Since 1965, the NBRL, Boston, MA has been supported by the U.S. Navy’s Bureau of Medicine and Surgery, the U.S. Navy’s Research and Development Command, the Office of Naval Research, and the U.S. Congress to preserve red blood cells and platelets to treat wounded casualties. For Food and Drug Administration (FDA) approval, the 24-hour posttransfusion survival of Group ABO and Rh compatible sterile red blood cells must be at least 75% and hemolysis less than 1% with no requirement that the red blood cells function.
The 24-hour posttransfusion survival in healthy volunteers can be measured by the 125I albumin and 51Cr; the 99mTc and 51Cr, the 111In-oxine and 51Cr, and the double 51Cr methods.
In the past 5 decades NBRL has published data on the survival and function of preserved RBC. Valtis DJ and Kennedy AC [1] reported on the increased oxygen affinity of red blood cells stored in acid citrate dextrose at 4 C for one week. The increased affinity for oxygen is produced by the reduction in the red blood cell 2,3 diphosphoglycerate (DPG) level [2,3]. Increase in red blood cell 2,3 DPG and red blood cell creatine levels in patients with red blood cell mass deficits or with cardiopulmonary insufficiency were reported by Valeri CR and Fortier NL [4] (Figures 1, 2, and 3). The increases in red blood cell 2,3 DPG and creatine levels measure tissue hypoxia in the patients with red blood cell mass deficits or patients with cardiopulmonary insufficiency [4].
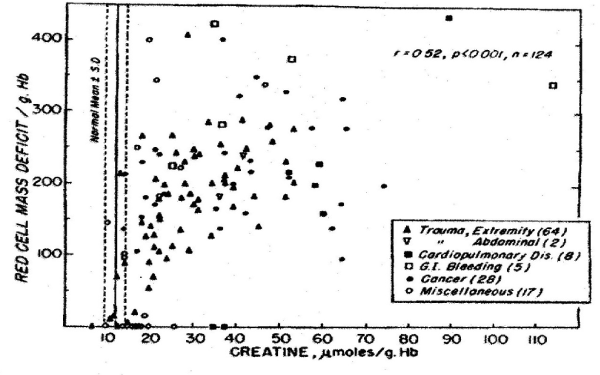
Figure 1. Relation between Red-Cell Mass Deficit and Red-Cell Creatine Level [4].

Figure 2. Relation between Red-Cell Mass Deficit and Red-Cell Creatine Level [4].
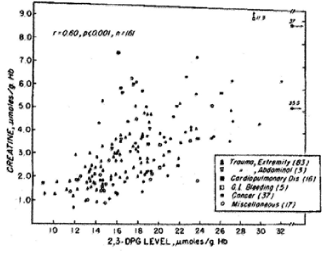
Figure 3. Relation between Creatine and 2,3 DPG Concentrations in Human Erythrocytes [4].
Our laboratory has shown that the level of red cell 2,3 DPG increased in proportion to the degree of red cell mass deficiency in patients who were not suffering from cardiopulmonary insufficiency. We found in addition that red cell 2,3 DPG was similarly elevated in patients with cardiopulmonary diseases who had no red cell deficits. Red cell creatine level was measured because in our laboratory it was previously incidentally observed that creatinuria in some patients disappeared after therapeutic transfusion to correct the red blood cell deficits. Hypoxia in muscle releases creatine which accumulates in the circulating red cells. The increased affinity of red blood cells for oxygen impairs the red blood cells to deliver oxygen to tissue following transfusion. The restoration of the red cell 2,3 DPG level following transfusion decreases the red cell affinity for oxygen.
For the past 5 decades the NBRL has reported the need to preserve the red blood cell 2,3 DPG level and red blood cell affinity for oxygen at the time of transfusion. The NBRL has published the rate of synthesis of red blood cell 2,3 DPG levels in patients transfused with red blood cells with reduced 2,3 DPG levels in Figures 4 and 5 [5,6]. During the time needed for the synthesis of the 2,3 DPG level in the transfused red blood cells, the patient needs to increase cardiac output following transfusion to maintain the oxygen tension in the brain, heart, and kidneys [7,8].
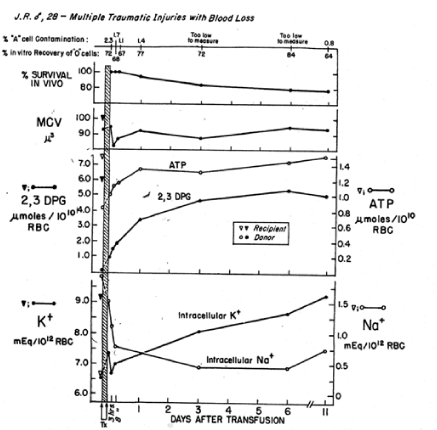
Figure 4. In vivo restoration of red blood cells transfused to J.R. after storage of whole blood for 16 days. Four units of O Rh-positive red blood cells were administered within 2¾ hr, and this represented 34.2% of the red blood cell volume after transfusion. Donor red blood cells were isolated from the recipient’s red blood cell population by a manual agglutination procedure [5].
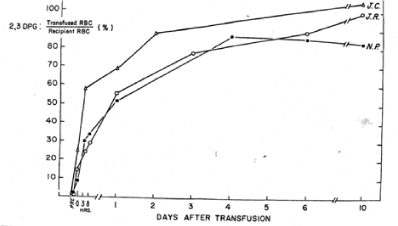
Figure 5. The in vivo restoration of 2,3 DPG-depleted red blood cells is shown is reported as a percentage of the recipient’s level of 2,3 DPG for each of the patients studied [6].
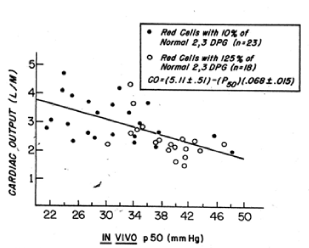
Figure 6. Relation between cardiac output and the in vivo P50 value in baboons subjected to haemorrhagic shock and resuscitated by the transfusion of preserved red cells with increased or decreased affinity for oxygen [7].
The NBRL has published procedures to biochemically treat red blood cells to provide red blood cells with normal and increased 2,3 DPG levels and normal and decreased affinity for oxygen at the time of transfusion [9,10]. The NBRL has recommended that acceptable preservation of red blood cells should provide 24-hour posttransfusion survival values of at least 75% with normal and decreased oxygen affinity at the time of transfusion.
The affinity for oxygen of baboon red cells is similar to that of human red cells. Like human red cells, baboon red cells can be biochemically modified to increase their 2,3 DPG and ATP levels. One such rejuvenation solution, PIGPA contains of pyruvate, inosine, glucose, phosphate, and adenine, has been used to prepare red blood cells for transfusions to baboons. Rice CL and collaborators studied baboons subjected to hemorrhagic hypovolemic shock and then transfused red cells with increased or decreased 2,3 DPG levels [7]. One group of baboons was given red blood cells that had been stored at 4 C for 3 weeks and washed before transfusion: these red cells had 10% of normal 2,3 DPG levels and increased affinity for oxygen. The other group of baboons was given fresh red cells that had been biochemically modified with the PIGPA solution and washed before transfusion: these red cells had 125% or normal 2,3 DPG levels and a decreased affinity for oxygen. The baboons that received the high 2,3 DPG red cells with decreased affinity for oxygen exhibited a significant reduction in cardiac output and work of the heart during the post resuscitation period (Figure 6). This reduction in cardiac output was almost equally divided between decreases in stroke volume and in heart rate. The baboons that received low 2,3 DPG red cells with increased affinity for oxygen showed a significant increase in cardiac output and work of the heart. Oxygen consumption was slightly greater in baboons that received high 2,3 DPG red cells. This significant finding suggests that red cells with improved oxygen delivery to tissue are able to immediately correct the oxygen debt produced by hemorrhagic shock. These investigators found a significant correlation between the cardiac output and the in vivo p50 values: at higher in vivo p50 values, less cardiac output was required to maintain oxygen consumption (Figure 6). The in vivo p50 value in the baboons that received the low 2,3 DPG red cells was about 30 ± 2.0 mm Hg. In the baboons that received the high 2,3 DPG red cells it was 38 ± 3.0 mm Hg. The improvement in oxygen delivery to tissue was comparable to a 15% increase in red cell volume. The high 2,3 DPG red cells produced an increase in the delivery of oxygen to tissues without demanding an increase in blood flow or a decrease in mixed venous blood oxygen tension.
The functional defect which develops in red blood cells during storage at 4°C is related to a loss of red cell 2,3 DPG and results in an increased affinity for oxygen by the red blood cells. This defect appears to develop sooner in blood stored in acid-citrate-dextrose (ACD), but after storage for about 10 days, the decreases in the 2,3 DPG level is significant whether ACD or CPD is used as the anticoagulant.
In a study reported by the NBRL, we transfused 2 to 4 units of liquid-preserved group O red cells with low 2,3 DPG levels to recipients of blood group A. We used a manual differential agglutination procedure to recover donor red cells and found that immediately upon completion of the 2 to 3 hour transfusion, the donor red cell 2,3 DPG level was about 15% of its final value [5]. About 24 hours after termination of the transfusion, the 2,3 DPG level was about 50% of the final value, and it continued to rise gradually for 10 more days. The ATP level of the donor red cells rose rapidly during the 3 hours of transfusion and continued to do so after the transfusion (Figures 4 and 5).
Valeri CR and Collins FB studied the circulatory changes in nonsurgical normotensive anemic patients after the transfusion of preserved red cells that were depleted of 2,3 DPG and had increased affinity for oxygen. No changes in systemic oxygen consumption were observed after transfusion of 3 to 5 units of red cells that had been stored at 4 C for 2 to 3 weeks. The cardiac index was increased immediately after the transfusion and 8 hours later it was within normal limits (Figure 7). Using the Fick principle, the cardiac output was calculated from the measured oxygen consumption and the measured difference in oxygen content in blood samples obtained from the femoral artery and pulmonary artery (the mixed venous blood). The oxygen content in blood obtained from the femoral artery and pulmonary artery was reduced immediately after the transfusion (Figure 7).
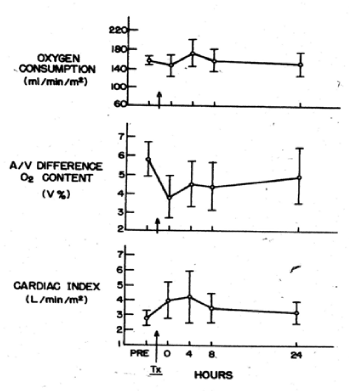
Figure 7. Oxygen consumption, arteriovenous difference in oxygen content, and cardiac output in stable anemic patients before and after transfusion of 3 to 5 units of preserved red cells with low 2,3 DPG levels and increased affinity for oxygen [10].
The current method to preserve red blood cells in the liquid state permits the storage for only 2 weeks at 4 C with 24-hour posttransfusion survival of 75%, slightly increased affinity for oxygen and less than 1% hemolysis [11]. Universal donor group O Rh positive and group O Rh negative red blood cells can be frozen with 40% W/V glycerol and stored at -80°C for at least 10 years, thawed, washed, and resuspended in the additive solution Nutricel at 4°C for 2 weeks with 24-hour posttransfusion survival of 75%, slightly increased affinity for oxygen, and hemolysis of less than 1% [11].
Preservation of RBC in the liquid state at 4°C for 2 weeks and in the frozen state at -80°C for at least 10 years, thawed, deglycerolized and stored at 4°C in AS-3 (Nutricel) for 2 weeks with 24-hour posttransfusion survival of 75% with slightly increased affinity for oxygen and with less than 1% hemolysis provide therapeutically effective and safe RBC [11]. In addition to its major role to deliver oxygen to tissue red blood cells exerts a hemostatic effect to reduce nonsurgical blood loss [12].
The hemostatic function of red blood cells to reduce nonsurgical blood loss has been studied at the NBRL for the past 50 years. Red blood cells scavenge endothelial cell nitric oxide by oxidation to perioxynitrite, nitrite, and nitrate and by binding of nitric oxide to hemoglobin. Red blood cells restore platelet function by the reduction in nitric oxide and the decrease in platelet cyclic guanosine monophosphate (cGMP) level. RBC, like platelets, provide both adenosine diphosphate (ADP) or arachidonic acid (AA) for platelet production of thromboxane at the bleeding time site. RBC increases blood viscosity and shear stress which may release ADP to aggregate platelets and may release endothelin, a potent vasoconstrictor substance from endothelial cells. RBC may be stimulated in vivo by agonists like derivatives of subendothelial glycocalyx to produced thrombin at the bleeding site. In the administration of erythropoietin (EPO) to patients with renal disease, the dose of erythropoietin to increase the hematocrit to 33 to 35 V% produces optimum hemostasis and improvement in platelet function. Transfusion trigger for prophylactic and therapeutic platelet transfusion in anemic thrombocytopenic patients need to assess the hematocrit value.
In 1998, the Nobel Prize in Medicine and Physiology was awarded to RF Furchgot, LJ Ignarro and F Murad. These recipients described the role of endothelial nitric oxide to vasodilate blood vessels and to inhibit platelet function. Endothelial derived relaxing factor (EDRF) was reported by Furchgott RF. Ignarro LJ reported that nitric oxide was the endothelial relaxing factor. Murad F reported that nitric oxide increased platelet cGMP which inhibited platelet function.
At the BT site tissue substances like calcium and derivatives of subendothelial glycocalyx like N-acetyl glucosamine may stimulate the red blood cells and platelets to expose phosphatidylserine which accumulates factor Va and factor Xa to produce prothrombinase activity to convert prothrombin to thrombin and fibrinogen to fibrin.
Activation of RBC by the agonists at the bleeding time site produce thrombin to convert fibrinogen to fibrin demonstrate the prothrombotic effect of RBC. The hematocrit to minimize the prothrombotic effect of RBC is about 35 V% which minimizes shear stress on the endothelial cells. The release at the nonsurgical bleeding site of thromboxane from the platelets and endothelin from the endothelial cells vasoconstrict the microcirculation.
The generation of thrombin by activation of the RBC and platelets convert the fibrinogen to fibrin to restore hemostasis. Nonsurgical bleeding diathesis in anemic thrombocytopenic patients is affected by temperature, hematocrit, platelet (Plt) count, Plt size, Plt function, and plasma clotting proteins factors V and VIII, von Willebrand’s factor and fibrinogen [12]. Bleeding time (BT) correlates to nonsurgical blood loss but does not correlate to surgical blood loss which requires surgical intervention and hemostatic agents with local external pressure to stop the bleeding. BT correlates with the volume of shed blood collected at the bleeding time site. The BT and the volume of shed blood collected at the BT site correlates better with the hematocrit than the Plt count in normal volunteer and in patients with bleeding disorders.
The current FDA standard for acceptable therapeutic effectiveness of preserved platelets is that they have in vivo recovery of 66% of fresh platelets and a lifespan of at least 50% of fresh platelets. There is no FDA requirement that the preserved platelets function. The basic assumption is that the in vivo recovery and lifespan of the preserved platelets correlate with their in vivo function.
Studies in aspirin treated normal volunteers, in aspirin treated baboons, and in hemodiluted anemic thrombocytopenic patients following CPB surgery demonstrated that the in vivo recovery and survival of preserved platelets did not correlate with their function to reduce the bleeding time, reduce nonsurgical blood loss, and increase the level of thromboxane at the bleeding time site. Khuri SF and associates reported that cyropreserved platelets had reduced in vivo recovery but improved hemostatic function compared to liquid preserved platelets in patients following cardiopulmonary bypass surgery [13].
Autologous baboon platelets stored at 22°C for 48 hours had in vivo recovery values similar to those stored for I8 hours, and they significantly reduced the bleeding lime (BT) and increased the shed blood thromboxane B2 (TxB2) level after transfusion in aspirin treated baboons.
Autologous baboon platelets stored at 22°C for 72 hours had in vivo recovery values similar to those stored for 18 hours, but the BT was not corrected after transfusion, although there was a significant increase in the shed blood TxB2 level in aspirin treated baboons.
Autologous cryopreserved baboon plate1et significantly reduced the BT and significantly increased the shed blood TxB2 level after transfusion in aspirin treated baboons. Cryopreserved autologous baboon platelets had better in vivo survival and function than the 5-day liquid stored autologous baboon platelets.
The survival of autologous fresh, liquid preserved, and cryopreserved baboon platelets did not correlate with their function to reduce an increased bleeding time in baboons treated with aspirin [14]. The hematocrit, hemoglobin concentration, platelet count, and mean platelet volume (MPV) did not change significantly during the 48-hour period for each of the eight groups of baboons that were studied. Acceptable platelet preservation should ensure that both survival and hemostatic function are maintained. Current guidelines only require satisfactory platelet survival. Studies have demonstrated that platelet survival does not correlate with platelet hemostatic function. Studies at the NBRL over the past 50 years have demonstrated that platelet function should be measured in aspirin treated male volunteers [18-21].
The hematocrit of 35 V% produces minimal shear stress on endothelial cells to minimize nitric oxide production which inhibits platelet hemostatic function. The optimum treatment of anemic thrombocytopenic patients should be to transfuse the patients with red blood cells to increase the hematocrit value to 35 V% to restore platelet hemostatic function prior to prophylactic and therapeutic transfusion of platelets which should circulate and function to restore hemostasis.
Aspirin treated normal volunteers have been studied by other investigators to assess the survival and hemostatic function of non-aspirin treated autologous washed and filtered platelets by measuring the in vivo recovery and lifespan and the reduction in the bleeding time in aspirin treated normal volunteers [12,15,16].
RBC must circulate and function to release oxygen at high tissue p02 tension to scavenge the endothelial cell produced nitric oxide a potent vasodilator substance that inhibits platelet function by increasing platelet cyclic GMP 1evel. The reduction in endothelial nitric oxide restores platelet hemostatic function by reduction in platelet GMP level and stimulates the release of endothelial cell endothelin, a potent vasoconstrictor substance to reduce blood flow to the bleeding time site [12].
Anemic thrombocytopenic patients have both reduced number of platelets and dysfunctional platelets. Treatment of anemic thrombocytopenic patients require viable and functional RBC to increase the hematocrit to restore platelet hemostatic function. Anemic thrombocytopenic patients need to be transfused with viable and functional RBC to achieve a hematocrit of 35 V% prior to prophylactic and therapeutic platelet transfusions. Transfusion trigger for platelets need to consider the patient’s hematocrit and the platelet hemostatic function [17].
The in vivo survival 1 to 2 hours after transfusion and the lifespan of the preserved platelets can be measured by 51Cr and 111In-oxine labeling of the platelets. The function of the fresh platelets, the liquid preserved platelets and the cryopreserved platelets can be measured in aspirin treated male volunteers transfused with allogeneic and autologous platelets [18-21]. The FDA has approved platelets stored in the liquid state at room temperature (22°C) for 5 days with agitation. Apheresed leukoreduced universal donor group O platelets treated with 5% and 6% DMSO, the supernatant DMSO removed by centrifugation at 1250 X g for 10 minutes and frozen at 2 to 3°C per minute by storage in a -80°C mechanical freezer for at least 2 years, thawed, and diluted with 0.9% NaCl and stored at room temperature for 6 hours without agitation provide platelets with in vitro recovery of 90% with 5-8% platelet microparticles, in vivo recovery of 25-30%, and lifespan of 7 days [11].
The hemostatic function of liquid preserved and cryopreserved platelets has been studied at the NBRL, Inc., Boston, MA for the past 50 years. Since 1968 aspirin treated male volunteers have been studied to measure the reduction in the increased bleeding time by allogeneic and autologous platelets. Studies performed in aspirin treated male volunteers have shown that frozen platelets are superior to fresh platelets and liquid preserved platelets to reduce the aspirin induced increased bleeding time [18-21]. A study performed in patients following cardiopulmonary bypass has reported that frozen platelets were superior to liquid preserved platelets stored at room temperature (22°C) for 3.4 days to reduce the nonsurgical blood loss and reduce the need for red blood cells and fresh frozen plasma transfusions [13].
The editorial Cryopreserved Platelets: Are We There Yet? by D.C. Marks reported the experience of freezing human platelets at the NBRL, Boston, MA over the past 45 years; the experience of Schiffer CA and associates to transfuse autologous cryopreserved platelets to support alloimmunized refractory patients after chemotherapy with more than 1600 autologous transfusions over a 10-year period; the 18-year experience of the Netherlands military to transfuse frozen platelets to wounded casualties in war zones; and the recent experience of Slichter SJ and associates using cryopreserved platelets to treat bleeding patients with thrombocytopenia [22].
The simple method for freezing human platelets with 6% DMSO at 2-3ºC per minute in a -80ºC mechanical freezer, thawed, washed and resuspended in plasma was published by Valeri and associates in 1974 [21]. In March 2000, the method to freeze human platelets treated with 6% DMSO frozen at 2-3ºC per minute by storage in a -80ºC mechanical freezer, thawed, washed and resuspended in plasma was modified to eliminate the post-thaw washing by concentration of the platelets by centrifugation at 1250 X g for 10 minutes to remove the supernatant DMSO prior to freezing at 2-3ºC per minute by storage in a -80ºC mechanical freezer. The thawed, non-washed platelets are diluted with 0.9% NaCl prior to transfusion was reported by Valeri CR and associates in 2005 [23].
Since 2001, Dr. C.C.M. Lelkens and the Netherlands military have utilized leukoreduced universal donor group O frozen platelets with universal donor group O frozen red blood cells and universal donor group AB frozen plasma all stored in a -80ºC mechanical freezer to treat wounded casualties in war zone in Afghanistan and Iraq for the past 18 years [24].
Slichter SJ and associates have reported on the safety and therapeutic effectiveness of cryopreserved platelets frozen with 6% DMSO, the supernatant DMSO removed prior to freezing at-80ºC, thawed and diluted with 0.9% NaCl prior to transfusion in bleeding thrombocytopenic patients. The results of the study of 24 bleeding patients with thrombocytopenia provide evidence that cryopreserved platelets were safe and stopped bleeding indicating that the cryopreserved platelets were not prothombotic [25].
Universal donor group O leuko reduced apheresed 2.5 to 3.0 X 1011 platelets stored at 22°C for 48 hours to allow for the mandated testing for infectious disease markers treated with 6% DMSO, the supernatant DMSO removed by centrifugation at 1250 X g for 10 minutes prior to freezing at 2-3°C per minute with storage at -80°C for at least 2 years, thawed and resuspended in 10 to 20 mL of 0.9% NaCl and stored at room temperature for 6 hours contain a bimodal population of platelets: one population of GPIb normal and annexin V reduced platelets which circulate like platelets stored at 22°C for 24 hours and a population of GPIb reduced and increased annexin V binding platelets which exert an immediate hemostatic effect like platelets stored at 4 C for 24 hours [11,23,24,26].
The clinical experience reported by Khuri [13] in patients following CPB surgery transfused with frozen platelets and the clinical experience by the Netherlands military in the treatment of wounded casualties in war zones in Afghanistan and Iraq with universal donor frozen group O RBC, frozen group O platelets, and frozen AB plasma have demonstrated the therapeutic effectiveness and safety of the frozen platelets for the past 18 years [11,22-26].
The NBRL modified procedure to freeze platelets was provided to the Netherlands military in March 2000 and this procedure was published by Valeri [23] to freeze universal donor group O Rh-positive and group O Rh-negative leukoreduced apheresed 2.5 to 3. 0 X 1011 platelets treated with 6% DMSO, centrifuged at 1250 X g for 10 minutes to remove the supernatant DMSO prior to freezing at 2 to 3°C per minute by storage at -80°C in mechanical freezers for at least 2 years, thawed, and diluted with 10 to 20 mL of 0.9% NaCl and stored at 22°C for 6 hours prior to transfusion provide platelets that function to immediately reduce nonsurgical blood loss. This method to freeze platelets is simple and a routine blood bank needs a -80°C mechanical freezer with a dual-cascade air-cooled compressor and a tank of liquid carbon dioxide to maintain the temperature of the frozen platelets at -80°C for at least 2 years. The platelets are thawed and resuspended in 10 to 20 mL of 0.9% NaCl and stored at room temperature (22°C) for 6 hours without agitation prior to transfusion.
The universal donor leukoreduced apheresed group O, 2.5 to 3.0 X 1011 frozen platelets should replace platelets in fresh whole blood stored at room temperature (22°C) for 4 hours and liquid preserved platelets stored at room temperature (22°C) with agitation for 5 days to provide platelets that circulate and function to treat nonsurgical bleeding [11].
For the past 18 years, the Netherlands military have utilized universal donor RBC, platelets and plasma all frozen and stored at -80°C in a mechanical freezer to treat wounded casualties with excellent clinical results and without adverse events. The data reported by the NBRL over the past 50 years and the Netherlands military over the past 18 years now recommend that the Food and Drug Administration, the American Red Cross, Health and Human Service, and the Department of Defense should support the use of universal donor frozen group O Rh positive and group O Rh negative RBC, frozen group O Rh positive and group O Rh negative leukoreduced platelets and frozen AB plasma. All the blood products frozen and stored at -80°C provide safe and therapeutic effective blood products to treat patients. The frozen blood products will eliminate or reduce the severe adverse events of mortality and morbidity associated with the current FDA approved red blood cell products, platelet products and plasma products. The universal donor group O Rh positive and group O Rh negative frozen RBC, group O Rh positive and group O Rh negative leukoreduced single donor frozen platelets, and AB frozen plasma obtained from male donors will provide blood products that are compatible with acceptable in vivo survival and function to treat patients and reduce or eliminate the severe adverse events associated with the current FDA approved blood products.
- Valtis DJ, Kennedy AC (1954) Defective gas-transport function of stored red blood-cells. Lancet 263: 119-124. [Crossref]
- Benesch R, Benesch RE (1967) The effect of organic phosphates from the human erythrocyte on the allosteric properties of hemoglobin. Biochem Biophys Res Commun 26: 163-167.
- Chanutin A, Curnish RF (1967) Effect of organic and inorganic phosphates on the oxygen equilibrium of human erythrocytes. Arch Biochem Biophys 121: 96-102.
- Valeri CR, Fortier NL (1969) Red-cell 2,3-diphosphoglycerate and creatine levels in patients with red-cell mass deficits or with cardiopulmonary insufficiency. N Engl J Med 281: 1452-1455. [Crossref]
- Valeri CR, Hirsch NM (1969) Restoration in vivo of erythrocyte adenosine triphosphate, 2,3-diphosphoglycerate, potassium ion, and sodium ion concentrations following the transfusion of acid-citrate-dextrose-stored human red blood cells. J Lab Clin Med 73: 722-733.
- Fortier NL, Hirsch NM, Valeri CR (1969) Restoration of 2,3 DPG and ATP in ACD-stored red blood cells. Forsvarmedicine 5:250-257. [Crossref]
- Rice CL, Herman CM, Kiesow LA, Homer LD, John DA, et al. (1975) Benefits from improved oxygen delivery of blood in shock therapy. J Surg Res 19: 193-198.
- Valeri CR, Collins FB (1971) Physiologic effects of 2,3-DPG-depleted red cells with high affinity for oxygen. J Appl Physiol 31: 823-827. [Crossref]
- Valeri CR (1976) Blood Banking and the Use of Frozen Blood Products. Chemical Rubber Company, Boca Raton, FL.
- Valeri CR (1974) Oxygen transport function of preserved red cells. Clin Haematol 3: 649-688.
- Valeri CR, Ragno Giorgio G (2013) Forty-five years of research at the NBRL, Boston, MA. Chaotic Observations, Serendipity, and Patience. Lulu Publishing Services.
- Valeri CR, Khuri S, Ragno G (2007) Non-surgical bleeding diathesis in anemic thrombocytopenic patients: role of temperature, RBC, platelets, and plasma clotting proteins. Transfusion 47: 206S-248S.
- Khuri SF, Healey N, MacGregor H, et al. (1999) Comparison of the effects of transfusions of cryopreserved and liquid preserved-platelets on hemostasis and blood loss after cardiopulmonary bypass. J Thorac Cardiovasc Surg 117: 172-184.
- Valeri CR, MacGregor H, Giorgio A, Ragno C (2002) Circulation and hemostatic function of autologous fresh, liquid preserved, and cryopreserved baboon platelets transfused to correct an aspirin induced thrombocytopathy. Transfusion 42: 1206-1216.
- Pineda AA, Zylstra VW, Clare DE, Dewanjee MK, Forstrom LA (1989) Viability and functional integrity of washed platelets. Transfusion 29: 524-527. [Crossref]
- Brecher ME, Pineda AA, Zylstra-Halling VW, Chowdhury S, Porstrom LA (1990) In vivo viability and functional integrity of filtered platelets. Transfusion 30: 718-721.
- Valeri CR, Ragno G (2010) An approach to prevent the severe adverse events associated with transfusion of FDA-approved blood products. Transfus Apher Sci 42: 223-233. [Crossref]
- Handin RI, Valeri CR (1971) Hemostatic effectiveness of platelets stored at 22°C. N Engl J Med 285: 538-543. [Crossref]
- Valeri CR (1974) Hemostatic effectiveness of liquid-preserved and previously frozen human platelets. N Engl J Med 290: 353-358.
- Spector JI, Yarmala JA, Marchionni LD, Emerson CP, Valeri CR (1977) Viability and function of platelets frozen at 2 to 3 C per minute with 4 or 5 per cent DMSO and stored at -80°C for 8 months. Transfusion 17: 8-15. [Crossref]
- Valeri CR, Feingold H, Marchionni LD (1974) A simple method for freezing human platelets using 6 per cent dimethylsulfoxide and storage at -80°C. Blood 43: 131-136. [Crossref]
- Marks DC (2018) Cryopreserved platelets: are we there yet? Transfusion 58: 2092-2094. [Crossref]
- Valeri CR, Ragno G, Khuri S (2005) Freezing human platelets with 6 percent dimethylsulfoxide with removal of the supernatant solution before freezing and storage at -80°C without post-thaw processing. Transfusion 45: 1890-1898.
- Lelkens CC, Koning JG, de Kort B, Floot IB, Noorman F (2006) Experiences with frozen blood products in the Netherlands military. Transfus Apher Sci 34: 289-298. [Crossref]
- Slichter SJ, Dumont LJ, Cancelas JA, et al. (2018) Safety and efficacy of cryopreserved platelets in bleeding patients with thrombocytopenia. Transfusion 58: 2129-2138. [Crossref]
- Valeri CR, Giorgio GR (2015) The safety and therapeutic effectiveness of universal donor frozen RBC, frozen platelets and frozen plasma stored at -80°C in mechanical freezers to treat wounded casualties. Trauma Cases Rev 1: 3-5.







