Abstract
Importance: Repeated intravitreal injections are administered for a number of diseases, many of which are bilateral.
Objective: Determine present opinion among retina specialists on bilateral same-day injections (BSI), how frequently BSI are performed, and what measures are most frequently employed to prevent complications.
Design: From March to May 2015, 84 retina specialists responded to an online survey.
Setting: An invitation to the survey was included in the American Society of Retina Specialists online newsletter. In addition, some surveys were personally emailed out.
Results: Of the 82 physicians who perform frequent injections, 66 (80%) report performing BSI, with 53 (65%) performing at least monthly BSI. Of those who reported using bevacizumab, 66% (51/77) reported using an accredited compounding pharmacy. More than half of respondents who perform bilateral bevacizumab injections (30/55, 54%) reported not taking any special measures with regards to lot, batch or vial number. A majority (54/82, 66%) felt that precautions related to lot and batch number were good but not necessary because the risk of bilateral complications is minimal.
Conclusion: BSI are performed frequently, but there is little consensus on measures to reduce the risk of bilateral complications.
Key words
survey, anti-VEGF, intravitreal injections, bilateral, endophthalmitis, safety precautions
Key Points
Question: How commonly do retina specialists perform bilateral same-day injections (BSI) and what precautions are being used?
Findings: 80% of surveyed retina specialists perform BSI. More than half do not take any special precautions with regards to lot, batch or vial number.
Meaning: BSI are performed frequently, but there is little consensus on measures to reduce the risk of bilateral complications.
Introduction
Anti-Vascular Endothelial Growth Factor (VEGF) injections are now standard treatment for vision loss from conditions like wet age-related macular degeneration (AMD) and diabetic macular edema (DME). The burden of bilateral disease combined with the need for monthly injections often over many months has led some physicians to offer bilateral same-day injections (BSI). Studies have suggested that this practice incurs no greater risk to patients than unilateral injections [1]. As of 2011, bilateral same-day intravitreal anti-VEGF injections were performed by nearly half of retina specialists in the United States [2]. The frequency with which BSI are performed is not well known. Furthermore, special techniques for BSI to reduce risk of complications are not universally agreed upon or standardized.
Not in question are the rare but potentially devastating effects of infectious or inflammatory complications of injections. A case series from India described six cases of endophthalmitis stemming from the injection of eight patients sourced from a single vial of bevacizumab. Only three out of six showed any improvement in vision with intravitreal antibiotics [3]. In fact, there have been numerous case reports in the United States and abroad detailing outbreaks of endophthalmitis following intravitreal injections. Both microbes causing infection and contaminants causing inflammation likely originated from the compounding process of bevacizumab [4]. A cluster of cases in Florida following bevacizumab injection identified the source of the outbreak as contamination during the compounding pharmacy’s preparation [5]. Even more devastating, there have also been recent case series and reports of patients with bilateral endophthalmitis from bilateral anti-VEGF injections [6]. One such case series from Iran described two patients each with bilateral culture-proven S. epidermidis endophthalmitis [7].
As a result of such outbreaks, the FDA issued warnings to ensure compliance with the procedures of the United States Pharmacopoeia Chapter 797 [8]. Many experts agree Chapter 797 holds the key to preventing the catastrophic events that can occur when compounding single vial bevacizumab for multiple injections. Recommendations include keeping documentation of whether the compounding pharmacy has the proper license, inspection forms and certifications to compound bevacizumab. Some pharmacists and retina experts recommend having at least 10% of the syringes from a lot independently tested for sterility with results sent with each shipment [9].
Additionally, some propose the precaution of using different lot or batch numbers for each injection of BSI to ensure each medication comes from a source independently produced and compounded. Lot number refers to a group of vials of bevacizumab produced by the supplier (Genentech). The compounding of each vial is then done under sterile conditions by compounding pharmacists for the off-label use of bevacizumab as an intravitreal injection. Each vial is subdivided into individual syringes for injection. The syringes compounded from a single vial represents a batch. It is important to note that the vials in a lot as well as the individual syringes in a batch are made of the same concentration of bevacizumab. No dilution is performed during compounding. For medications like ranibizumab and aflibercept that come in packaged vials designed for single use, there is no compounding process. Aseptic technique must be used in the clinic prior to injecting.
The process of preparing vials and preparing the eye for an injection may also contribute to cases of sporadic endophthalmitis. While exact steps may differ slightly, many physicians use a similar process to reduce infection risk. Green-Simms et al. published a large survey of over 700 AAO retina specialists in 2011 aimed at finding whether there was consensus on any of the steps of preparing and performing injections. The authors noted that most measures were not based on strongly supported evidence. Physician preference and experience largely guided before, during and after-injection care. The only measure that was almost universally used was some form of povidine-iodine solution for cleaning prior to the injection. There seemed to be great variation in individual technique, including whether to use an eyelid speculum or gloves. A small number of respondents (4%) even reported not injecting directly into the vitreous cavity [2].
We sought to determine present opinion among retina specialists of BSI, how frequently BSI are performed, and what measures are most frequently employed to prevent complications when BSI are performed
Methods
Survey participants were recruited in one of two ways: (1) via an invitation and link in Retina FYI, an American Society of Retina Specialists (ASRS) online newsletter, and (2) via an emailed invitation.
The online invitation appeared from March 2 to April 20, 2015, in three editions of FYI, a newsletter that reaches a large percentage of ASRS’s ~2600 United States and international retina specialists. In all, 70 respondents completed the survey via the link in FYI.
All members of the Dallas Academy of Ophthalmology (DAO) who self-identified as a medical or surgical retina specialist and published their email address in the DAO directory (70) were emailed an invitation and link. Additionally, 14 graduates of The University of Texas Southwestern Medical Center vitreoretinal surgery fellowship who kept an up-to- date email on file at UT Southwestern were emailed an invitation and link. An email reminder was sent once. Of the total 84 individuals emailed, 14 (17%) completed the survey between April 18 and May 8, 2015.
The survey was created, and the data were collected and stored using the website SurveyMonkey.com. Results were finalized on May 9, 2015.
Results
A total of 84 medical or surgical retina specialists completed the survey from March 2 to May 8, 2015. The respondents represented physicians who perform a high volume of injections. Nearly two thirds (54/84, 64%) reported performing greater than 30 injections per week. Only two (2%) reported performing less than 6 per week (Figure 1a).
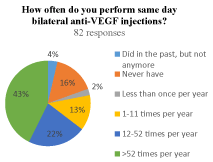
Figure 1. (a) How many anti-VEGF injections do you perform in an average week?,
Bevacizumab was used by the highest percentage of physicians (94%), while ranibizumab was used by the fewest (86%). Aflibercept was used by 91% of physicians (77/84).
Of the 82 who perform frequent injections, 53 (65%) reported performing at least monthly BSI. Nearly 43% (35/82) of physicians reported performing at least weekly BSI. Those that reported never performing BSI and those that once did but stopped represented 16% (13/82) and 4% (3/82) of the physicians, respectively (Figure 1b).
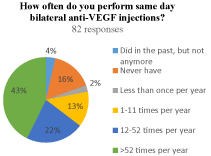
(b) How often do you perform same day bilateral anti-VEGF injections?
Compounding
Of those who reported using bevacizumab, 66% (51/77) reported using a compounding pharmacy accredited by the Pharmacy Compounding Accreditation Board (PCAB). A total of 23 physicians (30%) did not know if the PCAB accredited their source of bevacizumab, and 3 physicians reported either their pharmacy was not accredited (1, 1%) or reported using a single vial themselves to supply injections for multiple patients (2, 3%) (Figure 2a). Of those who used a compounding pharmacy for bevacizumab, 53% (40/75) reported that they knew their pharmacy performed microbiological aliquot testing to ensure sterility compared to 47% (35/75) who did not know whether their pharmacy did aliquot testing or not (Figure 2b).
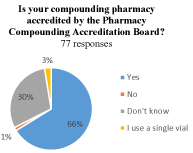
Figure 2. (a) Is your compounding pharmacy accredited by the Pharmacy Compounding Accreditation Board,
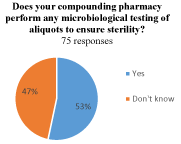
(b) Does your compounding pharmacy perform any microbiological testing of aliquots to ensure sterility?
Complications
A significant majority of physicians had cared for a patient with endophthalmitis or toxicity resulting from an anti-VEGF injection (69/82, 84%) compared to those who had not (13/82, 16%). Of those who had cared for a patient with endophthalmitis or toxicity, one physician reported having cared for a patient with bilateral endophthalmitis or toxicity. Overall, 99% (81/82) of those physicians who had cared for a case of unilateral endophthalmitis or toxicity had never cared for a bilateral case.
Safeguards
For the 66 physicians who perform BSI, the most common measure taken was finishing the injection in one eye then proceeding to the second eye using different materials (73%). Nearly half of respondents reported prepping both eyes at the same time (48%), and five out of 66 (8%) respondents reported using a different medicine for each eye when performing BSI (e.g. bevacizumab for one eye and ranibizumab for the other). For physicians performing BSI with bevacizumab, 20 out of 55 report using a different lot of medicine from the manufacturer for bevacizumab (36%), and nine out of 55 report using a different batch of aliquotted medicine from the same pharmacy for bevacizumab (16%). Two physicians reported drawing up bevacizumab from separate vials for BSI, bypassing the compounding pharmacy. Notably, more than half of physicians in this group reported not taking any special measures with regards to lot, batch or vial (30/55, 54%). When asked to explain their reason for not taking special measures the most common reasons were because the issue had never been raised (19/55, 34%) or because of a lack of resources (11/55, 20%) (Figure 3a).
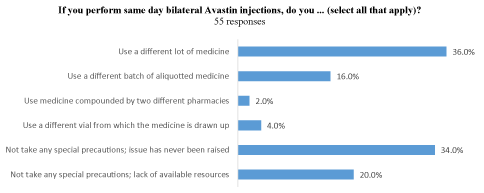
Figure 3. (a) If you perform same day bilateral Avastin injections, do you ... (select all that apply)?
Those physicians who perform bilateral same-day injections were asked about their choice of drug. Eleven out of 66 (17%) responded that they do not perform bilateral injections of bevacizumab, compared to four (6%) who do not perform bilateral injections of ranibizumab or aflibercept.
For BSI of ranibizumab and aflibercept, 68% (42/62) of physicians take no special precautions with regard to lot number; 22% (14/62) use different bottles from different lots; and 10% (6/62) use the same bottle for both eyes (Figure 3b).
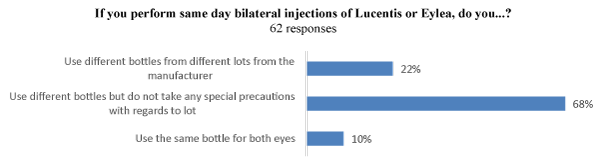
Figure 3. (b) If you perform same day bilateral injections of Lucentis or Eylea, do you...?
Opinions
Among the 16 physicians who reported having stopped performing BSI or never doing so, 75% (12/16) reported that they could not justify the risk of bilateral complications no matter what precautions were taken. One out of 16 mentioned decreased reimbursement as a reason for not performing BSI. Three out of 16 reported rarely caring for patients who needed bilateral injections.
All respondents (84) were asked at the end of the survey if they thought the practice of using different manufacturers' lots and different batches was necessary for the safe administration of bilateral injections. Two physicians skipped the question. Ten (12%) responded no, because bilateral injections should never be done. Eighteen (22%) responded yes, that it was necessary. Nearly two thirds (54/82, 66%) reported that while it a good idea, such a precaution was not necessary because the risk from using the same lot or batch was minimal (Figure 4).
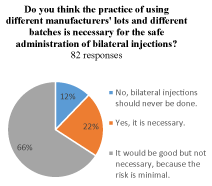
Figure 4. Do you think the practice of using different manufacturers' lots and different batches is necessary for the safe administration of bilateral injections?
Discussion
Injections
The population of physicians we sampled represented a group of physicians that perform high volume intravitreal injections of all three major anti-VEGF medications (bevacizumab, ranibizumab and aflibercept). Among this group BSI is fairly common with nearly two thirds of respondents reporting performing BSI at least monthly. This represents a higher percentage than the 2011 AAO study found when they sampled over 700 retina specialists. Only 46% of those physicians reported performing any BSI.
Compounding
2021 Copyright OAT. All rights reserv
A relatively large number of physicians did not know whether their pharmacy was accredited by the PCAB (30%). Of those who reported knowing they used a PCAB pharmacy, 46% did not know whether their pharmacy performed independent aliquot microbiologic testing of bevacizumab. The American Academy of Ophthalmology recommends independent PCAB inspection of facilities that compound bevacizumab [10].
Aliquot testing is recommended by retina specialists and pharmacists alike, as well as the United States Pharamcopeia (USP) 71 guidelines. Despite these strong recommendations, it would appear many physicians are not aware personally of whether their source of bevacizumab employs two major strategies used to reduce complication risk. The responsibility for ensuring compounding is done by a PCAB facility may intentionally fall on someone other than the injecting physician. More information, perhaps gained by surveying pharmacists who work for retina specialists, especially at teaching institutions, would be necessary to know a more accurate percentage of bevacizumab sourced from PCAB pharmacies.
Some physicians may have been using a single-use vial of bevacizumab like the ones employed by Veterans Affairs (VA) hospitals since 2011. In a study reporting their experience with bilateral injections in 2014, a group from the Miami VA reported no cases of endophthalmitis in either series of unilateral (3570 injections) and same-day bilateral injections (660 injections). They noted that midway through their study, a change in VA policy meant bevacizumab could no longer be sourced through compounding pharmacies that aliquotted single vials of bevacizumab into individual syringes. The VA physicians had to inject from an individual, commercially prepared single vial of bevacizumab. They used separate vials for bilateral injections [6]. Such a measure results in higher costs, though still much lower than alternative treatments (i.e., ranibizumab or aflibercept). Only 3% of physicians sampled reported using a different vial of bevacizumab sourced directly from Genentech for each eye when performing BSI.
We also asked physicians about their practices with commercially produced vials of ranibizumab and aflibercept, medicines that are only available as such. A relatively small percentage reported using the same vial for both eyes when performing BSI (6/62, 10%). We suspect from discussions with clinicians that at least some of these physicians are using “off-label” a single vial for both eyes and only charging the patient and insurance for one vial.
Complications
Not surprisingly, a large percentage of respondents reported taking care of a patient with endophthalmitis or toxicity from anti-VEGF injections. The physicians surveyed are all retina specialists and likely referred a number of cases of infection or inflammation after injections. However, only one physician (1%) reported taking care of a patient with bilateral endophthalmitis or inflammation as a result of an anti-VEGF injection. It would likely take the rare event of an outbreak to expose retina specialists to such cases. Studies seem to support the rarity of bilateral complications. The cohort of patients injected with BSI at the VA Hospital in Miami were followed and compared to patients who underwent unilateral injections. None of the patients followed (totaling 660 BSI and 4230 unilateral injections) developed endophthalmitis or toxicity in the average follow-up period of 26 months [6]. A number of older studies have pointed to the safety of bilateral injections and the similar complication rates of BSI compared to unilateral injections. Galler in a 2009 ARVO poster compared 102 patients receiving BSI and 102 receiving unilateral injections only. A total of 456 injections were performed on the bilateral group compared to 1017 in the unilateral group. No infectious or toxic complications developed in either group during follow-up [1].
Safeguards
Our data suggest there remains no consensus on whether using a different lot or batch number is important for each injection of BSI. This extra step would seem a common-sense measure of reducing the chance of bilateral complications. The odds of both samples being contaminated should be lower because two separate events in the compounding process would have to occur to result in contamination. Using different batch numbers for BSI is recommended by some retina specialists and organizations [5, 10], but may present a cost or time barrier to providing bevacizumab. The practice also does not appear to be a step scientifically proven to significantly reduce the risk of infectious complications. Physicians also reported variety in the process of prepping the eye. The most common safeguard overall was finishing the injection in one eye before proceeding to the second eye using different materials. The preparation process of both eyes was, however, commonly done at the same time.
Opinions
Unacceptable risk of bilateral complications was the most common reason physicians reported choosing not to perform BSI. Despite reporting never seeing a case of bilateral endophthalmitis after BSI, some physicians (12/84) still found it unacceptable to offer BSI because of the risk of bilateral complications. Perhaps some of this opinion stems from disaster instances of not only contamination of syringes but also misidentification of medications assigned for eye injections. Bortezomib (a multiple myeloma drug) has been mistakenly injected in place of bevacizumab with devastating consequences in Portugal in 2009 [4].
Interestingly, the vast majority of physicians, whether they performed BSI or not, did not feel using different manufacturers' lots and different batches were important or necessary. Only 22% of the total physicians queried felt paying attention to lot or batch number mattered.
Our study was limited by our response rate on the emailed invitations (14/84, 17%). There may have been a regional bias as well. The overall percentage of respondents known to have trained or currently work in the Dallas area was also 17% (14/84) and may have been somewhat higher as respondents via Retina FYI did not indicate their location in the survey.
Conclusion
Bilateral same-day injections of anti-VEGF medications are performed frequently among retina specialists and by a higher percentage of retina specialists than previously documented through survey. Despite recent outbreaks linked to compounding of bevacizumab, there remains little consensus in practice about whether using different lots or batches for BSI is important in reducing complication risk. Overall, just as with unilateral injection practices, BSI are performed with a variety of techniques for all anti- VEGF medications. Those physicians not performing BSI seem to fear bilateral infection most.
References
- Johnston C. DG News (2009) Bilateral injections of anti-VEGF drugs for treatment of bilateral macular degeneration appear safe, convenient. May 5 Available at: http://www.docguide.com/bilatera l-injections-anti-vegf- drugs-treatment-bilateral-macular-degeneration-appear-safe-convenient. Accessed October 12, 2014.
- Green-Simms AE, Ekdawi NS, Bakri SJ (2011) Survey of intravitreal injection techniques among retina specialists in the United States. Am J Ophthalmol 151: 329-332. [Crossref]
- Khan P, Khan L, Mondal P (2013) Cluster endophthalmitis following multiple intravitreal bevacizumab injections from a single use vial. Indian J Ophthalmol 57-59. [Crossref]
- Gonzalez S, Rosenfeld PJ, Stewart MW, Brown J, Murphy SP (2012) Avastin doesn't blind people, people blind people. Am J Ophthalmol 153: 196-203. [Crossref]
- Goldberg RA, Flynn HW Jr, Isom RF, Miller D, Gonzalez S (2012) An outbreak of Streptococcus endophthalmitis after intravitreal injection of bevacizumab. Am J Ophthalmol 153: 204-208. [Crossref]
- Chao DL, Gregori NZ, Khandji J, Goldhardt R (2014) Safety of bilateral intravitreal injections delivered in a teaching institution. Expert Opin Drug Deliv 11: 991-993. [Crossref]
- Tabatabaii A, Ahmadraji A, Khodabande A, Mansouri M (2013) Acute bilateral endophthalmitis following bilateral intravitreal bevacizumab (Avastin) injection. Mid East African J of Ophthalmol 20: 87-88. [Crossref]
- United States Food and Drug Administration. FDA alerts health care professionals of infection risk from repackaged Avastin intravitreal injections. August 30, 2011. Available at: http://www.fda.gov/Drugs/DrugSafety/ucm270296.htm. Accessed May 2, 2015.
- Cebulla CM. Compounding pharmacies: recent outbreaks of infection and implications for the care of patients with age-related macular degeneration. October 2013. Issue 62. Available at: http://www.visioncareprofessional.com/emails/amdupdate/index62.htm. Accessed October 12, 2014.
- American Academy of Ophthalmology. Verifying the source of compounded bevacizumab for intravitreal injections. February 2012. Clinical Statement: San Francisco, CA: AAO, 2012. http://one.aao.org/clinical-statement/verifying-source-of-compounded-bevacizumab-intravi-2. Accessed May 8, 2015.







