Chagas disease is a serious public health problem in Brazil and world. Caused by the protozoan Trypanosoma cruzi, it is estimated 6-7 million people infected worldwide. The only drug used to treatment is Benzonidazole, but this drug is only effective in the acute phase of the disease. This paper reports the synthesis and the anti-Trypanosoma cruzi activity of 4-thiazolidinone and 1,3-thiazole derivatives based in thiosemicarbazones previously described as potent trypanocidal agent, planned through the bioisosterism strategy. Therefore, the synthesis of 28 aryl-4-thiazolidinones (2a-r and 3a-j) was achieved, which aryl ring possesses halogens atoms in meta and para positions, and the heterocyclic system had lipophilic substituents. These compounds were thus evaluated as anti-T. cruzi agents against epimastigote, trypomastigote and amastigote forms of T. cruzi. Compounds were also tested its toxicity in L929 fibroblasts. In view to investigate a bioisosteric relationship between 1,3-thiazoles and 4-thiazolidinones, eighteen 1,3- thiazoles derived from trifluoromethyl thiosemicarbazone (4a-r) were also tested in the same model. It was possible to show that between the 46 tested compounds, those that possess a bromine (2a-r) atom showed better activity compared to compounds substituted by a trifluoromethyl group (3a-j) and to 1,3-thiazole derivatives (4a-r), which were inactive. In general, the 2a-r series showed low toxic profile in the cell line tested. Besides, compound 2h was the most active of then all when compared to the standard Benznidazole.
Chagas disease, Anti-T. cruzi agents, thiosemicarbazone, 1,3-thiazoles, 4-thiazolidinones
Chagas disease or American Trypanosomiasis is a serious disease resulting from parasitic infection by the protozoan Trypanosoma cruzi, and triatomine insects as vectors [1,2]. About 7 million people are infected worldwide and Latin America countries are endemic areas. It is estimated that 30% of chronically infected people develop cardiac disorders and up to 10% develop digestive, neurological or mixed changes that may require special treatment [1-3].
Currently, the only drugs used in Chagas disease therapy are Benznidazole (BZD) and nifurtimox, which are effective in the acute phase of the disease, but less effectivity in patients in the chronic phase, presenting only palliative effect [2,4-7]. Furthermore, BZD is not considered a suitable drug, since exhibits serious side effects such as hypersensitivity, rash, gastrointestinal disorder etc., leading to a large number of patients interruption the treatment [2,8]. Therefore, the search for new drugs and bioactive molecules more selective is pivotal.
Among the chemical structures studied for anti-T. cruzi activity, hydrazones and 4-thiazolidinones are notable because of their extensive biological activity [9-18], especially anti-parasitic activities [10,16-20]. Some papers already published investigated bioactive compounds against Chagas disease, however, a noticeable paper published by 21, identified twenty aryl thiosemicarbazones as potent anti-T. cruzi drug prototypes, with IC50 values below 200 nM for T. cruzi cruzain [21]. Among these compounds previously evaluated, 3-(bromopropiofenone)thiosemicarbazone (1i) and 3-(trifluoromethyl)thiosemicarbazone (3d) were identified as leading compounds (Figure 1).
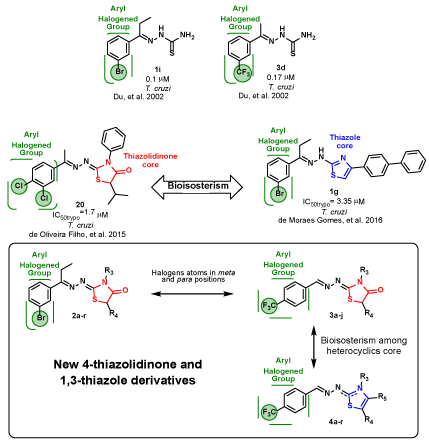
Figure 1. The influence of halogen groups in the anti T. cruzi activity and proposed compounds in this work (2a-r, 3a-j and 4a-r) [16-19].
In this context, it is well noticed that several drugs and drug candidates in clinical development possess halogen atoms in their structures [7,22]. In fact, they contribute favorably through a molecular interaction in the stabilization of the protein-ligand complex. In addition, synthesis of fluorine, chlorine, and bromine derivatives are well known in industrial scale and possess desirable stability and cost [23].
In the last few years, our research group had been used the building-block strategy in view to optimize the antiparasitical profile. Recently, the 1,3-thiazole core was obtained from 3'-bromopropiophenone- thiosemicarbazone and trifluoromethyl-thiosemicarbazone in view to access new lead generation of anti-T. cruzi agents. In fact, most of these compounds exhibited antiparasitic activity similar to BZD [24] (Figure 1).
Bearing in mind the bioisosteric relationship between 1,3-thiazole and 4-thiazolidinone cores and by the promising results obtained by Moraes (2016) and Du (2002), in this work, our group presents two series of 4-thiazolidinones, derived by 3'-bromopropriophenone- thiosemicarbazone and 3-(trifluoromethyl)thiosemicarbazone (2a-r and 3a-j, respectively), in view to achieve novel and more selective candidates against T. cruzi. In this approach, 28 compounds were synthesized with variations lipophilic groups inserted at N3 and C5 positions of the 4-thiazolidinone ring. In addition, these compounds were evaluated for their trypanocidal potential in the epimastigote, trypomastigote and amastigote forms of the parasite, and their cytotoxicity was tested in L929 fibroblast cells. Analyzing the activity against the three T. cruzi forms is important in an attempt to clarify the mechanism of action of compounds. In view to make a structure-activity biological profile between 1,3-thiazole and 4-thiazolidinone nucleus, we also evaluated the anti-T. cruzi properties of eighteen 1,3-thiazoles derived from (trifluoromethyl)thiosemicarbazone (4a-r).
Chemistry
For the synthesis of the desired compounds, the procedures previously reported by 10,25 were followed. First, the thiosemicarbazones 1d and 1e were prepared by reacting commercially available thiosemicarbazide or methyl thiosemicarbazide, with 4-(trifluoromethyl)benzaldehyde (1:1 mol ratio) using ethanol, under reflux, in the presence of catalytic amount of hydrochloric acid, for 3 hours. After the final reaction, water was added for precipitation of the compounds. This reaction led to a satisfactory yield (85% for compound 1d and 94% for compound 1e). The thiazolidin-4-ones (3a-j) were prepared by cyclization of compounds 1d or 1e and respective acid/ester, under reflux for 24 hours. The precipitate was filtered in Büchner funnel, washed with cold ethanol, and then dried over SiO2. The yields were satisfactory (>40%). The synthesis of 4a-r series is described by [25].
In view to set up the structural planning of the 4-thiazolidinone series 2a-r and 3a-j, the aimed perspectives were based on followed points: a) the choice of the halogen (bromine or fluorine), positions to be inserted (meta or para) in the aryl ring, employing 3-bromophenyl propanone or 4-(trifluoromethyl)benzaldehyde; b) the lipophilic and steric profile of the substituents at C5 position, adopting different esters; c) attachment of a methyl or phenyl group at nitrogen N3 by applying 4-methyl or 4-phenyl-thiosemicarbazide. About the ethyl group present at R2 position in the series 2a-r coming from the ketone used (Scheme 1). Therefore, two thiazolidinones series were synthesized: 2a-r and 3a-j. For synthesis of desired compounds (Scheme 1), it was followed procedures previously described by [10].
Regarding 1,3-thiazole series (4a-r), variations were made in the group linked to 4 position of the thiazole ring, through the insertion of aromatic rings with different substitutions in ortho, meta and para positions (Figure 2). The synthesis of series 4a-r followed the procedures described by de Oliveira [26], and is described by 25. The chemical structures of compounds were determined by nuclear magnetic resonance (NMR., 1H, and 13C), infrared (I.R.) and mass spectra (HR-MS), while purity was determined by elemental analysis (EA).
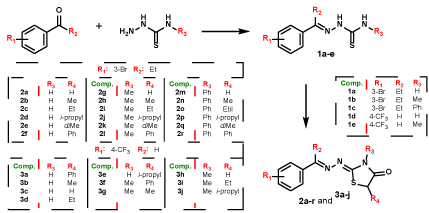
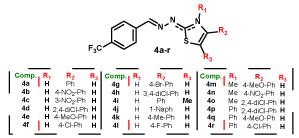
Figure 2. Series 4a-r, variations in the group linked to 4 position of the thiazole ring.
Biological evaluation
The in vitro anti-T. cruzi activity of compounds for epimastigote, trypomastigote and amastigote T. cruzi forms was evaluated, and their toxicity was evaluated in L929 fibroblasts. Among the tested compounds, the series 2a-r showed the best results when compared with 3a-j series. The 2a-r series possess a bromine atom at the position 3 of the aryl ring system and the 3a-j series possess a trifluoromethyl group at position 4 of aryl ring system.
In recent studies [24] a series of 1,3-thiazoles derived from 3-(bromopropiophenone) thiosemicarbazone were synthesized and their results demonstrated similar activity to BZD (T. cruzi trypomastigote form). Furthermore, compounds possessing halogens in their structure (fluorine and bromine), in general, exhibit interesting biological activities and interactions [23] in perspective of these promising outcomes, we decided to explore the anti-T. cruzi potential of bromine (series 2a-r) and trifluoromethyl group (series 3a-j) 4-thiazolidinone derivatives.
Considering the trypanocidal activity of the intermediates 1a-e, and the series 2a-r and 3a-j, for trypomastigote form, the intermediate thiosemicarbazones 1b and 1c, with IC50 = 5.0 µM and IC50 = 2.3 µM, respectively, presented the best results of all tested compounds. Regarding the series 2, compounds 2n (IC50 = 3.7 µM), 2l (IC50 = 7.9 µM), and 2d (IC50 = 8.1 µM) draw attention, all of them had anti-T. cruzi activity against trypomastigote form similar to BZD, however, no correlation of structural features can be drawn.
For series 3, which possesses the core thiazolidine-4-one, the compound 3d (IC50 = 7.8 µM) was the most active of 3 series. The insertion of an group containing halogen at the para position (series 3a-j), was not beneficial for the activity against the trypomastigote form when compared to series 2a-r.
Considering the inhibitory activity of amastigote forms for the entire series, only compound 2h was active, being more potent than BZD, with an IC50 = 2.4 μM. The notable feature of compound 2h is, besides Br atom present at the position 3 of the aryl ring, 4-thiazolidinone ring, possess a methyl group in N3 position and a methyl group in C5.
About the substitutions in N3 it was noticed that the insertion of the methyl group was responsible for the increase the activity. Also, between the compounds 2a, 2g, and 2m, it was possible to verify that the methyl group is pivotal to the reduction of IC50.
The compounds 1a-e, thiosemicarbazones that originated the respective 4-thiazolidinones, can be observed that intermediates 1a and 1d, which possess H in R3, presented the best IC50 values for amastigote form, suggesting that the presence of hydrogen bonds donors in the structure may be of interest for improving the biological activity in the amastigote form.
When we compare the trypanocidal activity in amastigote, the cyclic 4-thiazolidinone derivatives 2g (IC50=12.2µM) and 2h (IC50=2.4µM) were about 4 and 21-folds respectively more active than intermediate compound 1b (IC50=51.1µM). The same trend was observed to compound 2p (IC50=48.5µM) approximately 2-folds more potent than intermediate 1c (IC50=89.1µM).
In general, the compounds substituted at the N3 position of the 4-thiazolidinone ring with methyl, for the sub-series 2, were active against amastigote form (2g, 2h, 2j, 2k). It is interesting to note that, in contrast to our previous work [10], the 4-thiazolidinones of the present work, with phenyl in the N3 position were not the most active, with the exception of compound 2n (IC50trypo= 3.7 µM), which has a methyl group in C5 of the heterocyclic ring.
Described by [23], within the class of drugs that present halogen atom in their structure, those that possess a fluoride, are in greater quantity since the fluorination changes the physical, chemical and conformational parameters. This feature could eventually result in optimized pharmacological properties. The fluorine chemistry provides good opportunities for enhancing the binding affinity of potential drug candidates. These features have made the trifluoromethyl (CF3) useful chemical groups in the contemporary drug design [27]. Within this observation, we can say that the molecules which present trifluoromethyl (CF3) group in their structure (1d-e and 3a-j), were not the most active, as expected when compared to the series presenting the halogen bromine (1a-c and 2a-r).
In the sub-series 3, five compounds were active against trypomastigote form: 3b (IC50=61.9 µM), 3c (IC50=22.4 µM), 3d (IC50=7.8 µM), 3e (IC50=39.3 µM) and 3j (IC50=59.3 µM), with highlight 3d compound. In the amastigote form, three compounds were active: 3a (IC50=43.2 µM), 3c (IC50=121.8 µM) and 3j (IC50=22.4 µM). Contrary to series 2, structural characteristic is a methyl in N3 is not beneficial for series 3. The sub-series 2 had the most active compounds substituted in N3 by a methyl, generally, the most active compound being 2h (Table 1).
Table 1. Determination of cytotoxicity in T. cruzi and fibroblast cells
Cpd |
R1 |
R2 |
R3 |
R4 |
Epimastigote IC50 [µM]1 |
Trypomastigote IC50 [µM]2 |
Amastigote IC50 [µM]3 |
Fibroblast CC50 [µM] |
SI |
 |
1a |
3-Br |
Et |
H |
- |
28.7 |
31.8 |
5.7 |
70.1 |
12.2 |
1b |
3-Br |
Et |
Me |
- |
ND |
5.0 |
51.1 |
267.5 |
5.2 |
1c |
3-Br |
Et |
Ph |
- |
6.8 |
2.3 |
89.1 |
110.7 |
1.2 |
1d |
4-CF3 |
H |
H |
- |
ND |
20.2 |
<10.11 |
80.89 |
>8 |
1e |
4-CF3 |
H |
Me |
- |
ND |
191.4 |
- |
- |
Inactive |
1f |
4-CF3 |
H |
Ph |
- |
20.0 |
13.8 |
45.77 |
61.85 |
1.4 |
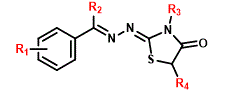 |
2a |
3-Br |
Et |
H |
H |
82.6 |
146.9 |
- |
- |
Inactive |
2b |
3-Br |
Et |
H |
Me |
ND |
33.7 |
80.2 |
235.9 |
2.9 |
2c |
3-Br |
Et |
H |
Et |
66.8 |
23.8 |
- |
- |
Inactive |
2d |
3-Br |
Et |
H |
i-propyl |
ND |
8.1 |
- |
- |
Inactive |
2e |
3-Br |
Et |
H |
diMe |
65.2 |
89.0 |
13.3 |
< 226.6ii |
< 17 |
2f |
3-Br |
Et |
H |
Ph |
140.7 |
17.8 |
89.5 |
199.4 |
2.2 |
2g |
3-Br |
Et |
Me |
H |
ND |
23.7 |
12.2 |
117.9 |
9.6 |
2h |
3-Br |
Et |
Me |
Me |
ND |
18.8 |
2.4 |
56.6 |
23 |
2i |
3-Br |
Et |
Me |
Et |
ND |
55.5 |
- |
- |
Inactive |
2j |
3-Br |
Et |
Me |
i-propyl |
46.0 |
113.9 |
79.5 |
< 524.8iv |
< 6.6 |
2k |
3-Br |
Et |
Me |
diMe |
69.6 |
46.3 |
175.7 |
217.9 |
1.2 |
2l |
3-Br |
Et |
Me |
Ph |
119.7 |
7.9 |
- |
- |
Inactive |
2m |
3-Br |
Et |
Ph |
H |
78.0 |
15.3 |
- |
- |
Inactive |
2n |
3-Br |
Et |
Ph |
Me |
ND |
3.7 |
- |
- |
Inactive |
2o |
3-Br |
Et |
Ph |
Et |
42.2 |
60.1 |
- |
- |
Inactive |
2p |
3-Br |
Et |
Ph |
i-propyl |
ND |
ND |
48.5 |
< 112.8i |
< 2,3 |
2q |
3-Br |
Et |
Ph |
diMe |
12.4 |
50.3 |
168.7 |
> 186.4iii |
> 1.1 |
2r |
3-Br |
Et |
Ph |
Ph |
ND |
53 |
- |
- |
Inactive |
3a |
4-CF3 |
H |
H |
Ph |
ND |
ND |
43.2 |
55 |
1.3 |
3b |
4-CF3 |
H |
H |
Me |
ND |
61.9 |
- |
- |
Inactive |
3c |
4-CF3 |
H |
H |
H |
ND |
22.4 |
121.8 |
139.2 |
1.1 |
3d |
4-CF3 |
H |
H |
Et |
ND |
7.8 |
- |
- |
Inactive |
3e |
4-CF3 |
H |
H |
i-propyl |
ND |
39.3 |
- |
- |
Inactive |
3f |
4-CF3 |
H |
Me |
Ph |
ND |
ND |
- |
- |
Inactive |
3g |
4-CF3 |
H |
Me |
Me |
ND |
ND |
- |
- |
Inactive |
3h |
4-CF3 |
H |
Me |
H |
ND |
ND |
- |
- |
Inactive |
3i |
4-CF3 |
H |
Me |
Et |
ND |
ND |
- |
- |
Inactive |
3j |
4-CF3 |
H |
Me |
i-propyl |
22.4 |
59.3 |
22.4 |
58.2 |
2.6 |
BZD |
- |
- |
- |
- |
48.8 |
6.3 |
3.8 |
2381 |
625 |
Concentration (mg/µL): I) < 50; II) < 80; III) > 80; IV) < 200. IC50: Concentration that inhibit by 50% the growth of T. cruzi. CC50: Concentration capable of causing cytotoxic effect on 50% of mammalian cells
CC50 and IC50 values were calculated using concentrations in triplicate and experiment was repeated, only values with a standard deviation < 10% mean were considered. SI[1] (Selectivity Index): CC50 of mammalian cells / IC50 T. cruzi BZD: Benznidazole; Inactive: Compound was not active at any tested dose
Regarding the 1,3-thiazole series, three compounds had lower IC50 values for the amastigote form of T. cruzi, compounds 4b (IC50 = 9.9μM), 4d (IC50 = 5.3μM) and 4h (IC50 = 8.9μM), they have very electronegative groups attached to the aromatic ring bound to the 1,3-thiazole as a characteristic, however, all compounds presented a very low selectivity index (Table 2). Two of these compounds (4d and 4h) have in common the presence of two chlorines in the aromatic ring bound to the 1,3-thiazole. One at 2 and 4 positions (4d) and the other at 3 and 4 positions (4h), which may indicate that di-substitution with halogens in the aromatic ring may be beneficial for anti-T. cruzi activity, this data corroborates with that recently observed by 10,26.
Table 2. Determination of cytotoxicity in T. cruzi and fibroblast cells
Cpd |
R1 |
R2 |
R3 |
Epimastigote IC50 [µM]1 |
Trypomastigote IC50 [µM]2 |
Amastigote IC50 [µM]3 |
Fibroblast CC50 [µM] |
SI |
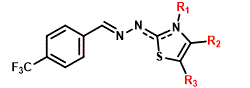 |
4a |
H |
Ph |
H |
24.3 |
83.06 |
- |
- |
Inactive |
4b |
H |
4-NO2-Ph |
H |
79.1 |
71.75 |
9.93 |
25.48 |
2.6 |
4c |
H |
3-NO2-Ph |
H |
32.7 |
80.87 |
13.50 |
50.97 |
3.8 |
4d |
H |
2,4-diCl-Ph |
H |
10.6 |
53.84 |
5.28 |
12.01 |
2.3 |
4e |
H |
4-MeO-Ph |
H |
6.9 |
9.65 |
52.99 |
>105.99 |
>2 |
4f |
H |
4-Cl-Ph |
H |
25.0 |
42.76 |
19.64 |
>209.53 |
>10.7 |
4g |
H |
4-Br-Ph |
H |
8.5 |
80.33 |
17.82 |
46.92 |
2.6 |
4h |
H |
3,4-diCl-Ph |
H |
29.6 |
40.75 |
8.88 |
24.02 |
2.7 |
4i |
H |
Ph |
Me |
10.9 |
79.36 |
- |
- |
Inactive |
4j |
H |
1-Naph |
H |
24.4 |
88.70 |
- |
- |
Inactive |
4k |
H |
4-Me-Ph |
H |
27.5 |
78.53 |
- |
- |
Inactive |
4l |
H |
4-F-Ph |
H |
10.6 |
32.98 |
22.71 |
54.74 |
2.4 |
4m |
Me |
4-MeO-Ph |
H |
18.6 |
57.52 |
51.09 |
>51.09 |
>1 |
4n |
Me |
4-NO2-Ph |
H |
83.7 |
169.66 |
49.21 |
>196.86 |
>4 |
4o |
Me |
2,4-diCl-Ph |
H |
NT |
NT |
11.62 |
<22.24 |
<1.9 |
4p |
Ph |
2,4-diCl-Ph |
H |
NT |
NT |
10.11 |
<20.22 |
<2 |
4q |
Ph |
4-MeO-Ph |
H |
NT |
NT |
- |
- |
Inactive |
4r |
Ph |
4-Cl-Ph |
H |
NT |
NT |
- |
- |
Inactive |
BZD |
- |
- |
- |
48.8 |
6.3 |
3.8 |
2381 |
626.6 |
Concentration (mg/µL): I) < 50; II) < 80; III) > 80; IV) < 200. IC50: Concentration that inhibit by 50% the growth of T. cruzi. CC50: Concentration capable of causing cytotoxic effect on 50% of mammalian cells
CC50 and IC50 values were calculated using concentrations in triplicate and experiment was repeated, only values with a standard deviation < 10% mean were considered. SI[1] (Selectivity Index): CC50 of mammalian cells / IC50 T. cruzi. BZD: Benznidazole; Inactive: Compound was not active at any tested dose.
Figure 3 below summarizes the pharmacological structure-activity relationships observed in this work.
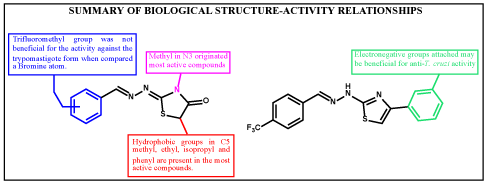
Figure 3. Summary of structure-activity relationships for series of 4-thiazolidinones and 1,3-thiazoles.
Physicochemical properties
We also evaluated the physicochemical properties to determine if they are compliant with the Lipinski’s rule [28,29]. This rule has important determinants to providing better pharmacokinetics and analyses promising future drug development. For this purpose, physicochemical and ADME properties were calculated using the SwissADME (a free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules). Compound obeying at least three of the four criteria are considered to adhere the Lipinski Rule [29]. All synthesized compounds are compatible with Lipinski Rule. Another interesting property is the number of rotatable bonds and the polar surface area (PSA). A large number of rotatable bonds (≥10) has been associated with poor oral bioavailability [30]. Compounds with a low PSA (≤ 140 Å2) tend to have higher oral bioavailability [30,31]. All synthesized compounds have appropriate PSA and number of rotatable bonds (Table 3 and Table 4).
Table 3. Physicochemical property profile of thiazolidinone derivatives, calculated by Property Calculator (https://mcule.com/apps/property-calculator/)
Cpd |
R1 |
R2 |
R3 |
R4 |
MW
(g/mol)
<500 |
Log P o/w
<5 |
H-bond donors
<5 |
H-bond acceptors
<10 |
Violation Lipinski
Rule of 5 |
Rotatable bonds
<10 |
*PSA
(Ų)
<140 |
1a |
3-Br |
Et |
H |
- |
258.14 |
2.71 |
2 |
3 |
0 |
4 |
82.50 |
1b |
3-Br |
Et |
Me |
- |
272.17 |
2.66 |
2 |
3 |
0 |
5 |
68.51 |
1c |
3-Br |
Et |
Ph |
- |
334.24 |
4.23 |
2 |
3 |
0 |
6 |
68.51 |
1d |
4-CF3 |
H |
H |
- |
247.24 |
2.96 |
2 |
3 |
0 |
4 |
82.50 |
1e |
4-CF3 |
H |
Me |
- |
261.27 |
2.91 |
2 |
3 |
0 |
5 |
68.51 |
2a |
3-Br |
Et |
H |
H |
326.21 |
3.11 |
1 |
4 |
0 |
3 |
79.12 |
2b |
3-Br |
Et |
H |
Me |
340.24 |
3.50 |
1 |
4 |
0 |
3 |
79.12 |
2c |
3-Br |
Et |
H |
Et |
354.27 |
3.89 |
1 |
4 |
0 |
4 |
79.12 |
2d |
3-Br |
Et |
H |
i-propyl |
368.29 |
4.14 |
1 |
4 |
0 |
4 |
79.12 |
2e |
3-Br |
Et |
H |
diMe |
354.27 |
3.88 |
1 |
4 |
0 |
3 |
79.12 |
2f |
3-Br |
Et |
H |
Ph |
402.31 |
4.85 |
1 |
4 |
0 |
4 |
79.12 |
2g |
3-Br |
Et |
Me |
H |
340.24 |
3.06 |
0 |
4 |
0 |
3 |
70.33 |
2h |
3-Br |
Et |
Me |
Me |
354.27 |
3.45 |
0 |
4 |
0 |
3 |
70.33 |
2i |
3-Br |
Et |
Me |
Et |
368.29 |
3.84 |
0 |
4 |
0 |
4 |
70.33 |
2j |
3-Br |
Et |
Me |
i-propyl |
382.32 |
4.09 |
0 |
4 |
0 |
4 |
70.33 |
2k |
3-Br |
Et |
Me |
diMe |
368.29 |
4.02 |
0 |
4 |
0 |
3 |
70.33 |
2l |
3-Br |
Et |
Me |
Ph |
416.34 |
4.80 |
0 |
4 |
0 |
4 |
70.33 |
2m |
3-Br |
Et |
Ph |
H |
402.31 |
4.76 |
0 |
4 |
0 |
4 |
70.33 |
2n |
3-Br |
Et |
Ph |
Me |
416.34 |
5.15 |
0 |
4 |
1 |
4 |
70.33 |
2o |
3-Br |
Et |
Ph |
Et |
430.36 |
5.54 |
0 |
4 |
1 |
5 |
70.33 |
2p |
3-Br |
Et |
Ph |
i-propyl |
444.39 |
5.79 |
0 |
4 |
1 |
5 |
70.33 |
2q |
3-Br |
Et |
Ph |
diMe |
430.36 |
5.54 |
0 |
4 |
1 |
4 |
70.33 |
2r |
3-Br |
Et |
Ph |
Ph |
478.41 |
6.51 |
0 |
4 |
1 |
5 |
70.33 |
3a |
4-CF3 |
H |
H |
Ph |
363.36 |
4.33 |
1 |
4 |
0 |
4 |
79.12 |
3b |
4-CF3 |
H |
H |
Me |
301.29 |
2.98 |
1 |
4 |
0 |
3 |
79.12 |
3c |
4-CF3 |
H |
H |
H |
287.26 |
2.59 |
1 |
4 |
0 |
3 |
79.12 |
3d |
4-CF3 |
H |
H |
Et |
315.32 |
3.36 |
1 |
4 |
0 |
4 |
79.12 |
3e |
4-CF3 |
H |
H |
i-propyl |
329.34 |
3.61 |
1 |
4 |
0 |
4 |
79.12 |
3f |
4-CF3 |
H |
Me |
Ph |
377.39 |
4.28 |
0 |
4 |
0 |
4 |
70.33 |
3g |
4-CF3 |
H |
Me |
Me |
315.32 |
2.93 |
0 |
4 |
0 |
3 |
70.33 |
3h |
4-CF3 |
H |
Me |
H |
301.29 |
2.54 |
0 |
4 |
0 |
3 |
70.33 |
3i |
4-CF3 |
H |
Me |
Et |
329.34 |
3.32 |
0 |
4 |
0 |
4 |
70.33 |
3j |
4-CF3 |
H |
Me |
i-propyl |
343.37 |
3.56 |
0 |
4 |
0 |
4 |
70.33 |
Table 4. Physicochemical property profile of thiazole derivatives, calculated by Property Calculator (https://mcule.com/apps/property-calculator)
Cpd |
R1 |
R2 |
R3 |
MW
(g/mol)
<500 |
Log P o/w
<5 |
H-bond donors
<5 |
H-bond acceptors
<10 |
Violation Lipinski
Rule of 5 |
Rotatable bonds
<10 |
*PSA
(Ų)
<140 |
1d |
H |
- |
- |
247.24 |
2.06 |
2 |
3 |
0 |
4 |
82.50 |
1e |
Me |
- |
- |
261.27 |
2.91 |
2 |
3 |
0 |
5 |
68.51 |
1f |
Ph |
- |
- |
323.34 |
4.49 |
2 |
3 |
0 |
6 |
68.51 |
4a |
H |
Ph |
H |
349.38 |
4.80 |
1 |
3 |
0 |
5 |
65.52 |
4b |
H |
4-NO2-Ph |
H |
392.36 |
5.78 |
1 |
6 |
1 |
6 |
111.34 |
4c |
H |
3-NO2-Ph |
H |
392.36 |
5.78 |
1 |
6 |
1 |
6 |
111.34 |
4d |
H |
2,4-diCl-Ph |
H |
416.25 |
6.00 |
1 |
3 |
1 |
5 |
65.52 |
4e |
H |
4-MeO-Ph |
H |
377.39 |
4.71 |
1 |
4 |
0 |
6 |
74.75 |
4f |
H |
4-Cl-Ph |
H |
381.80 |
5.35 |
1 |
3 |
1 |
5 |
65.52 |
4g |
H |
4-Br-Ph |
H |
426.26 |
5.46 |
1 |
3 |
1 |
5 |
65.52 |
4h |
H |
3,4-diCl-Ph |
H |
416.25 |
6.00 |
1 |
3 |
1 |
5 |
65.52 |
4i |
H |
Ph |
Me |
361.39 |
5.01 |
1 |
3 |
1 |
5 |
65.52 |
4j |
H |
1-Naph |
H |
397.42 |
5.85 |
1 |
3 |
1 |
5 |
65.52 |
4k |
H |
4-Me-Ph |
H |
361.39 |
5.01 |
1 |
3 |
1 |
5 |
65.52 |
4l |
H |
4-F-Ph |
H |
365.35 |
4.84 |
1 |
3 |
0 |
5 |
65.52 |
4m |
Me |
4-MeO-Ph |
H |
391.41 |
4.72 |
0 |
4 |
0 |
5 |
67.12 |
4n |
Me |
4-NO2-Ph |
H |
406.38 |
5.14 |
0 |
6 |
1 |
5 |
103.71 |
4o |
Me |
2,4-diCl-Ph |
H |
430.27 |
6.01 |
0 |
3 |
1 |
4 |
57.89 |
4p |
Ph |
2,4-diCl-Ph |
H |
492.34 |
7.47 |
0 |
3 |
1 |
5 |
57.89 |
4q |
Ph |
4-MeO-Ph |
H |
453.48 |
6.17 |
0 |
4 |
1 |
6 |
67.12 |
4r |
Ph |
4-Cl-Ph |
H |
457.90 |
6.81 |
0 |
3 |
1 |
5 |
57.89 |
As demonstrated in Table 5, the most active compounds shown variable permeability based on gastrointestinal absorption (GI), according to the BOILED-Egg predictive model (Brain Or IntestinaL EstimateD permeation method). Three compounds showed high gastrointestinal absorption (1a, 2h and 2n). With respect to oral bioavailability, it's expected 0.55 of the probability of oral bioavailability score > 10% in the rat for all compounds, similar to BZD. All these data, suggests a good in silico drug-likeness profile and great chemical stabilities for all compounds synthesized.
Table 5. ADME properties of most active compounds
|
Compound |
*BBB permeant |
**GI absorption |
Bioavailability Score |
1a |
No |
High |
0.55 |
2h |
Yes |
High |
0.55 |
2n |
Yes |
High |
0.55 |
4d |
No |
Low |
0.55 |
BZD |
No |
High |
0.55 |
*BBB - blood–brain barrier. **GI - Gastrointestinal absorption
This study has synthesized, characterized and identified 46 new heterocyclic compounds, based on previous results. These compounds were assayed against epimastigote, trypomastigote, and amastigote forms of T. cruzi, as well as their toxicity in L929 fibroblasts. For epimastigote form, the most active compound was 4e. Towards trypomastigote and amastigote forms of T. cruzi, the most active heterocycle compounds identified in this study were 2n (most active against trypomastigote form) and 2h (most active against amastigote form). The findings of this research provide insights into valuable strategies of synthesis and Structure-Activity relationships (SAR) for the planning of new anti-T. cruzi drugs candidates.
General
Most the chemicals were purchased from Sigma-Aldrich (St. Louis, USA), Merck (Berlin, Germany) or Alfa-Aesar (Massachusetts, USA). Reactions in ultrasound bath were performed in a Unique EM-804 TGR instrument, with a frequency of 40 kHz and a nominal power of 180 W, without external heating. Precoated aluminum sheets (silica gel 60 F254, Merck) were used for thin layer chromatography (TLC) and spots were visualized under UV light. IR spectra in KBr pellets were acquired at Bruker FT-IR spectrophotometer. 1H and 13C NMR were recorded on a UnityPlus 400 MHz and Bruker AMX-300 MHz spectrometer, using DMSO-d6 as a solvent and tetramethylsilane (TMS) as the internal standard. Splitting patterns were defined as s, singlet; d, doublet; t, triplet; q, quartet; m, multiplet. Chemical shift values were given in, ppm. DEPT was employed to confirm the carbon assignment. Melting points were measured using capillaries on a Thomas device Hoover.
Synthesis of intermediate thiosemicarbazones. Example for 3-bromophenyl propylidene hydrazine carbothioamide (1a)
In a round bottom flask for 50 mL, the 3-bromophenyl propanone (0.45 g, 2.12 mmol) was dissolved in 8 mL EtOH, following by the addition of catalytic HCl. The flask was placed in an ultrasound bath (40 kHz, 180 W) and under sonication, 0.23 g (2.12 mmol) of thiosemicarbazide was added in the reaction. After 3 h, distilled water was added, and the precipitate formed was cooled at 0° C and the precipitate was filtered in a Büchner funnel with a sintered disc filter, washed with ethanol and then dried over SiO2. Colorless crystals were formed in a yield of approximately 70%.
2-(1-(3-bromophenyl)propylidene)hydrazinecarbothioamide (1a)
Yellow crystals; Yield 68%; m.p.(ºC) 140-143; Rf: 0.71 (Hexane/ethyl acetate 7:3). IR (KBr, cm-1): 3416 (NH), 3202 and 3147 (NH2), 1598 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 1.01 (t, J = 7.6 Hz, 3H, CH3), 2.83 (q, J = 7.6 Hz, 2H, CH2), 7.34 (t, J = 7.8 Hz, 1H, Ar), 7.52 (d, J = 7.6 Hz, 1H, Ar), 7.83 (d, J = 7.6 Hz, 1H, Ar), 8.12 (s, 1H, Ar), 8.34 (s, H, NH), 10.32 (s, 2H, NH2). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 10.8 (CH3), 19.1 (CH2), 122.0 (CH, Ar), 125.7 (CH, Ar), 129.0 (CH, Ar), 130.3 (CH, Ar), 133.0 (CH, Ar), 136.2 (CH, Ar), 150.2 (C=N), 179.1 (C=S).
2-(1-(3-bromophenyl)propylidene)-N-methylhydrazinecarbothioamide (1b)
Yellow crystals; Yield 81%; m.p.(ºC) 143-146; Rf: 0.72 (Hexane/ethyl acetate 8:2). IR (KBr, cm-1): 3290 and 3192 (NH), 1551 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 1.02 (t, J = 7.4 Hz, 3H, CH3), 2.83 (q, J = 7.4 Hz, 2H, CH2), 3.45 (d, 3H, CH3-NH), 7.34 (t, J = 7.9 Hz, 1H, Ar), 7.53 (d, J = 7.8 Hz, 1H, Ar), 7.84 (d, J = 8.0 Hz, 1H, Ar), 8.12 (s, 1H, Ar), 8.51 (br.s, 1H, NH), 10.42 (br.s, 1H, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm: δ 10.7 (CH3), 19.1 (CH2), 31.1 (N-CH3), 122.1 (CH, Ar), 125.7 (BrC, Ar), 127.2 (CH, Ar), 130.4 (CH, Ar), 131.7 (CH, Ar), 138.8 (C, Ar), 149.9 (C=N), 178.6 (C=S).
2-(1-(3-bromophenyl)propylidene)-N-phenylhydrazinecarbothioamide (1c)
Yellow crystals; Yield 70%; m.p.(ºC) 150-151; Rf: 0.60 (Hexane/ethyl acetate 8:2). IR (KBr, cm-1): 3307 and 2971 (NH), 1522 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 1.12 (t, J = 7.4 Hz, 3H, CH3), 2.90 (q, J = 7.4 Hz, 2H, CH2), 7.21 (t, J = 7.2 Hz, 1H, Ar), 7.32 (m, 3H, Ar), 7.53 (m, 3H, Ar), 7.90 (d, J = 7.6 Hz, 1H, Ar), 8.21 (s, 1H, Ar), 10.12 (s, 1H, NH), 10.73 (s, 1H, ArNH). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 10.8 (CH3), 19.4 (CH2), 122.0 (CH, Ar), 125.4 (CH, Ar), 125.9 (BrC, Ar), 126.1 (CH, Ar), 128.0 (CH, Ar), 129.2 (CH, Ar), 130.3 (CH, Ar), 131.9 (CH, Ar), 138.6 (C, Ar), 139.1 (C-N, Ar), 151.1 (C=N), 177.2 (C=S).
4-(trifluoromethyl)phenyl]methylidene}amino]thiourea (1d)
White crystals; Yield 85%; m.p.(ºC) 166-170; Rf:0,41 (hexane/ethyl acetate 7:3). IR (KBr, cm-1): 3272.63 (NH), 1698.36 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 3.35 (s, 2H, NH2), 7.67 (d, J = 7.8 Hz, 2H, Ar), 7.80 (d, J = 8.1 Hz, 2H, Ar), 8.30 (s, 1H, HC=N), 11.60 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 125.3 (C, Ar), 125.3 (C, Ar), 127.7 (C, Ar), 130.0 (C, Ar), 134.5 (C, Ar), 138.1 (C, Ar), 140.2 (C=N), 178.3 (C=S).
3-methyl-1-{[4-(trifluoromethyl)phenyl]methylidene}aminothiourea (1e)
White crystals; Yield 94%; m.p.(ºC) 234-236; Rf:0,75 (hexane/ethyl acetate 7:3). IR (KBr, cm-1): 3158.76 (NH), 1539.93 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 3.02 (d, J = 4.2 Hz, 3H, CH3), 7.76 (d, J = 7.8 Hz, 2H, Ar), 8.02 (d, J = 7.5 Hz, 2H, Ar), 8.69 (s, 1H, HC=N), 11.69 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 30.9 (CH3), 125.5 (C, Ar), 127.7 (C, Ar), 138.4 (C, Ar), 138.8 (C=N), 177.9 (C=S).
Synthesis of aryl-thiazolinones (2a-2r). Example: 3-bromophenyl propylidene hydrazone - 3,5-dimethylthiazolidin-4-one: (2h):
In a round bottom flask for 50 mL, was added the compound INT-2 (0.61 g, 2.12 mmol), 20 mL of ethanol and anhydrous sodium acetate (0.71 g, 8.28 mmol). After heating for about 15 minutes, methyl 2-bromopropionate was added (0.65 g, 4.24mmol) and the reaction was stirred and refluxed (120° C) for approximately 20 hours (monitored by TLC). The reaction was cooled at 0° C for two days and, subsequently, the powder was separated from the supernatant Buchner funnel with a sintered disc filter, washed with ethanol and then transferred to a desiccator under vacuum. There was obtained a yellow coloured powder with a yield of 92%.
2-((1-(3-bromophenyl)propylidene)hydrazono)thiazolidin-4-one (2a)
Recrystallization in toluene afforded white crystals, yield = 22%. M.p. (° C): 142-144. Rf: 0.66 (Toluene/ethyl acetate 7:3). IR (KBr): 3201 (NH), 1601 (C=O), 1507 (C=N) cm-1. 1H NMR (300 MHz, DMSO-d6): 0.92 (t, J = 7.2 Hz, 3H, CH3), 2.93 (q, J = 7.2 Hz, 2H, CH2), 3.93 (s, 2H, CH2), 7.32 (t, J = 7.9 Hz, 1H, Ar), 7.64 (d, J = 7.5 Hz, 1H, Ar), 7.72 (d, J = 7.5 Hz, 1H, Ar), 7.91 (s, 1H, Ar), 11.92 (s, 1H, NH). 13C NMR (75.5 MHz, DMSO-d6): 11.6 (CH3), 20.7 (CH2), 32.8 (CH2, heterocycle), 122.0 (CH, Ar), 125.6 (BrC, Ar), 128.9 (CH, Ar), 130.7 (CH, Ar), 132.3 (CH, Ar), 138.8 (C, Ar), 163.5 (C=N), 165.5 (S-C=N), 173.9 (C=O).
2-((1-(3-bromophenyl)propylidene)hydrazono)-5-methylthiazolidin-4-one (2b)
The washing in ethanol afforded yellow powders, yield = 49%. M.p. (° C): 119-121. Rf: 0.68 (Toluene/ethyl acetate 8:2). IR (KBr): 3409 (NH), 1722 (C=O), 1565 (C=N) cm-1. 1H NMR (300 MHz, DMSO-d6): 1.02 (t, J = 7.65 Hz, 3H, CH3), 1.53 (d, J = 6.9 Hz, 3H, CH3–C5), 2.92 (q, J = 7.7 Hz, 2H, CH2), 4.14 (q, J = 7.3 Hz, 1H, CH heterocycle), 7.43 (t, J = 7.95 Hz, 1H, Ar), 7.62 (d, J = 9.9 Hz, 1H, Ar), 7.84 (d, J = 9.8 Hz, 1H, Ar), 7.92 (s, 1H, Ar), 11.82 (s, 1H, NH). 13C NMR (75.5 MHz, DMSO-d6): 11.6 (CH3), 18.6 (CH2), 20.6 (CH3, heterocycle), 41.9 (CH, heterocycle), 122.0 (CH, Ar), 125.6 (BrC, Ar), 126.2 (CH, Ar), 129.4 (CH, Ar), 130.8 (CH, Ar), 133.2 (C, Ar), 150.6 (C=N), 163.6 (S-C=N), 176.7 (C=O).
2-((1-(3-bromophenyl)propylidene)hydrazono)-5-ethylthiazolidin-4-one (2c)
Recrystallization in toluene afforded yellowish crystals, yield = 58%. M.p. (°C): 116-118. Rf: 0.70 (Toluene/ethyl acetate 8:2). IR (KBr): 1714 (C=O), 1613 (C=N) cm-1. 1H NMR (300 MHz, DMSO-d6): 0.93 (m, 6H, CH3), 1.92 (m, 2H, CH2-C5), 2.94 (q, J = 7.5 Hz, 2H, CH2), 4.12 (q, J = 4.0 Hz, 1H, CH heterocycle), 7.30 (t, J = 7.9 Hz, 1H, Ar), 7.63 (d, J = 7.8 Hz, 1H, Ar), 7.8 (d, J = 7.8 Hz, 1H, Ar), 7.92 (s, 1H, Ar). 13C NMR (75.5 MHz, DMSO-d6): 10.3 (CH3), 11.6 (CH3), 20.6 (CH2), 25.4 (CH2), 49.2 (CH heterocycle), 122.0 (CH, Ar), 125.5 (BrC, Ar), 128.8 (CH, Ar), 130.7 (CH, Ar), 132.1 (CH, Ar), 138.9 (C, Ar), 163.0 (C=N), 165.2 (S-C=N), 176.8 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-5-isopropilthiazolidin-4-one (2d)
The washing in ethanol afforded yellow powders, yield = 93%. M.p. (° C): 135-137. Rf: 0.74 (Toluene/ethyl acetate 8:2). IR (KBr): 3421 (NH), 1708 (C=O), 1613 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 0.92 (m, 9H, CH3), 2.43 (m, 1H, CH-C5), 2.81 (q, J = 5.9 Hz, 2H, CH2), 3.92 (d, J = 3.2 Hz, 1H, CH heterocycle), 7.32 (t, J = 7.8 Hz, 1H, Ar), 7.53 (d, J = 8.4 Hz, 1H, Ar), 7.74 (d, J = 7.6 Hz, 1H, Ar), 7.92 (s, 1H, Ar). 13C NMR (100 MHz, DMSO-d6): 11.5 (CH3), 16.3 (CH3), 17.7 (CH3), 20.3 (CH2), 21.3 (CH), 56.5 (CH heterocycle), 121.9 (CH, Ar), 125.1 (BrC, Ar), 128.4 (CH, Ar), 130.5 (CH, Ar), 131.3 (CH, Ar), 139.6 (C, Ar), 159.5 (C=N), 160.4 (S-C=N), 172.1 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-5,5-dimethylthiazolidin-4-one (2e)
Recrystallization in toluene afforded yellowish crystals, yield = 88%. M.p. (° C): 162-164. Rf: 0.45 (Toluene/ethyl acetate 8:2). IR (KBr): 1712 (C=O), 1615 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 1.12 (t, J = 7.4 Hz, 3H, CH3), 1.74 (s, 6H, CH3), 2.93 (q, J = 7.4 Hz, 2H, CH2), 7.23 (t, J = 6.4 Hz, 1H, Ar), 7.54 (d, J = 8.4 Hz, 1H, Ar), 7.74 (d, J = 7.6 Hz, 1H, Ar), 7.93 (s, 1H, Ar). 13C NMR (100 MHz, DMSO-d6): 11.8 (CH3), 22.0 (CH2), 28.0 (CH3 heterocycle), 28.3 (CH3 heterocycle), 49.6 (C, heterocycle), 122.0 (CH, Ar), 122.8 (BrC, Ar), 123.3 (CH, Ar), 129.9 (CH, Ar), 130.0 (CH, Ar), 133.1 (C, Ar), 161.7 (C=N), 166.2 (S-C=N), 175.1 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-5-phenylthiazolidin-4-one (2f)
The washing in ethanol afforded yellow powders, yield = 85%. M.p. (°C): 187-189. Rf: 0.82 (Toluene/ethyl acetate 8:2). IR (KBr): 1709 (C=O), 1619 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 1.02 (t, J = 7.4 Hz, 3H, CH3), 2.93 (q, J = 7.6 Hz, 2H, CH2), 5.42 (s, 1H, CH heterocycle), 7.14 (m, 6H, Ar), 7.63 (d, J = 7.2 Hz, 1H, Ar-Br), 7.75 (d, J = 7.9 Hz, 1H, Ar-Br), 7.93 (s, 1H, Ar-Br). 13C NMR (100 MHz, DMSO-d6): 11.6 (CH3), 20.7 (CH2), 51.1 (CH heterocycle), 122.0 (CH, Ar), 125.2 (BrC, Ar), 125.6 (CH, Ar), 128.0 (CH, Ar), 128.1 (CH, Ar), 128.4 (CH, Ar), 128.8 (CH, Ar), 128.9 (CH, Ar), 130.7 (CH, Ar), 132.3 (C, Ar), 138.7 (C=N), 163.4 (S-C=N), 175.3 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-3-methylthiazolidin-4-one (2g)
The washing in ethanol afforded yellow powders, yield = 74%. M.p. (°C): 129-131. Rf: 0.72 (Toluene/ethyl acetate 9:1). IR (KBr): 1707 (C=O), 1628 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 1.02 (t, J = 7.2 Hz, 3H, CH3), 1.84 (m, 2H, CH2), 2.93 (s, CH2 heterocycle), 3.34 (s, 3H, N-CH3), 7.42 (t, J = 7.8 Hz, 1H, Ar), 7.63 (d, J = 7.2 Hz, 1H, Ar), 7.82 (d, J = 7.9 Hz, 1H, Ar), 7.93 (s, 1H, Ar). 13C NMR (100 MHz, DMSO-d6): 11.9 (CH3), 20.6 (CH2), 25.4 (CH heterocycle), 49.2 (N-CH3), 121.9 (CH, Ar), 125.5 (BrC, Ar), 128.8 (CH, Ar), 130.7 (CH, Ar), 132.1 (CH, Ar), 138.9 (C, Ar), 163.0 (C=N), 165.1 (S-C=N), 176.8 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-3,5-dimethylthiazolidin-4-one (2h)
The washing in ethanol afforded yellow powders, yield = 92%. M.p. (°C): 120-122. Rf: 0.68 (Toluene/ethyl acetate 8:2). IR (KBr): 1716 (C=O), 1611 and 1573 (C=N) cm-1. 1H NMR (300 MHz, DMSO-d6): 1.02 (t, J = 7.6 Hz, 3H, CH3), 1.54 (d, J = 7.8 Hz, 3H, CH3), 2.93 (q, J = 7.4 Hz, 2H, CH2), 3.34 (s, 3H, N-CH3), 4.24 (q, J = 7.3 Hz, 1H, CH), 7.42 (t, J = 7.2 Hz, 1H, Ar), 7.63 (d, J = 8.4 Hz, 1H, Ar), 7.83 (d, J = 8.1 Hz, 1H, Ar), 7.93 (s, 1H, Ar). 13C NMR (75.5 MHz, DMSO-d6): 11.4 (CH3), 18.6 (CH2), 20.7 (CH3 heterocycle), 29.5 (N-CH3), 41.3 (CH heterocycle), 122.0 (CH, Ar), 125.7 (BrC, Ar), 128.9 (CH, Ar), 130.7 (CH, Ar), 132.4 (CH, Ar), 138.7 (C, Ar), 163.2 (C=N), 164.9 (S-C=N), 175.1 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-5-ethyl-3-methylthiazolidin-4-one (2i)
The washing in ethanol afforded yellow powders, yield = 69%. M.p. (°C): 187-189. Rf: 0.62 (Toluene/ethyl acetate 8:2). IR (KBr): 1722 (C=O), 1605 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 0.93 (t, J = 7.2 Hz, 3H, CH3), 1.04 (t, J = 7.6 Hz, 3H, CH3), 1.93 (m, 2H, CH2-C5 heterocycle), 2.93 (q, J = 3.2 Hz, 2H, CH2), 3.24 (s, 3H, CH3), 4.24 (q, J = 3.0 Hz, 1H, CH heterocycle), 7.44 (t, J = 7.8 Hz, 1H, Ar), 7.63 (d, J = 8.0 Hz, 1H, Ar), 7.84 (d, J = 7.6 Hz, 1H, Ar), 7.93 (s, 1H, Ar). 13C NMR (100 MHz, DMSO-d6): 10.3 (CH3), 11.4 (CH3), 20.8 (CH2), 25.4 (CH2), 29.4 (N-CH3), 48.2 (CH heterocycle), 122.0 (CH, Ar), 125.7 (BrC, Ar), 128.9 (CH, Ar), 130.7 (CH, Ar), 132.5 (CH, Ar), 138.7 (C, Ar), 163.0 (C=N), 165.2 (S-C=N), 174.2 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-5-isopropyl-3-methylthiazolidin-4-one (2j)
The washing in ethanol afforded yellow powders, yield = 92%. M.p. (°C): 136-138. Rf: 0.59 (Toluene/ethyl acetate 8:2). IR (KBr): 1716 (C=O), 1611 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 1.02 (t, J = 7.6 Hz, 3H, CH3), 1.52 (d, J = 7.2 Hz, 6H, CH3), 2.93 (q, J = 7.2 Hz, 2H, CH2), 3.34 (s, 3H, N-CH3), 4.24 (q, J = 7.2 Hz, 1H, CH heterocycle), 7.44 (t, J = 8.0 Hz, 1H, Ar), 7.63 (d, J = 7.2 Hz, 1H, Ar), 7.84 (d, J = 7.6 Hz, 1H, Ar), 7.94 (s, 1H, Ar). 13C NMR (100 MHz, DMSO-d6): 10.1 (CH3), 11.2 (CH3), 11.6 (CH3), 13.8 (CH), 20.6 (CH2), 29.1 (N-CH3), 46.3 (CH heterocycle), 122.0 (CH, Ar), 125.7 (BrC, Ar), 128.9 (CH, Ar), 130.7 (CH, Ar), 132.4 (CH, Ar), 138.7 (C, Ar), 163.2 (C=N), 164.9 (S-C=N), 175.1 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-3,5,5-trimethylthiazolidin-4-one (2k)
Recrystallization in toluene afforded yellowish crystals, yield = 31%. M.p. (°C): 133-135. Rf: 0.63 (Toluene/ethyl acetate 8:2). IR (KBr): 1715 (C=O), 1608 (C=N) cm-1. 1H NMR (300 MHz, DMSO-d6): 0.93 (t, J = 5.25 Hz, 3H, CH3), 2.54 (q, J = 7.7 Hz, 2H, CH2), 3.3 (s, 6H, diMe), 5.64 (s, 3H, N3-CH3), 7.54 (t, J = 7.8 Hz, 1H, Ar), 7.44 (d, J = 8.8 Hz, 1H, Ar), 7.63 (d, J = 8.1 Hz, 1H, Ar), 7.93 (s, 1H, Ar). 13C NMR (75.5 MHz, DMSO-d6): 10.4 (CH3), 11.6 (CH2), 21.6 (CH3-N3), 28.3 (CH3, heterocycle), 29.8 (CH3, heterocycle), 51.2 (C, heterocycle), 122.7 (CH, Ar), 124.9 (BrC, Ar), 125.4 (CH, Ar), 129.2 (CH, Ar), 129.6 (CH, Ar), 132.6 (C, Ar), 161.6 (C=N), 166.2 (S-C=N), 178.2 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-3-methyl-5-phenylthiazolidin-4-one (2l)
The washing in ethanol afforded yellow powders, yield = 90%. M.p. (°C): 128-130. Rf: 0.66 (Toluene/ethyl acetate 8:2). IR (KBr): 1709 (C=O), 1619 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 1.02 (t, J = 7.4 Hz, 3H, CH3), 2.22 (s, 3H, CH3), 2.93 (q, J = 7.5 Hz, 2H, CH2), 5.43 (s, 1H, CH heterocycle), 7.33 (m, 6H, Ar), 7.64 (d, J = 8.0 Hz, 1H, Ar-Br), 7.84 (d, J = 8.0 Hz, 1H, Ar-Br), 7.93 (s, 1H, Ar-Br). 13C NMR (100 MHz, DMSO-d6): 11.6 (CH3), 13.8 (CH2), 20.7 (CH3-N3), 26.3 (CH, heterocycle), 122.0 (CH, Ar), 125.2 (BrC, Ar), 125.6 (CH, Ar), 128.1 (CH, Ar), 128.2 (CH, Ar), 128.4 (CH, Ar), 128.8 (CH, Ar), 130.7 (CH, Ar), 132.3 (C, Ar), 137.0 (C, Ar), 138.7 (C=N), 163.6 (S-C=N), 175.1 (C=O).
2-((1-(3-bromophenyl)propilidene)hidrazono)-3-phenylthiazolidin-4-one (2m)
The washing in ethanol afforded yellow powders, yield = 61%. M.p. (°C): 168-170. Rf: 0.58 (Hexane/ethyl acetate 8:2). IR (KBr): 1720 (C=O), 1602 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 0.84 (t, J = 7.4 Hz, 3H, CH3), 2.63 (q, J = 8.8 Hz, 2H, CH2), 4.14 (s, 2H, CH2 heterocycle), 7.42 (m, 6H, Ar), 7.63 (d, J = 8.0 Hz, 1H, Ar-Br), 7.73 (d, J = 8.0 Hz, 1H, Ar-Br), 7.93 (s, 1H, Ar-Br). 13C NMR (100 MHz, DMSO-d6): 11.3 (CH3), 21.0 (CH2), 32.3 (CH2 heterocycle), 122.0 (CH, Ar), 125.7 (BrC, Ar), 127.9 (CH, Ar), 128.4 (CH, Ar), 128.8 (CH, Ar), 128.9 (CH, Ar), 130.7 (CH, Ar), 132.5 (CH, Ar), 135.0 (C, Ar), 138.6 (N3-C, Ar), 164.2 (C=N), 165.3 (S-C=N), 171.8 (C=O).
2-((1-(3-bromophenyl)propilidene)hidrazono)-5-methyl-3-phenylthiazolidin-4-one (2n)
Recrystallization in toluene afforded yellowish crystals, yield = 68%. M.p. (°C): 141-143. Rf: 0.69 (Hexane/ethyl acetate 8:2). IR (KBr): 1724 (C=O), 1605 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 0.84 (t, J = 7.4 Hz, 3H, CH3), 1.63 (d, J = 7.2 Hz, 3H, CH3), 2.6 (q, J = 7.6 Hz, 2H, CH2), 4.44 (q, J = 7.2 Hz, 1H, CH heterocycle), 7.33 (m, 5H, Ar), 7.54 (t, J = 7.8 Hz, 1H, Ar-Br), 7.63 (d, J = 7.0 Hz, 1H, Ar-Br), 7.75 (d, J = 7.6 Hz, 1H, Ar-Br), 7.9 (s, 1H, Ar-Br). 13C NMR (100 MHz, DMSO-d6): 11.3 (CH3), 18.7 (CH2), 20.9 (CH3), 41.3 (CH heterocycle), 122.0 (CH, Ar), 125.7 (BrC, Ar), 127.9 (CH, Ar), 128.5 (CH, Ar), 128.8 (CH, Ar), 128.9 (CH, Ar), 130.7 (CH, Ar), 132.5 (CH, Ar), 135.0 (C, Ar), 138.6 (N3-C, Ar), 162.9 (C=N), 165.3 (S-C=N), 174.8 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-5-ethyl-3-phenylthiazolidin-4-one (2o)
The washing in ethanol afforded yellow powders, yield = 90%. M.p. (°C): 121-123. Rf: 0.68 (Toluene/ethyl acetate 8:2). IR (KBr): 1731 (C=O), 1603 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 0.83 (t, J = 7.4 Hz, 3H, CH3), 1.03 (t, J = 7.0 Hz, 3H, CH3), 2.04 (m, 2H, CH2), 2.62 (q, J = 7.3 Hz, 2H, CH2), 4.43 (t, J = 5.4 Hz, 1H, CH heterocycle), 7.33 (m, 6H, Ar), 7.62 (d, J = 8.8 Hz, 1H, Ar-Br), 7.72 (d, J = 8.0 Hz, 1H, Ar-Br), 7.91 (s, 1H, Ar-Br). 13C NMR (100 MHz, DMSO-d6): 10.1 (CH3), 11.3 (CH3), 21.0 (CH2), 25.6 (CH2), 48.2 (CH heterocycle), 122.0 (CH, Ar), 125.7 (BrC, Ar), 127.9 (CH, Ar), 128.5 (CH, Ar), 128.8 (CH, Ar), 129.9 (CH, Ar), 130.7 (CH, Ar), 132.5 (CH, Ar), 134.9 (CH, Ar), 138.6 (N3-C, Ar), 162.7 (C=N), 165.5 (S-C=N), 173.9 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-5-isopropyl-3-phenylthiazolidin-4-one (2p)
Recrystallization in toluene afforded yellowish crystals, yield = 67%. M.p. (°C): 157-159. Rf: 0.55 (Toluene/ethyl acetate 8:2). IR (KBr): 1725 (C=O), 1566 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 0.82 (m, 6H, CH3), 1.02 (t, J = 7.6 Hz, 3H, CH3), 2.63 (q, J = 5.3 Hz, 2H, CH2), 3.12 (m, 1H, CH isopropyl), 4.52 (d, J = 3.8 Hz, 1H, CH heterocycle), 7.42 (m, 6H, Ar), 7.63 (d, J = 7.6 Hz, 1H, Ar-Br), 7.84 (d, J = 7.9 Hz, 1H, Ar-Br), 7.93 (s, 1H, Ar-Br). 13C NMR (100 MHz, DMSO-d6): 10.9 (CH3), 17.4 (CH3), 20.8 (CH2), 26.3 (CH, diMe), 53.9 (CH heterocycle), 122.0 (CH, Ar), 125.7 (BrC, Ar), 127.9 (CH, Ar), 129.3 (CH, Ar), 129.5 (CH, Ar), 130.1 (CH, Ar), 130.9 (CH, Ar), 132.9 (CH, Ar), 135.5 (CH, Ar), 138.7 (C, Ar), 139.2 (CH, Ar), 163.0 (C=N), 165.1 (S-C=N), 172.7 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-5,5-dimethyl-3-phenylthiazolidin-4-one (2q)
Recrystallization in toluene afforded yellowish crystals, yield = 40%. M.p. (°C): 122-124. Rf: 0.56 (Hexane/ethyl acetate 8:2). IR (KBr): 1732 (C=O), 1606 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 0.85 (t, J = 7.4 Hz, 3H, CH3), 1.63 (s, 6H, CH3), 2.63 (q, J = 7.5 Hz, 2H, CH2), 7.42 (m, 6H, Ar), 7.63 (d, J = 7.6 Hz, 1H, Ar-Br), 7.72 (d, J = 8.0 Hz, 1H, Ar-Br), 7.93 (s, 1H, Ar-Br). 13C NMR (100 MHz, DMSO-d6): 11.2 (CH3), 20.9 (CH2), 27.8 (CH3, heterocycle), 50.8 (C, heterocycle), 122.0 (CH, Ar), 125.7 (BrC, Ar), 128.0 (CH, Ar), 128.6 (CH, Ar), 128.8 (CH, Ar), 130.7 (CH, Ar), 132.5 (CH, Ar), 135.0 (CH, Ar), 138.5 (C, Ar),161.7 (C=N), 165.3 (S-C=N), 177.2 (C=O).
2-((1-(3-bromophenyl)propylidene)hidrazono)-3,5-diphenylthiazolidin-4-one (2r)
The washing in ethanol afforded yellow powders, yield = 61%. M.p. (°C): 183-185. Rf: 0.67 (Hexane/ethyl acetate 8:2). IR (KBr): 1728 (C=O), 1602 (C=N) cm-1. 1H NMR (400 MHz, DMSO-d6): 0.93 (t, J = 7.2 Hz, 3H, CH3), 2.63 (d, J = 7.6 Hz, 2H, CH2), 5.62 (s, 1H, CH heterocycle), 7.42 (m, 11H, ArH), 7.72 (d, J = 7.6 Hz, 1H, Ar-Br), 7.93 (s, 1H, Ar-Br). 13C NMR (100 MHz, DMSO-d6): 11.3 (CH3), 21.0 (CH2), 50.2 (CH, heterocycle), 122.0 (CH, Ar), 125.7 (BrC, Ar), 128.0 (CH, Ar), 128.4 (CH, Ar), 128.6 (CH, Ar), 128.9 (CH, Ar), 130.7 (CH, Ar), 132.6 (C, Ar), 135.0 (CH, Ar), 136.7 (CH, Ar), 138.5 (CH, Ar), 162.4 (C=N), 165.8 (S-C=N), 172.7 (C=O).
5-phenyl-2-[2-{[4-(trifluoromethyl)phenyl]methylidene}hydrazin-1-ylidene]-1,3-thiazolidin-4-one (3a)
White crystals; Yield 43%; m.p.(ºC) 212-215; Rf: 0.61 (Toluene / ethyl acetate 7:3). IR (KBr, cm-1): 2943.43 (NH), 1723.06 (C=O), 1637.62 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 5.48 (s, 1H, CH thiazolidin-4-one), 7.35-7.39 (m, 5H, Ar), 7.81 (d, J = 8 Hz, 2H, Ar), 7.96 (d, J = 7.2 Hz, 2H, Ar), 8.55 (s, 1H, HC=N), 12.10 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 51.9 (C, thiazolidin-4-one), 122.7 (CF3), 125.7 (C, Ar), 126.2 (C, Ar), 126.2 (C, Ar), 128.7 (C, Ar), 128.9 (C, Ar), 129.4 (C, Ar), 137.3 (N=C-S), 138.6 (C=N), 155.3 (C=O).
5-methyl-2-[2-{[4-(trifluoromethyl)phenyl]methylidene}hydrazin-1-ylidene]-1,3-thiazolidin-4-one (3b)
White crystals; Yield 45%; m.p.(ºC) 239-241; Rf: 0.71 (Toluene / ethyl acetate 7:3). IR (KBr, cm-1): 2939.54 (NH), 1727.77 (C=O), 1641.83 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 1.51 (d, J = 7.2 Hz, 3H, CH3), 4.23 (d, J = 7.2 Hz, 1H, CH thiazolidin-4-one), 7.83 (d, J = 8.4 Hz, 2H, Ar), 7.96 (d, J = 8.4 Hz, 2H, Ar), 8.51 (s, 1H, HC=N), 12.05 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 16.7 (C, CH3), 42.3 (C, thiazolidin-4-one), 122.7 (CF3), 125.4 (C, Ar), 125.7 (C, Ar), 128.2 (C, Ar), 130.0 (C, Ar), 130.3 (C=N), 137.3 (N=C-S), 154.9 (C=O).
2-[2-{[4-(trifluoromethyl)phenyl]methylidene}hydrazin-1-ylidene]-1,3-thiazolidin-4-one (3c)
White crystals; Yield 40%; m.p.(ºC) 251-254; Rf: 0.45 (Toluene / ethyl acetate 7:3). IR (KBr, cm-1): 2941.46 (NH), 1710.55 (C=O), 1644.05 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 3.92 (s, 2H, thiazolidin-4-one), 7.83 (d, J = 8.4 Hz, 2H, Ar), 7.96 (d, J = 8 Hz, 2H, Ar), 8.50 (s, 1H, HC=N), 12.08 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 33.1 (C, thiazolidin-4-one), 122.7 (CF3), 125.4 (C, Ar), 125.7 (C, Ar), 128.2 (C, Ar), 130.0 (C, Ar), 130.3 (N=C-S), 138.1 (C=N), 154.9 (C=O).
5-ethyl-2-[2-{[4-(trifluoromethyl)phenyl]methylidene}hydrazin-1-ylidene]-1,3-thiazolidin-4-one (3d)
White crystals; Yield 65%; m.p. (ºC) 235-237; Rf: 0.79 (Toluene / ethyl acetate 7:3). IR (KBr, cm-1): 2965.93 (NH), 1714.07 (C=O), 1641.98 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 0.96 (t, J = 7.2 Hz, 3H, CH3), 1.76-1.86 (m, 1H, CH2), 1.94-2.02 (m, 1H, CH2), 4.26 (q, J = 8 Hz, 1H, CH thiazolidin-4-one), 7.82 (d, J = 8 Hz, 2H, Ar), 7.97 (d, J = 8 Hz, 2H, Ar), 8.51 (s, 1H, HC=N), 12.07 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 10.3 (CH3), 25.4 (CH2), 49.3 (C, thiazolidin-4-one), 122.7 (CF3), 125.4 (C, Ar), 125.7 (C, Ar), 128.2 (C, Ar), 130.0 (C, Ar), 130.3 (N=C-S), 138.0 (C=N), 155.0 (C=O).
5-(propan-2-yl)-2-[2-{[4-(trifluoromethyl)phenyl]methylidene}hydrazin-1-ylide ne]-1,3-thiazolidin-4-one (3e)
White crystals; Yield 65%; m.p.(ºC) 203-205; Rf: 0.70 (Toluene / ethyl acetate 7:3). IR (KBr, cm-1): 2965.62 (NH), 1722.03 (C=O), 1637.52 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 0.88 (d, J = 6.4 Hz, 3H, CH3), 1.00 (d, J = 6.8 Hz, 3H, CH3), 2.47-2.50 (m, 1H, CH), 4.32 (d, J = 4 Hz, 1H, thiazolidin-4-one), 7.82 (d, J = 7.6 Hz, 2H, Ar), 7.80 (d, J = 8 Hz, 2H, Ar), 8.50 (s, 1H, HC=N), 12.08 (s, 1H, NH). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 16.4 (CH3), 20.5 (CH3), 29.9 (CH), 55.2 (C, thiazolidin-4-one), 122.7 (CF3), 125.7 (C, Ar), 128.2 (C, Ar), 131.0 (N=C-S), 138.2 (C=N), 154.7 (C=O).
3-methyl-5-phenyl-2-[2-{[4-(trifluoromethyl)phenyl]methylidene}hydrazin-1-ylidene]-1,3-thiazolidin-4-one (3f)
White crystals; Yield 40%; m.p.(ºC) 245-248; Rf:0.62 (Toluene / ethyl acetate 7:3). IR (KBr, cm-1): 2943.43 (NH), 1723.06 (C=O), 1637.62 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 3.33 (s, 3H, CH3), 5.53 (s, 1H, thiazolidin-4-one) 7.35-7.40 (m, 5H, Ar), 7.82 (d, J = 7,6 Hz, 2H, Ar), 7.97 (d, J = 7.6 Hz, 2H, Ar), 8.56 (s, 1H, HC=N). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 30.9 (C, CH3), 51.2 (C, thiazolidin-4-one), 122.7 (C, Ar), 125.4 (C, Ar), 125.7 (CF3), 125.7 (C, Ar), 128.2 (C, Ar), 128.4 (C, Ar), 128.9 (C, Ar), 129.8 (C, Ar), 130.1 (C, Ar), 130.4 (C, Ar), 136.6 (C, Ar), 137.9 (C, Ar), 155.3 (N=C-S), 64.8 (C=N), 174.7 (C=O).
3,5-dimethyl-2-[2-{[4-(trifluoromethyl)phenyl]methylidene}hydrazin-1-ylidene]-1,3-thiazolidin-4-one (3g)
White crystals; Yield 42%; m.p.(ºC) 114-118; Rf: 0.75 (Toluene / ethyl acetate 7:3). IR (KBr, cm-1): 2939.54 (NH), 1727.77 (C=O), 1641.83 (C=N). 1H NMR (300 MHz, DMSO-d6), δ, ppm: 1.53 (d, J = 6.9 Hz, 3H, CH3), 3.19 (s, 3H, CH3), 4.29 (q, J = 7.1 Hz, 1H, thiazolidin-4-one), 7.83 (d, J = 7.8 Hz, 2H, Ar), 7.98 (d, J = 7.5 Hz, 2H, Ar), 8.60 (s, 1H, HC=N). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 18.6 (C, CH3), 29.5 (C, CH3), 41.4 (C, thiazolidin-4-one), 125.8 (CF3), 125.7 (C, Ar), 128.3 (C, Ar), 137.9 (C, Ar), 156.2 (C=N), 165.1 (N=C-S), 175.3 (C=O).
3-methyl-2-[2-{[4-(trifluoromethyl)phenyl]methylidene}hydrazin-1-ylidene]-1,3-thiazolidin-4-one (3h)
White crystals; Yield 45%; m.p.(ºC) 156-158; Rf: 0.70 (Toluene / ethyl acetate 7:3). IR (KBr, cm-1): 2941.46 (NH), 1710.55 (C=O), 1644.05 (C=N). 1H NMR (300 MHz, DMSO-d6), δ, ppm: 3.18 (s, 3H, CH3), 3.99 (s, 2H, thiazolidin-4-one), 7.84 (d, J = 8.1 Hz, 2H, Ar), 7.99 (d, J = 7,5 Hz, 2H, Ar), 8.61 (s, 1H, HC=N). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 29.4 (CH3), 32.2 (CH2), 38.7 (C, thiazolidin-4-one), 125.7 (CF3), 125.8 (C, Ar), 128.3 (C, Ar), 138.0 (C, Ar), 156.2 (N=C-S), 166.5 (C=N), 172.3 (C=O).
5-ethyl-3-methyl-2-[2-{[4-(trifluoromethyl)phenyl]methylidene}hydrazin-1-ylidene]-1,3-thiazolidin-4-one (3i)
White crystals; Yield 70%; m.p.(ºC) 95.6- 98.9; Rf: 0.62 (Toluene / ethyl acetate 7:3). IR (KBr, cm-1): 2965.93 (NH), 1714.07 (C=O), 1641.98 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 0.83 (t, J = 7.4 Hz, 1H, CH3), 0.96 (t, J = 7.2 Hz, 1H, CH3), 1.03-1.09 (m, 1H, CH3), 1.44-1.62 (m, 1H, CH2), 1.77-2.07 (m, 1H, CH2), 3.19 (s, 3H, CH3), 4.31 (q, J = 4,1 Hz,1H thiazolidin-4-one), 7.84 (d, J = 8.4 Hz, 2H, Ar), 7.99 (d, J = 8 Hz, 2H, Ar), 8.61 (s, 1H, HC=N). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 10.4 (CH3), 25.6 (CH2), 30.9 (CH3), 49.4 (C, thiazolidin-4-one), 125.4 (CF3), 125.8 (C, Ar), 127.7 (C, Ar), 128.3 (C, Ar), 137.9 (C, Ar), 139.8 (C, Ar), 156.4 (N=C-S), 165.1 (C=N), 178.0 (C=O).
3-methyl-5-(propan-2-yl)-2-[2-{[4-(trifluoromethyl)phenyl]methylidene}hydrazin-1-ylidene]-1,3-thiazolidin-4-one (3j)
White crystals; Yield 50%; m.p.(ºC) 97.8-101; Rf: 0.72 (Toluene / ethyl acetate 7:3). IR (KBr, cm-1): 2965.62 (NH), 1722.03 (C=O), 1637.52 (C=N). 1H NMR (400 MHz, DMSO-d6), δ, ppm: 0.91 (d, J = 6.4 Hz, 3H, CH3), 1.07 (d, J = 6.8 Hz, 3H, CH3), 2.47-2.52 (m, 1H, CH), 4.47 (d, J = 4.4 Hz, 1H, thiazolidin-4-one), 7.89 (d, J = 8.4 Hz, 2H, Ar), 8.06 (d, J = 8.4 Hz, 2H, Ar), 8.68 (s, 1H, HC=N). 13C NMR (100 MHz, DMSO-d6), δ, ppm: 20.2 (CH3), 29.3 (CH), 30.9 (CH3), 54.0 (C, thiazolidin-4-one), 125.5 (CF3), 125.8 (C, Ar), 128.3 (C, Ar), 130.5 (C, Ar), 1388.3 (C, Ar), 164.9 (N=C-S), 173.8 (C=N), 177.9 (C=O).
Biological activity
Cytotoxicity in mouse L-929 fibroblasts: The active compounds were tested in vitro for determination of cytotoxic over L-929 cells using the alamarBlue® dye. Were used the same number of cells, time of the cell development and time of compound exposure used for the beta-galactosidase assay. The cells were exposed to compounds (dissolved in DMSO) at increasing concentrations (2.5 μg/mL until 160 μg/mL), starting at an IC50 value of the T. cruzi. The co mpounds were tested in quadruplicate. After 96 h of exposure, alamarBlue® was added and the absorbance at 570 and 600 nm was measured 6 h later. The cell viability was expressed as the percentage of difference in the reduction between treated and untreated cells CC50 values were calculated by linear interpolation and the selectivity index (SI) was determined based on the ratio between CC50 and IC50 values. Controls with uninfected cells, untreated infected cells, infected cells treated with BZD at 3.8 μM (positive control) or DMSO 1% were used.
Anti-T. cruzi activity (epimastigote): Epimastigotes (Dm28c strain) grown in LIT media were counted in a hemocytometer and then seeded at 106 cells/well into a 96-well plate. Compounds were dissolved in DMSO and then diluted in LIT “medium” in a serial dilution (1.23, 3.70, 11.11, 33.33 and 100 μg/mL) and added to respective wells, in triplicate. The final DMSO concentration in the plate was 1%. Plate was incubated for 5 days at 26 ºC, aliquots of each well were collected, and the number of viable parasites were counted in a Neubauer chamber and compared to untreated parasite culture. Inhibitory concentration for 50% (IC50) was calculated using nonlinear regression on Prism 4.0 GraphPad software. BZD were used as the reference drug.
Anti-T. cruzi activity (trypomastigotes): Metacyclic trypomastigotes were collected from the supernatant of infected LLC-MK2 cells and then seeded at 4 x 105 cells/well in RPMI-1640 medium. All compounds were dissolved in DMSO and then diluted in RPMI-1640 medium in a serial dilution (1.23, 3.70, 11.11, 33.33 and 100 μg/mL) and added to respective wells, in triplicate. The final DMSO concentration was 1%. Plate was incubated for 24 h at 37 ºC and 5% of CO2. Aliquots of each well were collected, and the number of viable parasites was counted in a Neubauer chamber. The percentage of inhibition was calculated in relation to untreated cultures. Cytotoxic concentration for 50% (CC50) was determined using nonlinear regression with Prism 4.0 GraphPad software. BZD were used as the reference drug
Anti-T. cruzi activity (amastigotes/trypomastigotes): T. cruzi (Tulahuen strain) expressing the Escherichia coli beta-galactosidase gene were grown on a monolayer of mouse L-929 fibroblasts. Cultures assayed for beta-galactosidase activity were grown in RPMI 1640 medium without phenol red plus 10% fetal bovine serum and glutamine. Ninety-six-well tissue culture microplates were seeded with L-929 fibroblasts at 4.0 x 103 per well in 80 µL and incubated overnight at 37 ºC, 5% CO2. Beta-galactosidase-expressing trypomastigotes were then added at 4.0 x 104 per well in 20 µL. After 2 h, the medium with trypomastigotes that have not penetrated in the cells was discarded and replaced by 200 µL of fresh medium. After 48 h, the medium was discarded again and replaced by 180 µL of fresh medium and 20 µL of test compounds dissolved in DMSO. Each compound was tested in quadruplicate. After 7 days culture development, chlorophenol red beta-D-galactopyranoside at 100 µM and Nonidet P-40 at 0.1% were added to the plates and incubated overnight, at 37 ºC. The absorbance was measured at 570 nm in an automated microplate reader. BZD at its IC50 (1 µg/µL = 3.81 µM) was used as positive control. The results are expressed as a percentage of parasite growth inhibition. Two independent experiments were performed.
Statistical analysis: To determine the statistical significance of each group in the in vitro experiments, the one-way ANOVA test and the Bonferroni correction for multiple comparisons were used. A P value < 0.05 was considered significant. The data are representative of at least two or three experiments run in triplicate.
This work was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico and Fundação de Amparo à Ciência e Tecnologia de Pernambuco (FACEPE). Ana Cristina Lima Leite is receiving a CNPq senior fellowship. Authors are thankful for the Departamento de Química Fundamental (DQF-UFPE) for recording the 1H NMR, 13C NMR, and IR spectra of compounds and Centro de Tecnologias Estratégicas do Nordeste (CETENE) for recording LCMS of all compounds. All authors declare no competing financial interest.
- WHO, Chagas disease (American trypanosomiasis).
- Pérez-Molina JA, Molina I (2018) Chagas disease. Lancet 391: 82-94. [Crossref]
- Schmunis GA, Yadon ZE (2010) Chagas disease: a Latin American health problem becoming a world health problem. Acta Trop 115: 14-21. [Crossref]
- Urbina JA (2009) New advances in the management of a long-neglected disease. Clin Infect Dis 49: 1685-1687. [Crossref]
- Fabiana S, Jelicks LA, Kirchhoff LV, Shirani J, Nagajyothi F (2013) Cardiol Rev 20: 53-65.
- Marin-Neto JA, Rassi A Jr, Avezum A Jr, Mattos AC, Rassi A, et al. (2009) The BENEFIT trial: testing the hypothesis that trypanocidal therapy is beneficial for patients with chronic Chagas heart disease. Mem Inst Oswaldo Cruz 104 Suppl 1: 319-324. [Crossref]
- Scarim CB, Jornada DH, Chelucci RC, de Almeida L, Dos Santos JL, et al. (2018) Current advances in drug discovery for Chagas disease. Eur J Med Chem 155: 824-838. [Crossref]
- Pinazo MJ, Muñoz J, Posada E, López-Chejade P, Gállego M, et al. (2010) Tolerance of benznidazole in treatment of Chagas' disease in adults. Antimicrob Agents Chemother 54: 4896-4899. [Crossref]
- Leite ACL, Santos LMF, Barbosa FF (2006) Biomed. Pharmacother 60: 121-126.
- de Oliveira FGB, de Oliveira MVC (2015) Bioorg. Med. Chem 23: 7478-7486.
- Andres CJ, Bronson JJ, D'Andrea SV, Deshpande MS, Falk PJ, et al. (2000) 4-Thiazolidinones: novel inhibitors of the bacterial enzyme MurB. Bioorg Med Chem Lett 10: 715-717. [Crossref]
- Küçükgüzel SG, Oruç EE, Rollas S, Sahin F, Ozbek A (2002) Synthesis, characterisation and biological activity of novel 4-thiazolidinones, 1,3,4-oxadiazoles and some related compounds. Eur J Med Chem 37: 197-206. [Crossref]
- Bondock S, Khalifa W, Fadda AA (2007) Synthesis and antimicrobial evaluation of some new thiazole, thiazolidinone and thiazoline derivatives starting from 1-chloro-3,4-dihydronaphthalene-2-carboxaldehyde. Eur J Med Chem 42: 948-954. [Crossref]
- Verma A, Saraf SK (2008) 4-thiazolidinone--a biologically active scaffold. Eur J Med Chem 43: 897-905. [Crossref]
- Aubé J (2012) Drug repurposing and the medicinal chemist. ACS Med Chem Lett 3: 442-444. [Crossref]
- Kaiser M, Maes L, Tadoori LP, Spangenberg T, Ioset JR (2015) Repurposing of the open access malaria box for kinetoplastid diseases identifies novel active scaffolds against trypanosomatids. J Biomol Screen 20: 634-645. [Crossref]
- Alvarez G, Varela J (2015) Antimicrob. Agents Chemother 59: 1398-1404.
- Alvarez G, Varela J, Márquez P, Gabay M, Arias Rivas CE, et al. (2014) Optimization of antitrypanosomatid agents: identification of nonmutagenic drug candidates with in vivo activity. J Med Chem 57: 3984-3999. [Crossref]
- GCS, UFD (2009) Antiinfect. Agents Med. Chem 8: 345-366.
- Magalhaes DRM, de Oliveira ADT, de Moraes PATG (2014) Eur. J. Med. Chem 75: 467-478.
- Du X, Guo C, Hansell E, Doyle PS, Caffrey CR, et al. (2002) Synthesis and structure-activity relationship study of potent trypanocidal thio semicarbazone inhibitors of the trypanosomal cysteine protease cruzain. J Med Chem 45: 2695-2707. [Crossref]
- Álvarez-Hernández DA, Franyuti-Kelly GA, Díaz-López-Silva R (2018) Gen. México 81: 154-164.
- Hernandes MZ, Cavalcanti SM, Moreira DR, de Azevedo Junior WF, Leite AC (2010) Halogen atoms in the modern medicinal chemistry: hints for the drug design. Curr Drug Targets 11: 303-314. [Crossref]
- de Moraes PATG, de Oliveira MB, Farias ES, de Oliveira MVC (2016) Eur. J. Med. Chem 121: 387-398.
- de Santana TI, Barbosa MO, Gomes PATM, da Cruz ACN, da Silva TG, et al. (2018) Synthesis, anticancer activity and mechanism of action of new thiazole derivatives. Eur J Med Chem 144: 874-886. [Crossref]
- de Oliveira GBF (2017) Eur. J. Med. Chem 141: 346-361.
- Jagodzinska M, Huguenot F, Candiani G, Zanda M (2009) Assessing the bioisosterism of the trifluoromethyl group with a protease probe. ChemMedChem 4: 49-51. [Crossref]
- CALA (2004) 337-341.
- Oprea TI, Gottfries J (1999) Toward minimalistic modeling of oral drug absorption. J Mol Graph Model 17: 261-274, 329. [Crossref]
- Veber DF, Johnson SR, Cheng HY, Smith BR, Ward KW, et al. (2002) Molecular properties that influence the oral bioavailability of drug candidates. J Med Chem 45: 2615-2623. [Crossref]
- Meanwell NA (2011) Improving drug candidates by design: a focus on physicochemical properties as a means of improving compound disposition and safety. Chem Res Toxicol 24: 1420-1456. [Crossref]







