Abstract
Drug discovery and development (DDD) is a collaborative, dynamic process of great interest to researchers, but an area where there is a lack of formal training. The Drug Development Educational Program (DDEP) at New York University was created in 2012 to stimulate an improved, multidisciplinary DDD workforce by educating early stage scientists as well as a variety of other like-minded students. The first course of the program emphasizes post-compounding aspects of DDD; the second course focuses on molecular signaling pathways. In five years, 196 students (candidates for PhD, MD, Master’s degree, and post-doctoral MD/PhD) from different schools (Medicine, Biomedical Sciences, Dentistry, Engineering, Business, and Education) completed the course(s). Pre/post surveys demonstrate knowledge gain across all course topics. 26 students were granted career development awards (73% women, 23% underrepresented minorities). Some graduates of their respective degree-granting/post-doctoral programs embarked on DDD related careers. This program serves as a framework for other academic institutions to develop compatible programs designed to train a more informed DDD workforce.
Keywords
career, drug development, drug discovery, education, graduate student, translation
Introduction
There is a revived optimism despite many challenges in drug discovery and development (DDD) [1-3]. DDD educational programs can influence growth and development of novel therapies and market transition [4]. Due to escalating complexity and feasibility barriers, there is a significant need for DDD educational programs. The median cost and time to develop a potential molecule from its discovery to approval is 2.6 billion dollars and 36 years, respectively [5,6]. Only 10 to 20% of health-related research projects reach human trials; only 6% of drugs that enter phase I trials reach approval status [7,8]. The new drug approval rate has remained low[9,10].Therefore, to promote successful translation in DDD, scientists need skills and training [11]. Few DDD training programs integrate aspects of both of discovery and development and expose students to opportunities and experts in the field [12].
In September 2012 with funding from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), we began enrollment for the Drug Development Educational Program (DDEP) at New York University (NYU). We aim to teach graduate and post-graduate students the essentials of DDD and inspire these motivated and talented individuals to get more involved in the translation of scientific discoveries and enter the DDD workforce as trained scientists, engineers, business analysts, and entrepreneurs. This improved, informed DDD workforce will be better equipped to address the nation’s biomedical and clinical research needs.
Our educational objectives were met, consistent with reported data from our two-year analysis [13]. Furthermore, we explored why students enrolled in each course, what they learned, and the course’s relevance to their career goals. We also traced the career paths of students who received DDEP’s career development awards. DDEP aims to teach essential skills and is the only known program of its kind within the Clinical and Translational Science Institutes (CTSIs).
Materials and methods
Description of course series
The NYUSOM DDEP was created in 2012 with support from NIDDK. Currently in its sixth year, the DDEP was initially comprised of a two-course series designed to highlight the essential and innovative features of the DDD process. Our goals are: 1) to teach a new generation of multidisciplinary students at the graduate and post-graduate levels about DDD; 2) to potentiate and expedite the translation of therapeutics and other applications, both novel and repurposed; and 3) to bridge the translational gap.
Enrollment is open to all NYU graduate and post-graduate students. The first course of the two-course series is Drug Development in a New Era (DDNE). This course is held in the fall term and focuses on the post-compounding aspects of DDD such as intellectual property, regulatory aspects, and marketing. The spring term course, Molecular Signaling in the Discovery and Development of Therapeutics (MSDDT), expands on molecular signaling pathways as potential sites for drug targets as well as viability testing (Table 1). Each course grants three credits. Classes are two hours in duration, where each begins with a lecture given by an expert in the field followed by an interactive discussion. Students are required to arrive prepared, having completed the assigned reading and coursework.
Table 1. Lecture topics
Drug Development in a New Era (DDNE) |
- Big Data, Supercomputing, Artificial Intelligence
- Biostatistics and Study Design: New Statistical Approaches
and the Application to New Drug Targets Including Metabolic Syndrome/Obesity
- Biotechnology/Entrepreneurship in Science
- Brown Adipogenesis as a Target for New Diabetes Drugs
- Challenges in Drug Discovery for the Treatment of Diabetic Complications
- Clinical Trials
- Case Study: Finding Fibromyalgia
- De-orphanization of GPCRs and Ligand Identification
- Epigenetic Basis of Metabolic Syndrome and Prenatal Environmental Exposures
and Design of Translational Animal Models to Bridge Biomarkers from Mouse to Man
and Targeted Drug Selection Prediction
- Finding the Missing Heritability Wide-Locus GWAS in Pharmacogenomics
- Fraud and Misconduct
- Genomic Medicine and Repurposing of Drugs
- Healthcare in the New Economy
- Human Subject Protection in Research
- Infliximab: How a TNF Inhibitor Advanced from Modest Beginnings to
Unforeseen Therapeutic Success
|
- Intellectual Property Relating to Clinical Data in Drug Development
- Modifiers of Gene Expression in Genetic Disease
and Measuring Effects on Phenotype
- Drug Development in Orphan Disease
- Lysosomal Storage Disorders: Novel Therapeutics
- Ethics in Clinical Trials, FDA and Conflicts of Interest, PDUFA
- Moving from Big Data to Better Models of Disease
and Drug Response
- New Diabetes and Obesity Drugs and
the FDA, Industry Drug Development
- Next Generation Data Mining in Pharmacogenomics
- Patenting Clinical Data
- Pharmaco-kid-netics: Pediatric Drug Development, an Industry Perspective
- Pharmacovigilance in Drug Safety, Application of Statistical
Data Mining Techniques to Monitor and Predict Drug Safety
- Phase I/II Developmental Trials
- Repurposing Failed Drugs to Create Successful
Medicines in Women's Health
- Drug Discovery and Development: From Target to IND and NDA
|
Molecular Signaling in the Development and Discovery of Therapeutics (MSDDT) (MSDDT) |
- Targeting the PI3k-Akt-mTOR Pathway
- Targeting Metabolic Disease - Primary Efficacy Endpoints for Cardiometabolic Trials
- Structured-Base Drug/Vaccine Design Targeting HIV /AIDS
- Startup Biotech: a First Person Perspective on the Risks
and Rewards of Starting Your Own Company
- Signaling Transduction and Signal Management in Pharmacovigilance
- RNAi for Drug Discovery
- Receptors and Drug Binding, Pharmacology of Autonomic Nervous System
- Ras/Cancer
- RAGE and Diabetic Complications and Therapeutics Approaches
- Protein Kinases as Targets for Drug Development
- Principles of G Protein Coupled Receptor signaling
- Preventative Cardiology
- Pharmacology of Addiction
- Personalized Medicine
- Opioid Receptor Heterodimerization in Analgesia and Addiction
- Nuclear Hormone Receptors
- Neuronal Control of Eating Behavior
- Molecular Signatures Disease
- Metabolism and Cancer
|
- Medicinal Chemistry
- Immunotherapy for Tauopathies
- Glycosaminoglycan Modulation of Signaling Pathways:
Implications for Drug Development
- Drug Distribution, Kinetics, Metabolism, and Cytochrome P450s
- Drug Addiction: Insights Obtained from Basic Science Research
- Discovery and Rational Development of an Antagonist to Phosphaturic Tumors
- Discovery and Rational Development of an
FGF23 Hormone Antagonist
- Diabetic Neuropathy trials and Choice of Endpoints
- Diabetes and Obesity
- Computational Drug Discovery
- Bivalent Approaches to Drug Discovery
- Approaches and Consideration for Biologic
Therapeutic Development - Targeting the FGF Pathway
- Aldose Reductase and Diabetes Complications
- Adenosine Receptor
- Immunomodulators: Discussion between
Developer and Industry Partner
- Macrocyclic Kinase Inhibitors
|
(Abbreviations (in alphabetical order): Acquired Immunodeficiency Syndrome (AIDS), Fibroblast Growth Factor (FGF), Food and Drug Administration (FDA), G-Protein-Coupled Receptor (GPCR), Genome-wide association study (GWAS), Human Immunodeficiency Virus (HIV), Investigational New Drug (IND), Mechanistic Target of Rapamycin (mTOR), New Drug Application (NDA), Phosphoinositide 3-kinase(PI3k), Prescription Drug User Fee Act (PDUFA), Protein Kinase B (Akt), Receptor for Advanced Glycation Endproduct (RAGE), RNA interference (RNAi), Tissue Necrosis Factor (TNF) )
DDNE (fall) course
The goal of the DDNE course is to provide an overview of the regulatory, economic, ethical, and business aspects of DDD from the preclinical phase through post-approval marketing and monitoring. Students are exposed to a wide range of topics including protocol planning, safety monitoring, cost and pricing analysis, and intellectual property. Students also learn how basic and clinical sciences, statistical analysis, business management, legal, and marketing departments converge in this interdependent, multidisciplinary process. At the end of the term, students are required to complete a final project that entails submission of a “mini” Investigational New Drug Application (IND) [7].
MSDDT (spring) course
In the MSDDT course, the principles of discovering and developing therapeutics in the lab as well as essential signaling pathways such as RAS and AKT are explored. Students learn about entity-specific paradigms that can help predict successful DDD trajectories and how to plan target selection via experimental testing. To ensure students are ready for the different scientific aspects of DDD, students are exposed to topics such as developing receptor and pathway networks, prediction models, new technologies, and challenges of designing animal models and clinical trials. Students are required to submit a final paper on research methods or approaches used to design therapy [8].
Student demographics
Demographic information, training level, and graduate school affiliation was collected from students who enrolled between Fall 2012 and Spring 2017.
Program evaluation
Pre/Post course surveys and analysis: To evaluate students’ self-reported contextual knowledge, each student completed a pre course survey on the first day of the course and a post course survey on the last day of the course. The surveys asked students to rate how well they understood each of the topics/knowledge items. The response options were: (1) nothing, (2) almost nothing, (3) some and (4) a great deal. Responses to all knowledge questions were summed to create a total knowledge score. Each survey also included a question about the relevance of the course in terms of career goals. The choices were: (1) not at all relevant, (2) only a little relevant, (3) somewhat relevant and (4) very relevant. IBM SPSS was used to analyze the differences between the pre and post course surveys. Due to small sample size and non-normally distributed data, Wilcoxon Ranked Sum Test was used for all data analyses.
Lecture ratings: On the last day of each course, students received surveys asking them to rate each of the lectures in 4 different domains: content, presentation, relevance, and overall. The options for each response were: (1) poor, (2) lower than expected, (3) satisfactory, (4) above expectations, (5) superior. The response to the question “Was the lecture free of commercial bias?” was also analyzed.
Free-response text: To capture more qualitative information, each survey contained free-response questions. In the pre course survey, students were asked, “What are the main things you hope to get out of this course?” They were also asked to explain their rating for the career relevance question. In the post course survey, students were asked, “What are the main take home points you got out of this course?”
IBM SPSS Text Analytics for Surveys was used to analyze the answers to free–response questions. The responses were grouped into categories for query and associated with keywords the students utilized in the response. We manually excluded words that contained components of search terms. Likewise, some responses that were not automatically grouped into the corresponding categories were grouped manually based on content. For the questions “What are the main things you hope to get out of this course” and “What are the main take home points you got out of this course”, Chi-square test was used to compare the differences between the pre and post course surveys.
Career development award: Enrolled students were eligible for a career development award. The goal of the award was to provide support for students to pursue activities to advance their careers. Uses included tuition remission, workshops, course/conference attendance, and publication support. Online search and telephone follow-up were used to track recipient’s career trajectories.
Results
Student demographics
From September 2012 through June 2017, 139 students completed the DDNE course, and 75 students completed the MSDDT course. A total of 196 students completed at least one course in the DDEP; 18 (9%) students completed both DDNE and MSDDT.
DDNE (fall) course
The 139 DDNE students were comprised as such: 48 (35%) post-doctoral PhD, 30 (22%) PhD or MD/PhD candidate, 29 (21%) MD/ Masters of Science in Clinical Investigation dual-degree candidate (MD/MSCI), 14 (10%) other Master’s degree candidate, 11 (8%) post-doctoral MD, and 7 (5%) faculty. 90 students (65%) were either enrolled or held positions at NYUSOM, 29 (21%) at NYU Sackler Institute of Graduate Biomedical Sciences, 7 (5%) at NYU Tandon School of Engineering, 5 (4%) at NYU Graduate School of Arts and Sciences (GSAS), 4 (3%) at NYU Stern School of Business, 3 (2%) at NYU College of Dentistry, and 1 (1%) at NYU Steinhardt School of Culture, Education, and Human Development (Figure1).
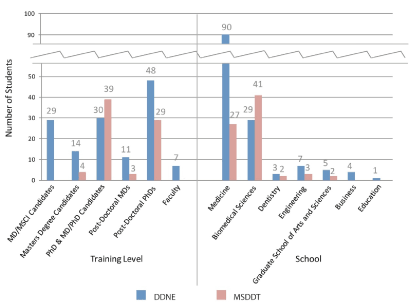
Figure 1. Student demographics. This bar graph shows the student demographic data: enrollment numbers, training levels, and affiliated schools for both DDNE and MSDDT courses.
MSDDT (spring) course
The 75 students who completed the MSDDT course were comprised as such: 39 (52%) PhD or MD/PhD candidate, 29 (39%) post-doctoral PhD, 4 (5%) Master degree candidate, and 3 (4%) post-doctoral MD. 41 students (55%) were either enrolled or held positions from NYU Sackler, 27 (36%) from NYUSOM, 3 (4%) from NYU Tandon, 2 (3%) from NYU GSAS, and 2 (3%) from NYU Dentistry (Figure1).
Program evaluation
DDNE (fall) course
Pre/Post course surveys: 139 students completed DDNE between September 2012 and June 2017. Of those, 127 (91%) completed the pre course survey, 98 (71%) completed a post course survey, and 89 (64%) completed both pre and post course surveys. Responses to all the individual knowledge questions, the total knowledge score, and the career relevance question were not normally distributed (pre course total knowledge score: Kolmogorov- Smirnov p = 0.006 and Shapiro-Wilk p = 0.114; all others: Kolmogorov-Smirnov p ≤ 0.001 and Shapiro-Wilk p ≤ 0.001). Students rated themselves significantly higher in each of the knowledge areas as well as overall knowledge after taking the course. The mean pre course career relevance (3.59 ± 0.57) was not different from the post course career relevance (3.51 ± 0.62) (Z=-0.480; p = 0.631) (Figure2A, Table 2).
Table 2. Pre-course and post course surveys results.
Drug Development in a New Era (DDNE) |
Question |
Pre Course Mean
(SD) |
Post Course Mean
(SD) |
Mean Difference |
Wilcoxon Signed Rank
Test Z-Score |
Wilcoxon Signed Rank
Test Significance (p) |
Knowledge
Question
|
1 |
2.57
(0.79) |
3.59
(0.43) |
1.02 |
-7.795 |
<0.001 |
2 |
2.41
(0.72) |
3.76
(0.52) |
1.35 |
-7.248 |
<0.001 |
3 |
2.23
(0.73) |
3.71
(0.46) |
1.48 |
-7.886 |
<0.001 |
4 |
2.61
(0.73) |
3.65
(0.52) |
1.04 |
-7.282 |
<0.001 |
5 |
2.39
(0.69) |
3.35
(0.58) |
0.96 |
-6.801 |
<0.001 |
6 |
1.91
(0.75) |
3.22
(0.56) |
1.31 |
-7.496 |
<0.001 |
7 |
2.03
(0.82) |
3.58
(0.5) |
1.55 |
-7.786 |
<0.001 |
Total Knowledge
Score |
16.13
(3.64) |
24.69
(2.33) |
8.56 |
-8.099 |
<0.001 |
Career
Relevance |
3.59
(0.57) |
3.51
(0.62) |
-0.08 |
-0.480 |
0.631 |
Molecular Signaling in the Development and Discovery of Therapeutics (MSDDT) |
Question |
Pre Course Mean
(SD) |
Post Course Mean
(SD) |
Mean Difference |
Wilcoxon Signed Rank
Test Z-Score |
Wilcoxon Signed Rank
Test Significance (p) |
Knowledge
Question
|
1 |
2.07
(0.82) |
3.15
(0.57) |
1.08 |
-5.078 |
<0.001 |
2 |
2.16
(0.80) |
3.35
(0.68) |
1.19 |
-5.116 |
<0.001 |
3 |
2.27
(0.79) |
3.27
(0.57) |
1.00 |
-4.824 |
<0.001 |
4 |
2.10
(0.82) |
3.38
(0.63) |
1.28 |
-5.182 |
<0.001 |
5 |
2.56
(0.72) |
3.46
(0.61) |
0.90 |
-4.885 |
<0.001 |
6 |
2.31
(0.84) |
3.08
(0.57) |
0.77 |
-4.098 |
<0.001 |
7 |
1.93
(0.75) |
3.00
(0.66) |
1.07 |
-5.232 |
<0.001 |
8 |
2.09
(0.77) |
3.23
(0.58) |
1.14 |
-5.114 |
<0.001 |
Total Knowledge
Score |
16.47
(5.44) |
25.15
(3.32) |
8.68 |
-5.757 |
<0.001 |
|
Career
Relevance |
3.64
(0.55) |
3.40
(0.85) |
-0.24 |
-1.206 |
0.228 |
|
Abbreviation: Standard Deviation (SD)
Lecture ratings: 94 (68%) students completed the lecture ratings (Range 1-5). The mean ratings for each domain were: content 4.17 (±0.80), presentation 4.12 (±0.87), relevance 4.25 (±0.81), and overall 4.18 (±0.81). 95% (1062 of 1116 responses to all lectures) reported that lectures were free of commercial bias.
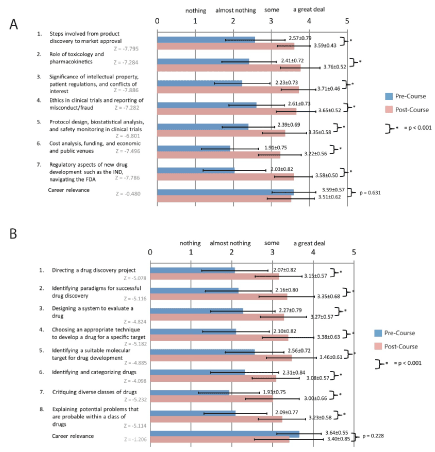
Figure 2. Pre and post course survey results. (A) This figure shows the results of the pre and post course surveys for the DDNE Course. (B) This figure shows the results for the MSDDT Course. The survey choices were (1) nothing, (2) almost nothing, (3) some, and (4) a great deal. The mean ratings and standard deviations are shown. Students rated themselves to be more knowledgeable in all areas after taking both the DDNE and MSDDT courses respectively (p < 0.001). There is no statistical difference in career relevance for either course.
Free response text: 113 (89%) of the 127 students who completed the pre course survey explained their career relevance ratings. 66 (58% of the 113 responses with explanations) indicated interest in working in research and DDD related careers. 40 (35%) indicated interest in a career outside academia (Table 3).
Table 3. Free text responses – Career Relevance Question.
Drug Development in a New Era (DDNE) |
Total Enrollment |
139 |
Number of Pre Course Surveys Completed |
127 (91%)* |
Number of Students Answered the Career Relevance Free Text Question |
113 (89%)** |
Categorized Answers |
Number of students indicated interest in career outside academia
(keywords: biotech, industry, company, pharma, consulting, biotechnology, pharmaceutical, business) |
40 (35%***) |
Number of students indicated interest in working for research and DDD related careers
(keywords: research, drug development, clinical trials, basic science) |
66 (58%***) |
Molecular Signaling in the Development and Discoveryof Therapeutics (MSDDT) |
Total Enrollment |
75 |
Number of Pre Course Surveys Completed |
68 (91%*) |
Number of Students Answered the Career Relevance Free Text Question |
55 (81%**) |
Categorized Answers |
Number of students indicated interest in career outside academia
(keywords: biotech, industry, company, pharma, consulting, biotechnology, pharmaceutical, business) |
17 (31%***) |
Number of students indicated interest in working for research and DDD related careers
(keywords: research, drug development, clinical trials, basic science) |
37 (67%***) |
* % of students who completed the course;
** % of students who completed the survey;
*** % of students who answered the free response question).
Abbreviations: Chi-square (Χ2), Drug Discovery and Development (DDD), NewDrug Application (NDA), Investigational New Drug (IND), Intellectual Property (IP)
In the pre course survey, 94 (74% of the 127 responses) students responded to the question “what they hope to get out of the class.” 35 (37% of the 94 responses to this question) indicated they were interested in the business and/or marketing aspects of DDD, 14 (15%) were interested in learning about the regulatory aspects, and 1 (1%) indicated interest in intellectual property (Table 4). In the post course survey, 92 (94% of the 98 responses) students answered, “what were the main takeaways of the class.” 31 students (34% of the 92 responses to this question) indicated they learned about the business and marketing aspects of DDD, 20 (22%) said they learned about regulatory aspects, and 13 (14%) learned about intellectual property. In the post course surveys, there is a significant increase (χ2 p = 0.034) in the number of responses that include keywords in the intellectual property category (Table 4).
Table 4. Free response question - What Students Hope to Get Out of the Class (Pre-Course) and What They Got Out of the Class (Post Course).
Drug Development in a New Era (DDNE) |
Total Enrollment |
139 |
Type of Survey |
Pre Course |
Post Course |
Number of Surveys Completed |
127 (91%*) |
98 (71%*) |
Number of Students Answered the Free Text Question |
94 (74%**) |
92 (94%**) |
Categorized Answers |
Type of Survey |
Pre Course |
Post Course |
Number of students wanted to learn/learned the business and marketing aspects of DDD
(keywords: property, business, industry, entrepreneur, startup, market, biotech, pharma)
Χ2 p-Value = 0.884 |
35 (37%***) |
31 (34%***) |
Number of students wanted to learn/learned regulatory aspects of DDD
(keywords: regulatory, regulation, approve, approval, FDA, NDA, IND)
Χ2 p-Value = 0.178 |
14 (15%***) |
20 (22%***) |
Number of students wanted to learn/learned about intellectual property
(keywords: IP, protect, intellectual, property, patent)
Χ2 p-Value = 0.034 |
1 (1%***) |
13 (14%***) |
Molecular Signaling in the Development and Discovery of Therapeutics (MSDDT) |
Total Enrollment |
75 |
Type of Survey |
Pre Course |
Post Course |
Number of Surveys Completed |
68 (91%*) |
52 (69%*) |
Number of Students Answered the Free Text Question |
63 (93%**) |
47 (90%**) |
Categorized Answers |
Type of Survey |
Pre Course |
Post Course |
Number of students wanted to learn/learned how to discover a drug
(key words: inhibit, pathway, target, discovery)
Χ2 p-Value = 1.000 |
32 (51%***) |
24 (51%***) |
Number of students wanted to learn/learned the business and marketing aspects of DDD
(keywords: property, business, industry, entrepreneur, startup, market, biotech, pharma)
Χ2 p-Value = 0.132 |
9 (14%***) |
9 (19%***) |
Number of students wanted to learn/learned regulatory aspects of DDD
(keywords: regulatory, regulation, approve, approval, FDA, NDA, IND)
Χ2 p-Value = 0.317 |
2 (3%***) |
3 (6%***) |
Number of students wanted to learn/learned about intellectual property
(keywords: IP, protect, intellectual, property, patent)
Χ2 p-Value = 0.317 |
0 (0%***) |
1 (2%***) |
* % of students who completed the course;
** % of students who completed the survey;
*** % of students who answered the free response question.
Abbreviations: Chi-square (Χ2), Drug Discovery and Development (DDD), NewDrug Application (NDA), Investigational New Drug (IND), Intellectual Property (IP)
MSDDT (spring) course
Pre/Post course surveys: 75 students completed the MSDDT course over five years, 68 (91%) completed the pre course survey, 52 (69%) completed a post course survey, and 46 (61%) completed both pre and post course surveys. Responses to all the individual knowledge questions, the total knowledge score, and the career relevance question were not normally distributed (pre course total knowledge: Kolmogorov-Smirnov p = 0.004 and Shapiro-Wilk p = 0.005; post course total knowledge: Kolmogorov-Sminov p = 0.004 and Shapiro-Wilk p = 0.226; all the others: Kolmogorov-Smirnov p ≤ 0.001 and Shapiro-Wilk p ≤ 0.001). As was the case in DDNE, students rated themselves significantly higher in each of the knowledge areas as well as total knowledge score after taking the course. Similarly, there was no difference between the pre and post course career relevance ratings (pre course: 3.64 ± 0.55; post course: 3.40 ± 0.85; Z = -1.206, p = 0.228) (Figure2B, Table 2).
Lecture ratings: 51 (68%) students in the MSDDT course completed the lecture ratings (Range 1-5). The mean ratings for each domain were: content 3.96 (±0.85), presentation 3.95 (±0.92), relevance 4.06 (±0.85), and overall 4.01 (±0.88). 98% (489 of 498 responses to all lectures) of responses reported that lectures were free of commercial bias.
Free response text: 55 (81%) of the 68 students who completed the pre course survey explained their career relevance ratings. A majority of responses (37, 67% of the 55 responses with explanations)indicated interest in working in research and DDD related careers. 17 (31%) students indicated interest in a career outside academia (Table 3).
In the pre course survey, 63 (93% of the 68 responses) responded to the question, “what they hope to get out of the class.” 32 (51% of the 63 responses to this question) indicated they were interested in learning how to discover a drug, 9 (14%) were interested in the business and/or marketing aspects of DDD, 2 (3%) were interested in learning about the regulatory aspects (Table 4). In the post course survey, 47 (90% of the 52 responses) students answered “what were the main takeaways of the class.” 24 (51% of the 47 responses to this question) students indicated they learned how to discover a drug, 9 (19%) learned about the business and marketing aspects of DDD, 3 (6%) learned about regulatory aspects, and 1 (2%) learned about intellectual property (Table 4).
Career development award
26 students were granted funds for career development activities. A total of $92,250 was awarded. 8 (31%) awardees were PhD candidates, 7 (27%) post-doctoral PhDs, 5 (19%) MD/MSCI candidates, 4 (15%) post-doctoral MDs, and 2 (8%) were Master’s degree candidates. 19 (73%) of the award recipients were women. 6 (23%) of the awardees were under-represented minorities (URM). 16 (62%) of the students used the funds to attend conferences, 9 (35%) for partial tuition remission, 4 (15%) for grant writing workshops, 2 (8%) to enroll in mini-courses, and 1 (4%) for publication support (Figure3).
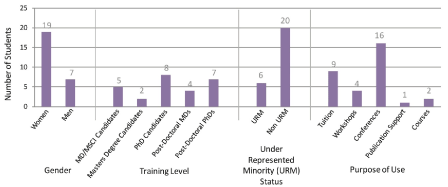
Figure 3. Career development award recipients. This bar graph displays the number of career development awardees categorized by gender, training level, underrepresented minority status, and purpose of use. Of note is that some students used career development funds for more than one purpose.
Where are the students who received career development support after graduation from their primary matriculated program/appointment?
The five graduated medical students who received support are all currently enrolled in US residency programs. Two of the eight PhD students are now graduated; one has a position as a post-doctoral trainee and one is a consultant at a healthcare company. Two Master’s degree students graduated; one is a project coordinator at a healthcare company and the other is a clinical trials research coordinator. All four post-doctoral MD students are now assistant professors at academic institutions. One of the seven post-doctoral PhD students is a manager at a pharmaceutical company; the other six continue their post-doctoral training (Table 5).
Table 5. Current fields and disciplines of graduated students who received career development funds.
MD/MSCI Dual Degree Candidates |
Index |
Year Received
Award |
Year of
Graduation |
Position |
Department |
Institution |
1 |
2013 |
2014 |
Resident |
Radiology |
NYUSOM, NY, NY |
2 |
2014 |
2015 |
Resident |
Radiology |
Perelman SOM at the University of Pennsylvania, Philadelphia, PA |
3 |
2014 |
2015 |
Resident |
Pediatrics |
Mount Sinai SOM, NY, NY |
4 |
2015 |
2015 |
Resident |
Dermatology |
NYUSOM, NY, NY |
5 |
2016 |
2017 |
Resident |
Ophthalmology |
Johns Hopkins SOM, Baltimore, MD |
PhD Candidates |
Index |
Year Received
Award |
Year of
Graduation |
Position |
Department |
Institution |
6 |
2016 |
2016 |
Post-doctoral
fellow |
Cardiothoracic surgery |
Weill Cornell Medical College, NY, NY |
7 |
2016 |
2016 |
Consultant |
N/A |
ClearView Healthcare Partners (healthcare/
consulting) Boston, MA |
Masters Degree Candidates |
Index |
Year Received Award |
Year of Graduation |
Position |
Department |
Institution |
8 |
2013 |
2014 |
Senior Clinical Project Coordinator |
N/A |
QuintilesIMS (healthcare/technology/
consulting), Overland Park, KS |
9 |
2016 |
2017 |
Clinical Trial
Research Coordinator |
N/A |
NYU Dental School, NY, NY |
Post-Doctoral MDs |
Index |
Year Received Award |
Year of Graduation |
Position |
Department |
Institution |
10 |
2013 |
2012 |
Assistant Professor |
Internal Medicine/
Preventative Medicine/Tropical Medicine |
Weill Cornell Medical College, NY, NY |
11 |
2013 |
2003 |
Assistant Professor |
Neurology |
NYUSOM, NY, NY |
12 |
2014 |
2006 |
Assistant Professor |
Internal Medicine/
Nephrology |
NYUSOM, NY, NY |
13 |
2015 |
2008 |
Assistant Professor |
Internal Medicine/ Cardiology |
NYUSOM, NY, NY |
|
|
|
|
|
|
Post-Doctoral PhDs |
Index |
Year Received Award |
Year of Graduation |
Position |
Department |
Institution |
14 |
2016 |
2013 |
Senior Manager |
Field Analytics & Operations |
Genentech, San Francisco, CA |
15 |
2016 |
2014 |
Post-Doctoral PhD |
Basic Science and Craniofacial Biology |
NYU Dental School, NY, NY |
16 |
2016 |
1999 |
Post-Doctoral PhD |
Microbiology |
NYUSOM, NY, NY |
17 |
2017 |
2014 |
Post-Doctoral PhD |
Pathology |
NYUSOM, NY, NY |
18 |
2017 |
2013 |
Post-Doctoral PhD |
Endocrinology |
NYUSOM, NY, NY |
19 |
2017 |
2016 |
Post-Doctoral PhD |
Cell Biology |
NYUSOM, NY, NY |
20 |
2017 |
2010 |
Post-Doctoral PhD |
Microbiology |
NYUSOM, NY, NY |
Abbreviation: School of Medicine (SOM)
Discussion
Our program continues to attract talented and motivated students from a variety of backgrounds at an early career stage. Initially, enrollment included students from Schools of Medicine, Biomedical Sciences, and Arts and Sciences. Currently, the student base has expanded to include the Schools of Dentistry, Engineering, Business, and Education. Moreover, our lecturers have diverse, yet specific, expertise from the various realms of DDD including academia, government/regulatory agencies, industry (large and small), and the business/economic sector. As a result, the lectures and discussions provide a platform for the multidisciplinary students to exchange ideas and share experiences toward a common goal.
The students who received career development awards are arguably our most enthusiastic students. Funds were used most often for conference attendance, which shows that recipients have strong interests in networking and becoming more fluent in their field of interest. Some of these awardees have embarked on careers in competitive medical fields and others are directly involved in DDD. It has been shown that MD/MSCI students apply to more competitive residency programs, and the current institutions and specialties of our MD/MSCI graduate recipients reflect this tendency [9]. Our program is successful in preparing these students to be key players in DDD. In addition, our career development funding encourages URM students. 23% of our recipients are URMs, while only 8.3% of all science, technology, engineering and mathematics PhDs are URMs [10].Likewise, women have historically been underrepresented in science and industry but 73% of our career development recipients are women [11].
In the first two years, there were 37 and 23 students in DDNE and MSDDT, respectively [6]. After five years, these numbers have increased to 139 and 75, respectively, reflecting increased annual enrollment in both courses without compromising the quality of lectures and discussions. With a larger sample size, we found consistently high lecture ratings in both courses, and students demonstrated learning in all areas queried. The high post course survey knowledge ratings suggest that our course can give students the knowledge, networking abilities, and confidence needed to successfully participate in DDD projects. In addition to gained knowledge, we can ascertain from the free text responses to “what students got out of the course” that students learned about the regulatory, business, economic, marketing, and intellectual property aspects. Furthermore, in regards to the latter, the number of responses that contained the keyword intellectual property increased significantly in the post-course DDNE survey. This is likely due to the course’s highly rated lecture on intellectual property [14-19].
Regarding career relevance, the majority of students (58% DDNE, 67% MSDDT) stated a desire to pursue a research and/or DDD related career. Approximately 1/3 of students indicated interest in working outside academia. Current trends in PhD training programs show that only 12.8% of graduates ultimately end up in academic careers [12]. Additional educational programs such the DDEP are important programs to enable students to gain skills and potentiate the DDD workforce.
The authors acknowledge that since there are no final exams (post-test) in our course series (and we did not administer a pre-test), we do not have a quantitative measurement of knowledge gained. The career trajectories of only those students who received the career development awards were examined.
Conclusion
Our DDEP program is successful in teaching DDD to students at an early career stage. Despite the fact that our students are at different levels of training and from different schools/fields, the material is appropriate and relevant. By inviting speakers from different areas of DDD and maintaining a discussion-based forum with multidisciplinary students, our program is able to provide collaborative, yet efficient training. We hope to produce highly trained scientists, engineers, educators, business analysts, and other innovators in DDD to further fuel the process. We will continue to expand the program. The next expansion will include an additional course, Biotechnology Industry: Structure and Strategy. The NYU Drug Development Educational Program serves as a framework for other institutions to develop similar, compatible programs to train early stage researchers in this critical area.
Acknowledgement
We would like to acknowledge Claudia Galeano and Daniel Cobos for their assistance in data acquisition.
Funding information
Financial support for this program was provided by the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), award number: R25DK092170 (co-PIs Gold-von Simson and Ramasamy), and the National Center for the Advancement of Translational Science (NCATS), award number: UL1TR00038. Gloria Lee is a trainee supported by NIDDK T35 Medical Student Training Program, award number: T35DK007421, and Gabrielle Gold-von Simson is a mentor in NIDDK T35 Medical Student Training Program, award number: T35DK007421. Ravichandran Ramasamy is further supported by National Heart, Lung, and Blood Institute (NHLBI) and NIDDK, award numbers: R01HL135987, P01HL060901, R01HL132516, R24DK103032, and R01DK109675.
Competing interest
The authors declare no conflicts of interest.
References
- Pammolli F, Magazzini L,Riccaboni M (2011) The productivity crisis in pharmaceutical R&D. NatureReviews Drug Discovery10: 428-438.
- Coelho E, Arrais J,Oliveira J (2016) Computational Discovery of Putative Leads for Drug Repositioning through Drug-Target Interaction Prediction. PLOS Computational Biology12: e1005219.
- Qi Y, Wang D, Wang D, Jin T, Yang L, Wu H, et al. (2016)HEDD: the human epigenetic drug database. Database0: baw159.
- Duda G, Grainger D, Frisk M, Bruckner-Tuderman L, Carr A, et al. (2014) Changing the mindset in life sciences toward translation: A Consensus. Science Translational Medicine6: 264.
- McNamee L, Walsh M, Ledley F (2017) Timelines of translational science: From technology initiation to FDA approval. PLOS ONE12: e017737.
- Avorn J (2015)The $2.6 Billion Pill — Methodologic and Policy Considerations. New England Journal of Medicine372: 1877-1879.
- KMR Group (2012) Annual R&D general metrics study highlights new success rate and cycle time death. [pdf] Chicago, IL: KMR Group. Available at: https://www.kmrgroup.com/
- PressReleases/2012_08_08%20KMR%20PBF%20Success%20Rate%20&%20Cycle%20Time%20Press%20Release.pdf [Accessed 19 Jul 2017].
- U.S. Food and Drug Administration (2015) The drug development process. [pdf] Silver Spring, MD: U.S. Food and Drug Administration. Available from: https://www.fda.gov/ForPatients/Approvals/Drugs/default.htm [Accessed 19 Jul 2017].
- U.S. Food and Drug Administration (2017)New Drugs at FDA: CDER’s New Molecular Entities and New Therapeutic Biological Products. [online] Silver Spring, MD: U.S. Food and Drug Administration. Available from: https://www.fda.gov/ForPatients/Approvals/Drugs/default.htm[Accessed 19 Jul 2017].
- U.S. Food and Drug Administration (2013)Summary of NDA Approvals & Receipts, 1938 to the present [online] Silver Spring, MD: U.S Food and Drug Administration. Available from:
https://www.fda.gov/aboutfda/whatwedo/history/productregulation/summaryofndaapprovalsreceipts
1938tothepresent/default.htm [Accessed 19 Jul 2017].
- Dolgos H, Trusheim M, Gross D, Halle J, Ogden J, et al. (2016) Translational medicine guide transforms drug development processes: The recent Merck experience. Drug Discovery Today21: 517-526.
- Clair R.S, Hutto T, Macbeth C, Newstetter W, McCarty NA, et al. (2017) Correction: The "new normal": Adapting doctoral trainee career preparation for broad career paths in science. PLOS ONE12: e0177035.
- Plaksin J, Cymerman R, CasoCaso R, Galeano C, Ramasamy R, et al. (2016) New York University School of Medicine Drug Development Educational Program: 2-year benchmark. Clinical and Translational Science9: 274-280.
- Gold-von Simson G (2016)SYLLABUS: Drug Development in a New Era: NYU GSAS, MSCI Program, Sackler, SOM 2016. [pdf] New York, NY: NYU School of Medicine. Available from: https://med.nyu.edu/research/postdoctoral-training/sites/default/files/PDF/drug-development-fall-2016-syllabus.pdf[Accessed 19 Jul, 2017].
- Gold-von Simson G (2017) SYLLABUS: Molecular Signaling in the Development and Discovery of Therapeutics: NYU GSAS, MSCI Program, Sackler, SOM 2017. [pdf] New York, NY: NYU School of Medicine Web site. Available from: https://med.nyu.edu/research/sackler-institute-graduate-biomedical-sciences/sites/default/files/syllabus-molecular-signalling-development-discovery-therapeutics-spring-2017.pdf [Accessed 19 Jul, 2017].
- Gillman J, Pillinger M, Plottel C, Galeano C, Maddalo S, et al. (2015) Teaching translational research to medical students: The New York University School of Medicine's Master's of Science in Clinical Investigation Dual-Degree Program. Clinical and Translational Science8: 734-739.
- Flynn D (2016) STEM field persistence: The impact of engagement on postsecondary STEM persistence for underrepresented Minority Students. Journal of Educational Issues2: 185-214.
- Nimmesgern H (2016) Why are women underrepresented in STEM fields? Chemistry- A European Journal22: 3529-3530.
- Larson R, Ghaffarzadegan N, Xue Y (2014) Too many PhD graduates or too few academic job openings: the basic reproductive number R0 in academia. System Research and Behavioral Science31: 745-750.



