Abstract
Over the past decades, attaching of carbon nanotube with metal oxide is an effective way to construct hybrid carbon with interesting new properties. MWCNT/MnO2/POAP nanocomposite thin film is synthesized using a pulsed potential electrodeposition technique on a graphite electrode. The electrochemical properties of the composite are evaluated by cyclic voltammetry and galvanostatic charge/discharge respectively. The results of cyclic voltammograms, and SEM demonstrate that the doping process is effective and nanocomposite has exciting potentials for high performance supercapacitors. The MWCNT/MnO2/POAP nanocomposite exhibits substantially improved conductivity, which is greater than that of it’s of pure POAP. The specific capacitance of the MWCNT/MnO2/POAP nanocomposite reaches 412 F g-1 at a current density of 0.5 Ag-1, which is significantly higher than that of pure POAP. An increase in electrochemical supercapacitor properties of the nanocomposite is due to highly dispersed MWCNT/MnO2 nanoparticles in the composite film, which improves the electric conductivity of the film electrode.
Key words
conducting polymer, manganese dioxide, electrochemical properties, nanocomposite, supercapacitor
Introduction
Conductive polymers (CPs) are active materials that can be used in various fields due to high electrical conductivity, ease of preparation, low cost, high chemical stability, and wide variety of applications in light-emitting, biosensor chemical sensor, separation membrane and electronic devices. CPs based supercapacitors have remarkable material properties in redox supercapacitor. In recent years, the researchers have focused on the combination of organic phases and inorganic mineral phases due to their high charge storage capabilities. CPs can be simply prepared electrochemically and have unique electrical conductivity, redox property, and good stability [1-5]. Owing to the remarkable material properties of conducting polymer, the development of novel composites has focused on the combination of organic phases and inorganic mineral phases in order to improve the performance of CPs based supercapacitors [6-9]. Changing of the morphology and surface area of these composites are expected to be utilized in supercapacitors to increase the conductivity, storage ability, specific capacitance, and creating excellent capacitance retention during cycling. In recent years, CPs based supercapacitors have attracted increasing attention due to their high charge storage capabilities [10-11]. For example, supercapacitors based on graphene-polyaniline derivative nanocomposite and manganese dioxide (MnO2) embedded poly pyrrole nanocomposites have been reported [12]. MnO2 and their derivative compounds have attracted special attention and are used in various application batteries, sensors and electrochemical supercapacitors [13-20]. There are increasing attentions in development of chemical methods for synthesis MnO2 nanoparticles to be used in fabrication of films and composites [21,22]. Metal oxides usually provide high modulus and strength at much higher temperature, which make them appropriate for several applications. Despite such features, individual metal oxides cannot perform all requirements to expand advanced technologies and to solve the world’s most immediate problems. Multi-walled carbon nanotubes (MWCNTs) have been commonly studied as electrode materials for electrochemical capacitors owing to their high surface area, inert, and electric conductance that are responsible for store and release energy by nanoscopic charge separation at electrochemical interface between an electrode and electrolyte [23,24]. For this reason, one field of research that has been attracting very quickly is the design, development and characterization of inorganic hybrid carbon-based nanostructures, generally consisting of metal oxides and CNTs. Binding CNTs with metal oxides cause to new functionalities in terms of electronic, optical and mechanical properties [25,26].
This strongly attracts us to modify conducting polymers with MWCNT/MnO2 for the enhancement of capacitive behaviors. In the present work, poly o-aminophenol (POAP) doped with MWCNT/MnO2 was synthesized via pulsed potential electrodeposition technique by one- step way. It used as electrode material for the construction of electrochemical supercapacitor in the aqueous electrolyte. The electrochemical properties of the polymer, as well as its capacitive behaviors were investigated. The specific capacitance of MWCNT/MnO2/POAP can reach high up to 412 Fg-1 at a current density of 0.5 Ag-1, which is attributed to the enhancement of the electrochemical properties of the polymer by MWCNT/MnO2.
Methods
Chemical experiments
The chemicals used in this work were obtained from Merck Chemical Co. as analytical grades. Solutions were prepared using doubly distilled water. The electrochemical experiments were performed using a conventional three-electrode cell powered by a μ-Autolabpotentiostat/galvanostat and a frequency response analyzer (Metrohm, model 12/30/302, The Netherlands). Graphite (G) with an area of 0.0675cm2 was used as the working electrode and a platinum foil as auxiliary electrode, while Ag/AgCl was used as the working electrode. The morphologies of the electrosynthesized conductive polymer obtained were analyzed using field emission scanning electron micro analyzer model KYKY-EM3200.1.
MnO2/(MWCNT) synthesis
MnO2 was synthesized by redox reaction of 0.1 M KMnO4 and ethanol in aqueous medium. In a typical synthesis in aqueous medium, certain amounts of N-Cetyle-N, N, N-trimethylammonium bromide (CTAB), MWCNT nanopartical and KMnO4 solution were mixed with ethanol solution and stirred continuously for 8h. A dark-brown precipitate thus formed, was washed several times with distilled water, centrifuged for remove potassium ions and then it was dried at 500°C for 10 h.
Preparation of POAP/MnO2/MWCNT
POAP was synthesized using a pulsed potential electrodeposition technique on the cleaned graphite disk electrode by cyclic voltammetry from an aqueous solution containing 0.02M o-aminophenol, 0.02M sodium dodecyl sulfate, 0.5 M perchloric acid for 50 cycles. Before each experiment, the bare graphite electrode was polished with 0.05 µm ∝-Al O on a piece of leather and thoroughly washed with distilled water and then rinsed ultrasonically in ethanol and water for 5 min. The MnO/(MWCNT)/(POAP) electrode was fabricated by the same procedure as graphite/POAP electrode, but with addition of 2% MnO/MWCNTs. The MnO/MWCNTs (1% (w/w)) were dispersed into a solution of 5 mM SDS by ultrasonic agitation for 30 min to obtain a homogeneous suspension. SDS was used as an additive in order to suspend MWCNT and MnO particles and improve the stability and electroactivity of the resulting films.
Results and discussion
Figure 1 shows scanning electron micrographs (SEM) images of the pure POAP, MnO2/MWCNT, and POAP/MnO2/MWCNT thin films. As can be seen, the morphologies of POAP, MnO2/MWCNT, and POAP/MnO2/MWCNT are quite different from each other and the surface morphology is changed and gives a clear evidence for the successful fabrication of nanocomposite on the surface. So it can be concluded that the introduction of MnO2/MWCNT to POAP changes the microstructure of the polymer.

Figure 1. SEM of pure POAP (a), MnO2/ MWCNT (b), and POAP / MnO2/ MWCNT (c) obtained via the electrodeposition.
For further investigation, UV-vis spectrum of POAP, and POAP/MnO/MWCNT nanocomposite were recorded (Figure 2). As seen in figure 2 the shapes of UV spectra polymer showed strong absorption band in the UV region. It can be seen by addition of MnO/MWCN, intensity of the peaks decreased due to interaction between MnO/MWCNT and POAP. This is because of the metal oxides and CNTs presence in composite film. It reveals that attaching of carbon nanotube with metal oxide nanoparticles in the polymer matrix, a considerable change is measured in the absorption spectrum, indicating interaction of metal nanoparticles with the polymer chain.
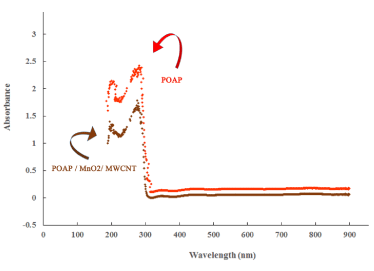
Figure 2. UV-vis spectrum of the POAP, and POAP / MnO2/ MWCNT.
Figure 3 shows cyclic voltammetry (CV) curves of POAP, MnO2/MWCNT, and POAP/MnO2/MWCNT electrodes with scan rates of 25 mVs-1. As can be seen the peak currents at POAP/MnO2/MWCNT are obviously higher than those at POAP. This is because of MnO2/MWCNT doping into the POAP during the polymerization process, which functions as the redox active catalyst of the polymer. The comparison of the current between POAP electrode and other modified electrodes shows that composite has the large background and identified peak currents. An enhancement in current of composite CV confirms a strong effect of MnO2/MWCNT in POAP matrix polymer.
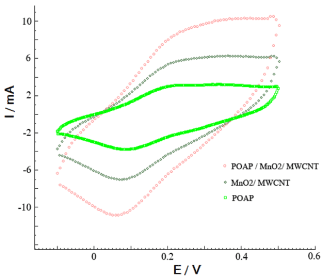
Figure 3. Cyclic voltammograms of POAP (a), MnO2/ MWCNT (b), and POAP / MnO2/ MWCNT (c) electrodes in 0.1M H2SO4 at the sweep rate of 25 mV/s.
The electrical conductivity of POAP and POAP/MnO2/MWCNT were measured by direct four probe method at room temperature. The expected conductivity is due to the incorporation of MnO2/MWCNT nanoparticles in the polymer matrix. After the POAP nanoparticles were modified with MnO2/MWCNT, the conductivity of the MnO2/MWCNT/POAP nanocomposite increases from 0.15 to 0.78 S/cm.
Figure 4 shows the CVs of POAP/MnO2/MWCNT at varying scan rates. Two pairs of redox peaks appear on the CVs of POAP/MnO2/MWCNT (Figure 3), and it increases especially at high potential scan rates. Moreover, it should be noted that the anodic and the cathodic peak potentials change to a more positive and negative direction, respectively, with the increase in the potential scan rate. As is evident from Figure 4 for POAP/MnO2/MWCNT, the shift in the peak potential is smaller, indicating that the POAP/MnO2/MWCNT has a better electrochemical reversibility.
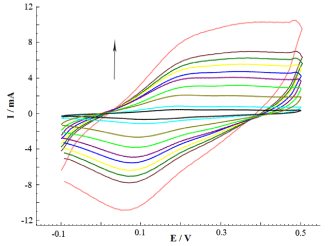
Figure 4. Cyclic voltammograms of POAP/ MnO2/ MWCNT in 0.1 molL-1 H2SO4 at different scan rates of 5, 10, 30, 50, 70, 90, 110, 130, 140, and 150mVs-1.
The specific capacitance of the electrodes can be calculated from the CV curves by using the following equation [27]:
 (1)
(1)
Where i, s, and m are average current, scan rate, and mass active electrode, respectively. The specific capacitance values of the pure POAP, MnO/MWCNT, and POAP/MnO/MWCNT samples are calculated as 209, 118, and 412 F/g, respectively. This outcomes reflect that the decoration of MnO/MWCNT onto the POAP supports induces a synergistic effect, enhances the electrical conductivity, and provides a large surface area more active sites available for formation of charge transfer in the composite electrode. The calculated capacitance of POAP, MnO/MWCNT, and POAP/MnO/MWCNT are shown in Figure 5:
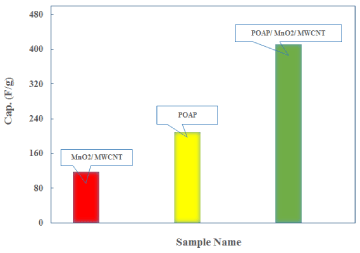
Figure 5. Bar diagram of calculated capacitance values of the POAP, MnO2/ MWCNT, and POAP/ MnO2/ MWCNT samples.
The galvanostatic charge/discharge plots of POAP and POAP/MnO/MWCNT at a constant current density of 0.5 Ag-1 are displayed in Figure 6. The charge/discharge experiments for all the electrode materials were carried out within range of 0.2 – 0.8 V. As Figure 6 shows, during the charging and discharging steps, the charge curves of POAP/MnO/MWCNT composite and POAP electrodes are almost symmetric to its corresponding discharge correlate, denoting the characteristic of a good capacitance. The specific capacitance values of the active material (F g-1) was evaluated from the charge/discharge by using the following equation:
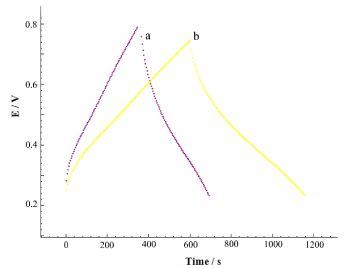
Figure 6. Charge/discharge curves of POAP (a), and POAP/ MnO2/ MWCNT (b) at a current density of 0.5 Ag-1 in 0.1M H2SO4.
 (2)
(2)
Where I, ∆t, m, and ∆V are current (A), discharge time (s), mass of the active material (g), and potential difference (V) respectively. POAP/MnO/MWCNT gives a specific capacitance of 476 F g-1 at a current density of 0.5 Ag-1, which is higher than those of POAP (249 F g-1). The significant increase in specific capacitance of POAP/MnO/MWCNT is imputed to the introduction of MnO/MWCNT during the polymerization process.
Conclusion
The POAP/MnO/MWCNT nanocomposite was synthesized using a pulsed potential electrodeposition technique by a simple one–step method. Scanning electron micrographs and UV-vis spectrum were utilized for characterization of the manufactured composites, indicating presence of MnO/MWCNT in the POAP matrix which is in agreement with the electrochemical results. The synthesized nanocomposite had a higher electric conductivity, and faster charge-transfer than the pure polymer film. The electrical conductivity of synthesized nanocomposite and pure polymer are 0.15, and 0.78 S/cm, respectively. Electrochemical properties of films were investigated by using cyclic voltammetry. Excellent electrochemical capacitor property was observed for nanocomposite against pure polymer. A specific capacitance as high as 476 Fg-1 was obtained at a constant current density of 0.5 Ag-1.
Acknowledgement
The author are gratefully to Payame Noor University and Iranian Nano Council for support this work.
References
- Mohammad Shiri H, Ehsani A (2016) A simple and innovative route to electrosynthesis of Eu2O3 nanoparticles and its nanocomposite with p-type conductive polymer: Characterisation and electrochemical properties. J Colloid Interface Sci 473: 126-131. [Crossref]
- Jarjes ZA, Samian MR, Ghani SA (2015) Arabian J Chem. 8: 726-731.
- Ajami N, Ehsani A, Babaei F, Safari R (2016) J Mol Liq 215: 24-30.
- Wang L (2016) Sens Actuators A 247: 199-205.
- Lange U, Mirsky VM (2012) Electroanalytical measurements without electrolytes: conducting polymers as probes for redox titration in non-conductive organic media. Anal Chim Acta 744: 29-32. [Crossref]
- Zhao H, Du A, Ling M, Battaglia V, Liu G (2016) Electrochim Acta 209: 159-162.
- Song Y, Jiang L, Qi W, Lu C, Zhu X (2012) Synth. Met. 162: 368-374.
- Lokhande VC, Lokhande AC, Lokhande CD, Kim JH, Ji T (2016) J Alloys Compd. 682: 381-403.
- Suleman M, Kumar Y, Hashmi SA (2015) Mater Chem Phys 161-171.
- Shayeh JS, Siadat SOR, Sadeghnia M, Niknam K, Rezaei M, et al. (2016) J Mol Liq 489-494.
- Zhou H, Han G (2016) Electrochim Acta 448-455.
- Basnayaka PA, Ram MK, Stefanakos EK (2013) Electrochim Acta 92: 376-382.
2021 Copyright OAT. All rights reserv
- Hill LI, Portal R, Verbaere A, Guyomard D (2001) Electrochemical and Solid State Letters 4: A180.
- Luo XL, Xu JJ, Zhao W, Chen HY (2004) Anal Chim Acta 512: 57.
- Broughton JN, Brett MJ (2005) Electrochim Acta 50: 4814.
- Kim K, Park S (2012) J Solid State Electrochem 16: 2751.
- Sumboja A, Foo C, Yan J (2012) J Mater Chem 22: 23921.
- Kim K, Park S (2012) Synth Met 162: 2107.
- Zhou S, Mo S, Zou W (2011) Synth Met 161: 1623.
- Li X, Gan W, Zheng F (2012) Synth Met 162: 953.
- Zhao Y, Jiang P, Xie SS (2013) J Power Sources 239: 393-8.
- Dubal DP, Holze R (2013) Energy 51: 407-12.
- Cao X, Shi Y, Shi W, Lu G, Huang X, et al. (2011) Preparation of novel 3D graphene networks for supercapacitor applications. Small 7: 3163-3168. [Crossref]
- Huang X, Yin Z, Wu S, Qi X, He Q, et al. (2011) Graphene-based materials: synthesis, characterization, properties, and applications. Small 7: 1876-1902. [Crossref]
- Wu J, Zhang H, Zhang Y, Wang X (2012) Mechanical and thermal properties of carbon nanotube/ aluminium composites consolidated by spark plasma sintering, Mater Des 41: 344-348.
- Michálek M, Sedlácek J, Parchoviansky M, Michálková M, Galusek D (2014) Ceram Int 40: 1289-1295.
- AI I, YS K, SM P, JH K, H I (2011) J Power Sources 196: 2393-7.






