) An abscopal response to radiation and ipilimumab in a patient with metastatic non-small cell lung cancer. Cancer Immunol Res 1: 365-372. [Crossref]
- Jones EW (1976) Dowling oration 1976. Malignant vascular tumours.Clin Exp Dermatol1: 287-312. [Crossref]
- Cerroni L, Peris K, Legge A, Chimenti S (1991) Angiosarcoma of the face and scalp. A case report with complete spontaneous regression.J Dermatol Surg Oncol17: 539-542. [Crossref]
- Brandes LJ, Friesen LA (1995) Can the clinical course of cancer be influenced by non-antineoplastic drugs?CMAJ153: 561-566. [Crossref]
- Sluzevich JC, Gloster HJ, Mutasim DF (2008) A case of regressing central facial cutaneous angiosarcoma. J Am Acad Dermatol 58: S113-S115. [Crossref]
- Thong PS, Ong KW, Goh NS, Kho KW, Manivasager V, et al. (2007) Photodynamic-therapy-activated immune response against distant untreated tumours in recurrent angiosarcoma. Lancet Oncol 8: 950-952. [Crossref]
- Mlitz V, Strasser B, Jaeger K, Hermann M, Ghannadan M, et al. (2014) Trichohyalin-like proteins have evolutionarily conserved roles in the morphogenesis of skin appendages. J Invest Dermatol 134: 2685-2692. [Crossref]
- Meunier S, Shvedunova M, Van Nguyen N, Avila L, Vernos I, et al. (2015) An epigenetic regulator emerges as microtubule minus-end binding and stabilizing factor in mitosis. Nat commun 6: 7889. [Crossref]
- Yamamoto S, Hirai K, Hasegawa-Oka Y, Hirai Y (2009) Molecular elements of the regulatory control of keratin filament modulator AHF/trichohyalin in the hair follicle. Exp Dermatol 18: 152-159. [Crossref]
- Minoda Y, Sakurai H, Kobayashi T, Yoshimura A, Takaesu G (2009) An F-box protein, FBXW5, negatively regulates TAK1 MAP3K in the IL-1beta signaling pathway.Biochem Biophys Res Commun381: 412-417. [Crossref]
- Kim HS, Maeda N (1986) Structures of two HaeIII-type genes in the human salivary proline-rich protein multigene family.J Biol Chem261: 6712-6718. [Crossref]
- Bera TK, Saint Fleur A, Lee Y, Kydd A, Hahn Y, et al. (2006) POTE paralogs are induced and differentially expressed in many cancers.Cancer Res66: 52-56. [Crossref]


