Quantitative electroencephalogram (QEEG) and the QEEG normative database help in characterization of normal versus neurocognitive diseases, in diagnosis and prognosis and in treatment tailoring. Constructing QEEG normative databases and standardization of QEEG protocols for use in both research and clinical settings has proven challenging over the last 61 years. The present paper focuses on a) historical and technical milestones the field had to overcome, b) standards to be followed when constructing and validating a normative databases, c) commonly used normative databases, and d) provides an illustrated step-by-step guide to QEEG normative database validation and comparison.
While clinical evaluation and correlation is key to diagnosis of mental and neurocognitive disorders, it is subjective. Among objective markers (biochemical, imaging and genetic tests) the electroencephalography (EEG) and in particular the digital EEG (dEEG) has evolved as a sensitive diagnostic and prognostic tool meeting the American Academy of Neurology (AAN) standards (Class III evidence, Type C recommendation) [1,2]. Nuwer defined dEEG as “. . . . the paperless acquisition and recording of the EEG via computer-based instrumentation, with waveform storage in a digital format on electronic media, and waveform display on an electronic monitor or other computer output device” [2]. The advent of the dEEG paved the way for quantitative electroencephalography (QEEG) with both serving complementary to each other. The dEEG captures an individual’s brain wave patterns, frequency, resting-state, and event- or evoke-related responses to visual, auditory, tactile, error, Go/No GO etc stimuli in healthy and disease states.
American Academy of Neurology defined qEEG as …., “the mathematical processing of digitally recorded EEG in order to highlight specific waveform components, transform the EEG into a format or domain that elucidates relevant information, or associate numerical results with the EEG data for subsequent review or comparison” [2], QEEG requires an individual patient’s numerical EEG results to be transformed from the time domain into the frequency domain and Gaussian approximation and cross-validation be carried out. Following this Z-scores are computed with relation to an appropriate normative database followed by construction of a topographic/brain map to be used either for diagnosis, prognosis or treatment tailoring [1,2]. The assessment of changes in QEEG brain maps is especially suited for differential diagnosis in cross-border diseases / diseases with symptom overlap for example in differentiating between delirium, dementia and depression [3-7].
Other areas where QEEG has made unique contributions include; epilepsy screening and in drug-resistant epilepsy, in court sentencing, pharmaco-QEEG, neurocognitive issues, traumatic brain injury (TBI) severity, post-concussion syndrome, mood disorders, exo- or endogenously induced behavioral disorders, attention deficit disorder (ADD/ADHD), schizophrenia, depression, tinnitus, encephalopathies and alcohol and/or substance abuse [3-7]. On the issue of differential diagnosis of cross-border diseases another parallel development which has bearing on QEEG’s usefulness as a diagnostic and prognostic tool is the Diagnostic and Statistical Manual of Mental Disorders (DSM) (Figure 1) [8]. Changes in disease definitions and classification as per the DSM influence cross-study comparability, QEEG derived biomarker reliability and validity. However, DSM-5, released in 2013 keeping in mind neurocognitive developments in the field has helped lay to rest many of the issues pertinent to disease classification [8].
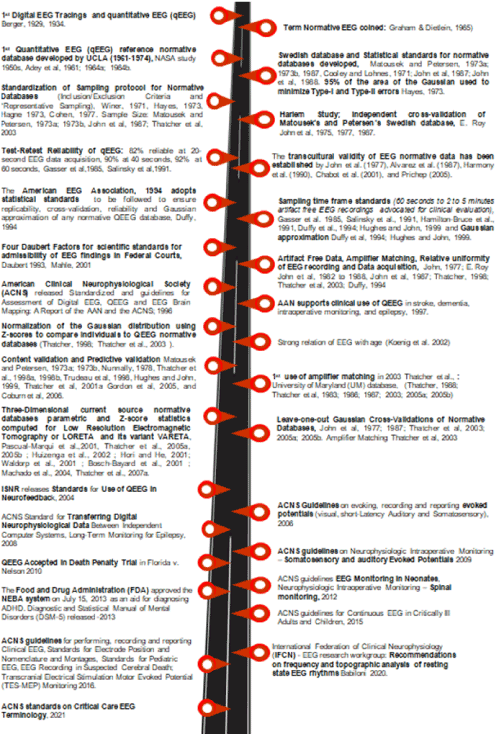
Figure 1. History of the scientific, technical and statistical improvements in constructing QEEG normative databases
The backbone of the QEEG is the normative database (a term coined by Graham and Dietlien in 1965) used in drawing comparisons [9]. In the hands of the untrained (operators, data analyzers and interpreters), the QEEG can yield results that are not of clinical relevance [10]. Therefore, over the last 61 years several QEEG standards have been developed to ensure.
- The validity and reliability of QEEG for research and clinical use in diagnosis, prognosis and pharmaco-QEEG,
- That a balance between “standardized medicine” and “precision medicine” is struck so as to meet the World Health Organization (WHO) “High 5s Project” goals to ensure patient safety and finally [11].
- To meet health insurance requirements [11-17].
- Standard methods of EEG data acquisition, visualization (synchronization, connectivity and topographic features), processing (de-artifaction, extraction and classification), storage and statistical comparisons have been and are in continuous development (Figure 1) [9-17].
- Standard methods of normative databases construction, guidelines on the same and FDA registered normative databases.
- World-wide efforts are on to generate long-tailed data and merge them to generate big data that will allow for both cross-study and cross-cultural comparisons [16-18].
The goal of this paper is to present; i) a brief historical review of technical and statistical milestones and standards that apply to QEEG and QEEG normative databases over the last 61 years (Figure 1 and 2) (Table 1), ii) protocols involved in normative database evaluation and comparison (Figure 3), iii) common normative QEEG databases in use and iv) to provide a step-by-step guide to normative database evaluation and comparison from EEG recording to Z-score computing, followed by construction of topographic maps using EEG machines like BrainView by Medeia (Figure 4a-4b).
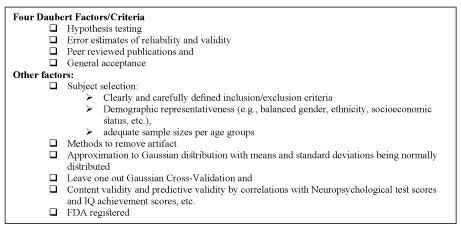
Figure 2. Scientific standards for admissibility of EEG normative databases in federal courts
Table 1. Findings for QEEG studies on selected Neurocognitive Disorders
The online search engines used were Google, Google Scholar, PubMed, MEDLINE. In keeping with the goal of this paper the keywords and phrases used to conduct the literature search were “EEG”, “electroencephalogram”, “quantitative electroencephalogram”, “QEEG”, “history of EEG/QEEG”, “guidelines”, “standards,” “normative database”, “technical standards EEG/QEEG”, “protocols for normative database construction EEG/QEEG”, “normative database comparison EEG/QEEG”, “FDA registered normative EEG/QEEG database”, “Z-score computing EEG/QEEG”.
First the titles and then abstracts that met the study goals were shortlisted from each of the above keyword-based searches: The preference was for articles whose full text was available in English and free. If any article was extremely important for the study but was not available for free, we obtained the article. Text books, book chapters, published original and review articles, standards and guidelines American Clinical Neurophysiology Society (ACNS), American Electroencephalographic Society (American EEG Society, AEEGS), American Academy of Neurology (AAN), white and grey papers, focusing on QEEG normative databases, their construction, comparison, FDA registered normative databases (e.g. https://www.accessdata.fda.gov/cdrh_docs/pdf4/K041263.pdf) and the historical developments in the field of QEEG were included in the study. The BrainView in-house manual by Medeia on the historical developments in the field of QEEG, normative database construction and comparison was also used. Articles beyond the scope of interest were excluded from the study.
The results and discussion is presented in four parts; the first part deals with the history of the scientific standards followed in constructing QEEG normative databases. The second part deals with QEEG normative databases validation and comparison and the third presents a list of commonly used normative databases. The fourth and final section deals with a step-by-step guide to evaluation and comparison of QEEG normative databases.
History of the scientific standards followed in constructing QEEG normative databases
In 1929 the human EEG was first measured and the first QEEG study was carried out by Hans Berger (Figure 1) (Table 1) using the Fourier transform to spectrally analyze EEG data and to compare different EEG measures to a normative database [9,19-21]. The first quantitative EEG (QEEG) reference normative database was developed in the 1950s at the UCLA Brain Research Institute by Ross Adey between 1961-1974. Its drawback was it was intended for selection of astronauts for NASA space travel and not clinical use (Figure 1) [22-24]. The statistical tests run on the database included calculation of means and standard deviations, measures to determine if the data followed the normal/Gaussian distribution, complex demodulation, Fourier spectral analysis and basic statistical parameters necessary for any reference normative database.
The first known statistical standards for normative databases and the first peer reviewed publication of a normative database was by two Swedish Neurologists Dr. Milos Matousek and Dr. Ingemar Petersen in 1973 [25,26]. They measured QEEG in n=401 subjects (Female%: 54.4%) aged between 2 months to 22 years with a sample size of n=18 to 49 per one year age grouping, all subjects lived in Stockholm, had no clinical histories and performed at grade level [25,26]. The sample sizes varied from 18 to 49 per one year age groupings. The Swedish pair set the standards for clinical inclusion/exclusion criteria, parametric statistical tests and peer reviewed publications.
The Swedish database was independent culturally cross-validated and deemed reliable by E. Roy John and colleagues in 1975 using EEG from 9- to 11-year-old Harlem black children, also performing at grade level with no history of neurological disorders (Figure 1) [27-29]. E. Roy John and colleagues formed a consortium of universities (1982 to 1988) to address the “need for standardization” [27-31]. In 1994 the American EEG Association, 1994 adopted the statistical standards mentioned below to ensure replicability, cross-validation, reliability and Gaussian approximation of any normative QEEG database [31]. Between 1993 and 2001 the four Daubert factors (Figure 2) for scientific standards for admissibility of EEG findings in federal Courts were derived [32-35]. The standards mentioned below set the stage for the evolution of QEEG and EEG standards currently advocated by the International Federation of Clinical Neurophysiology (IFCN) [15].
- In term of sampling time frames and intra and inter test-retest reliability, QEEG has proved to be highly reliable and reproducible [10-22]. Sampling/acquisition time frames were 82% reliable at 20-second EEG data acquisition, 90% reliable at 40 seconds, and 92% reliable at 60 seconds [13,21]. Current standards recommend at least 60 seconds to- preferably 2 to 5 minutes of artifact free EEG recordings for clinical evaluation [30,31]. Predictive accuracy and error rates depend on the data that make up a given EEG database as well as the statistical methods used to produce and compare QEEG normative databases. Split-half reliability and test re-test reliability measures (>0.9) are also important to demonstrate the internal consistency and reliability of the normative database [28,29,31,36-40].
- QEEG-database sample size is dictated by “effect size” and “power” i.e. the sample size required to detect a particular effect, the sample size required to achieve Gaussian distribution and cross-validation, cost and duration available for sample collection (Figure 1 and 3) [19,29 40-42]. Careful screening of the subjects that comprise a representative normative database is critical to prevent bias and prevent miss-classification of healthy versus disease individuals. (Figure1 and 3). “Representative sampling” means obtaining a demographically balanced sample in terms of gender, ethnic-background, socio-economic status and age. However, ensuring hyper-healthy normals or controls is unrealistic and instead “street normal” subjects that meet the exclusion criteria are more the norm. Another key issue pertinent to sample size is encountered with pediatric databases due to growth spurts in mental development. Thus, in pediatric databases sample size may at times differ by months instead of years as dramatic developmental changes occur over relatively short time intervals while in adult databases even 2-year differences in age-grouping are valid [25,26,36,40,43,44]. “Age Regression” is another method used to adjust for age related variations in QEEG [27-29].
- Manual de-artifaction is subjective, involving marking segments containing artifacts; the drawback is it can result in suboptimal inter- and intra-rater reliability. Automated de-artifacting methods can be either “semiautomatic” or “fully automatic” involving artifact “correction” or artifact “rejection” methods (Figure 1 and 3). Artifact rejection methods remove segments of EEG that are identified as being contaminated by artifacts, while artifact correction methods apply techniques that remove artifacts without removing the underlying EEG signal. One example of an artifact correction method is the use of “blind source separation” (BSS) that identifies different independent sources of variance in the EEG. The benefit of fully automatic de-artifacting methods is that they eliminate inter- and intra-rater variability thus and guarantee that each EEG will be de-artifacted using the exact same set of criteria.
- In the 1980s primitive analytic software hindered EEG comparability resulting in QEEG users using relative power versus absolute power. It was not until the mid-1990s that computer speed and software development made amplifier matching and normative database amplifier equilibration a possibility (Figure 1 and 3). Each channel has three electrical contacts: a ground contact and two other contacts that go directly into the differential pre-amplifier [45,46]. Different frequency response curves exist for different amplifiers and there is no one “gold standard” for EEG amplifiers. To circumvent this issue a universal equilibration process was developed so that micro-volts in a given amplifier could be converted/ equilibrated to microvolts in all other amplifiers and more importantly to normative database amplifiers. Calibrated sine waves are injected into the input of the EEG amplifiers to be compared to the normative database ensuring that amplifiers frequency range matches the normative database amplifiers. Then take the ratio of the micro-volt values at each frequency are obtained and the ratios is used as gain or amplitude scalars in the FFT to exactly equate the spectral output values to the normative database amplifiers. Following equilibration amplifiers used for recording a subject’s EEG can be directly compared with the normative database means and standard deviations.
- The combination of electrode inputs, summed to show the whole set of electrodes being studied, is called the “montage”. A montage is selected to most clearly demonstrate the EEG pattern being monitored. One example is the Laplacian/Hjorth montage [47]. In a set of differential amplifiers, one is the “active” electrode and the other the “reference” electrode. “References” electrodes include linked ear, ipsilateral and contralateral ear, the Cz or “vertex” reference, and the sequential or “bipolar” references, common average or global average and the weighted average reference montages and montages on the tip of nose, the mastoid process [46]. As each montage has its own strengths and weakness it has to be tailored to suit the need however the montage selected has to match the montage of the normative EEG database to which the data is being compared. Proper montage selection will allow a good EEG recording.
- Depending on the mental or neurocognitive disorder being studied data acquisition can either be resting EEGs with eyes-closed or eyes-open conditions or active Tasks i.e. Go-NoGO (inhibition), visual or auditory tasks, or cognitive task, evoked potentials (EPs) and event related potential (ERP) and Go-No while a subject performs a task (Figure 1, 2 and 3).
- Many a times QEEG analysis is used as evidence in court. In 1993 the Supreme Court in Daubert, stipulated the statistical foundations regarding admissibility of scientific evidence in court. The Four Daubert Factors for scientific standards of admissibility in Federal Courts are presented in Figure 2 [32-35]. In 2010 QEEG was accepted in the Grady Nelson death penalty trial in Florida and its findings led to change in sentencing from “death penalty” to “lifetime sentencing without parole” [6,7].
- From 1996 till date the American Clinical Neurophysiological Society (ACNS), American Academy of Neurology (AAN) and International Federation of Clinical Neurophysiology (IFCN) have released several guidelines and standards (Figure 1) (Table 1) to ensure both standardized and personalized healthcare is achieved in the area of QEEG and diagnosis, management and monitoring of neurocognitive disorders [48]. This has in turn allowed for cross-study comparability of QEEG findings in various neurocognitive disorders (Figure 1) (Table 1). Among them worth mentioning is the Food and Drug Administration (FDA) approval of the NEBA system on July 15, 2013 for diagnosis of ADHD in conjunction with clinical tests and the FDA approval of two commonly used normative databases qEEG
- the “qEEG-Pro” database (qEEG-Pro B.V.) and the “Lifespan” database (Applied Neuroscience, Inc) [49-51].

Figure 3. Standard protocol followed in database construction and validation
QEEG normative databases validation and comparison
Matousek and Petersen in their Swedish study were the first to compute means, standard deviations and Z-scores in one-year age-groups and use t-tests to compare an individual to a normative database (Figure 3 4a and 4b) [25,26]. E. Roy John and collaborators from 1974 to 1977 carried out the independent cross-validation of normative QEEG databases when they compared data from their Harlem study with the Swedish database [27-29,52]. Following this in 1994 the American EEG Association and the IFCN reiterated these methods as acceptable basic standards to be met by any normative QEEG database [15,27-29,31,52-81]. Data normalization to the Gaussian distribution using Z-scores helps in comparing individuals to a QEEG normative database. The values of Z within ±2SD i.e. 95% of the area of the Gaussian aids in minimizing Type-I and Type-II errors and in determining the sensitivity, false positives and false negatives of a normative database (Figure-1, Figure-4a-4b) [39,40,42,53]. Due to the expense to acquire independent data, most cross-validations are computed using a leave-one-out cross-validation procedure following equilibration using amplifier matching [13,27-29,43,44,56-81]. Figure 3 presents and overview of protocols followed in normative database construction and evaluation, Figure 4a-4b provide a step-by-step guide from EEG data acquisition to construction normative data to construction of topographic maps and normative database validation using EEG machines like Brain View.
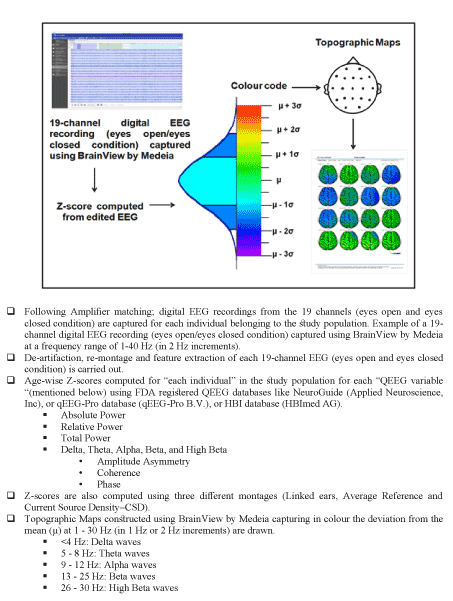
Figure 4a. Step-by- step guide to construction of QEEG topographical maps.
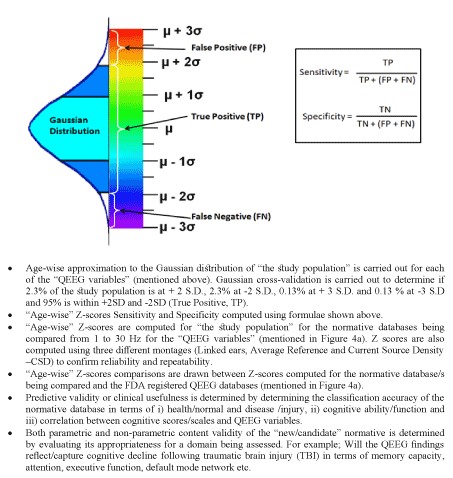
Figure 4b. Step-by- step guide to construction/comparison/validation of two or more normative database
In brief, following visual analysis of the dEEG, manual or automated deartifaction and feature extraction and data and statistical processing spectral analysis is carried out. Following this univariant or multivariant comparisons to a normative database is carried out. More advanced comparisons include cluster analysis where individuals are grouped by EEG features. Cluster analysis often aids in distinguishing between subtypes. For example, it helps differentiate between individuals with the same disorder (eg, attention deficit hyperactivity disorder (ADHD), obsessive-compulsive disorder (OCD)) one group of whom responded to medication and the other was drug-resistant [82-85]. In the area of mental and neurocognitive health; omission errors in the GO/NOGO test discriminated between subjects with Attention Deficit/Hyperactivity Disorder (ADHD) and controls [3,86,87]. The NEBA® system (received FDA approval on 2013) wherein resting theta/beta ratio (TBR) recorded at Cz (international 10-20 EEG system) is ratified by Blue Cross Blue Shield Association to clinically diagnose/indicate if further tests are required in children and adolescents with ADHD [49,88,89]. Amplitude, power and synchronization can be used to differentiate mild (sensitivity 85% and specificity 78%) and moderate Alzheimer’s disease (AD) (sensitivity 89% and specificity 88%), from healthy controls [90]. Another study carried out by Stylianou et al. illustrated QEEGs ability to differentiate between AD, dementia with Lewy bodies (DLB), Parkinson’s disease dementia (PDD) patients and healthy controls and identify QEEG signatures of cognitive fluctuations (CFs) in DLB with a diagnostic accuracy of 94%, sensitivity of 92.26% and specificity of 83.3% [91]. Yet another study showed that spectral analysis (spectA) was more sensitive than coherence (Coh) in differentiating 40 subjects with mild to moderate AD from 40 healthy elderly controls (91). A unique retrospective study on AD (n=169, female%:65.1%) carried out by Houmani et al. used neuropsychological tests, brain imaging and blood sampling to first diagnose AD following which retrospective normative EEG data was acquired between 2009 and 2013. Epoch-based entropy and bump modeling (automatic discrimination) exhibited a classification accuracy of 91.6% (specificity = 100%, sensitivity = 87.8%) when discriminating subjective cognitive impairment (SCI) from possible AD patients [92]. In terms of clinical usefulness of QEEG and normative databases Figure 1 and Table 1 presents a snapshot of key milestones crossed and key QEEG findings in selected neurocognitive disorders [2,20-27,32-35,36-40,48,52-77,93-159].
Common normative databases
Normative reference databases form the veritable backbone of QEEG analysis increasingly used in diagnosis or prognosis or Neurofeedback or Pharmaco-QEEG. Listed below are a few of the commonly used normative databases:
- UCLA Brain Research Institute database was the first of its kind developed by Ross Adey between 1961-1974 it was used to select astronauts for NASA space travel [22-24].
- The Swedish database was developed by Dr. Milos Matousek and Dr. Ingemar Petersen in 1973 [25,26]. It measured QEEG in n=401 subjects (Female%: 54.4%) aged between 2 months to 22 years.
- The BrainDX (BrainDX, L.L.C.) database, formerly the NXLink – NYU database was developed between1970’s-1980’s, it has a total of 464 subjects and manual deartifacting was carried out.
- The Neurometrics database measured delta, theta, alpha, and low frequency beta bands, absolute power, relative power, coherence, mean frequency within band, and symmetry (left-right and front-back) extracted from approximately two minutes of data in n=782 “normal” individuals with n=356 aged between 6-16 years and n=426 aged from 16 to 90 [29]. It has received a 510(k) clearance by the FDA (July 1998, #K974748), indicating that construction of the database has been scrutinized for good manufacturing practices (GMPs). However, only information about delta, theta, alpha, and low frequency beta bands are available.
- Thatcher Lifespan Normative EEG Database (LSNDB/NeuroGuide), a.k.a NeuroGuide, Appli ed Neuroscience, Inc; the University of Maryland (UM) database (Thatcher et al., 2003) was developed by Robert W. Thatcher (Thatcher, 1998). Eyes closed (EC) and eyes open (EO) resting-state recordings acquired from 1979 to1987 and in 2000 include n=625 individuals (2 months to 82 years of age). In 2008 an additional 53 adult subjects aged between 18.3 years to 72.6 years were added to the database bringing the numbers up to 678 subjects [37,40,159-162]. NeuroGuide has FDA 510 (k) clearance.
- The Sterman-Kaiser (SKIL) Database: includes 135 adults (18 to 55 years of age) and is comprised of students and laboratory personnel (50%), volunteers recruited from the community (25%), and U.S. Air Force personnel (25%) [163].
- The International Brain Database: is being developed (n=1000 controls and n=1000 normals) by a consortium of leading neuroscientists from 50 laboratories across U.S.A, United Kingdom, Holland, South Africa, Israel and Australia. The database will include EEG (EO and EC), ERP and autonomic activity data and data on 50 ADHD subjects. Psychophysiology Paradigms that will be used include Startle paradigm (fight and flight reflex) – Go-NoGO (inhibition) – Resting EEG (cortical stability) – Visual tracking task (automatic tracking) – Habituation paradigm (novelty learning) – Auditory oddball (efficiency of target processing) – Visual oddball (visual novelty target processing) – Conscious and subconscious processing of facial emotions – Visual working memory task (memory and sustained attention) – Executive maze task (planning and error correction) [164,165].
- qEEG-Pro by qEEG-Pro B.V. uses automatic deartifacting and client-based. It includes resting-state recordings acquired between 2004-2013, EC: n=1482 and EO: n=1232 and the age range is 6-82 years.
- HBI by HBImed AG, data was collected in the 1990’s and automatic deartifaction was carried out. 5 active tasks (two GO/NOGO tasks, arithmetic and reading tasks, auditory recognition and auditory oddball tasks) and EC and EO resting-state recordings were carried out on n=1000, children and adolescents (age 7-17): n=300, adults (18-60): n=500, and seniors (61+):n=200 [10,166]
- Cuban Human Brain Mapping Project (CHBMP): EEGs of 30 minutes duration including the following conditions: eyes closed, eyes open, hyperventilation and subsequent recovery. 56 participants, reaction times were recorded using a go-no- go paradigm which consisted in a visual attention task. High-density (64-120 channels) resting state electroencephalograms (EEG), magnetic resonance images (MRI), psychological tests (MMSE, Wechsler Adult Intelligence Scale -WAIS III, computerized reaction time tests using a go nogo paradigm) were carried out in 282 healthy participants (age range 18–68 years) acquired from 2004 to 2008 [167].
- EEG tomographic analysis called “LORETA” (low resolution EEG tomography analysis). The NovaTechEEG database has n=84 cases.
- Hudspeth offers the “Neurorep AQR” (Adult QEEG Reference Database, see: www.neurorep.com). The database measured absolute and relative power for 19 scalp electrodes, n=171 [168].
- BrainView QEEG normative database: It is a client-based QEEG database. EC (n=1965) and EO (n=2303) resting-state recordings were acquired between 2018 and 2020 (age range: 4 to 80 years; male %:48.5%) and delta, theta, alpha, and low frequency beta bands were measured. Spectral analysis was between 1 to 40Hz. Age regression method was by age bins. Deartifaction was both by manual and automatic methods.
A step-by-step guide to evaluation and comparison of QEEG normative databases
Figure 4a-4b i-iii present a step-by-step guide to construction, evaluation and comparison of QEEG normative databases (15,52-81). Following data acquisition using EEG machines like BrainView, artifact cleaning, and reliable dEEG data conversion to time series after which it may be re-referenced or re-montaged, it is then analyzed in either the time domain or the frequency domain. The selected normal subjects are grouped by age. The means and standard deviations of the EEG time series and/or frequency domain analyses are computed for each age group. Transforms are applied to approximate a Gaussian distribution of the EEG measures that comprise the means. Once approximation to Gaussian is completed, Z-scores are computed for each subject in the database and leave one out Gaussian cross-Validation is computed in order to arrive at optimum Gaussian cross-validation sensitivity (Figure 4a -4b). Finally, the Gaussian validated norms are subjected to content and predictive validation procedures such as correlation with neuropsychological test scores and intelligence, etc. and also discriminant analyses, neural networks and outcome statistics, etc., [61]. Content validation is carried out with respect to clinical measures such as intelligence, neuropsychological test scores, school achievement etc., (Figure 1, Figure 3 and Figure 4a-4b) [57]. Predictive validation is carried out with respect to discriminative, statistical or neural network clinical classification accuracy (Figure 1, Figure 3 and Figure 4a-4b). Both parametric and non-parametric statistics are used to determine the content and predictive validity of a normative EEG database (Figure 1, Figure 3 and Figure 4a-4b).
QEEG today provides information about the underlying neurophysiological correlates of psychological disorders. The development and integration of standardized protocols for EEG and QEEG: processing, analysis and interpretation and for normative database: construction, comparison and evaluation over the last 61 years have contributed to the current validity, reliability, and usability of QEEG. Technical and statistical improvements in the field since the inception of QEEG have greatly contributed to it fast becoming a personalized and precise medicine tool with enormous clinical and research potential.
- No authors listed (1989) Assessment: EEG brain mapping. Report of the American Academy of Neurology, Therapeutics and Technology Assessment Subcommittee. Neurology 39: 1100-1101. [Crossref]
- Nuwer M (1997) Assessment of digital EEG, quantitative EEG, and EEG brain mapping: Report of the American academy of neurology and the American clinical neurophysiology society. Neurology 49: 277-292.
- Coburn KL, Lauterbach EC, Boutros NN, Black KJ, Arciniegas DB (2006) The value of quantitative electroencephalography in clinical psychiatry: a report by the Committee on Research of the American Neuropsychiatric Association. J Neuropsychiatry Clin Neurosci 18: 460-500.
- Constantin D, Digital EEG (2008) Quantitative EEG techniques and brain mapping. Conventional and modern electroencephalogram in adult and child. București, Medical Publishing House 161-169.
- Jobert M, Wilson J, Ruigt GSF, Prichep LS (2012) Guidelines for the recording and evaluation of pharmaco-EEG data in man: the international Pharmaco-EEG Society (IPEG). Neuropsychobiology 66: 201-220.
- State of Florida v. Grady Nelson No. F05-00846 (11th Fla. Cir. Ct., 4 Dec 2010).
- Du Yu (2020) "The Application of Neuroscience Evidence on Court Sentencing Decisions: Suggesting a Guideline for Neuro-Evidence," Seattle Journal for Social Justice 18: 19.
- American Psychiatric Association (2013) Diagnostic and Statistical Manual of Mental Disorders. 5th ed. Arlington: Washington, DC, USA: American Psychiatric Association.
- Graham, MA, Deitlein LF, (1965, Technical details of data acquisition for normative EEG reference library, Analysis of centrao nervous system and cardiovascular data using computer methods. NASA SP 72: 433.
- Nuwer MR (2003) Clinical use of QEEG. Clin Neurophysiol 114: 2225.
- WHO Action on patient safety—High 5s. Accessed on 16 January 2021.
- Johnstone J, Gunkelman J (2003) Use of databases in qEEG evaluation. Journal of Neurotherapy 7: 31-52.
- Thatcher RW, Lubar JF (2008) History of the scientific standards of QEEG normative databases. In: Introduction to QEEG and neurofeedback: Advanced theory and applications. Thomas Budzinsky, H. Budzinski, J. Evans and A. Abarbanel editors, Academic Press, San Diego, CA.
- Keizer AW (2019) Standardization and Personalized Medicine Using Quantitative EEG in Clinical Settings. Clin EEG Neurosci 11: 1550059419874945.
- Babiloni C, Barry RJ, Başar E, Blinowska KJ, Cichocki A, et al. (2020) International Federation of Clinical Neurophysiology (IFCN) - EEG research workgroup: Recommendations on frequency and topographic analysis of resting state EEG rhythms. Part 1: Applications in clinical research studies. Clin Neurophysiol 131: 285-307.
- Thompson PM, Jahanshad N, Ching CRK (2020) ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl Psychiatry 10: 100.
- Alvarez A, Valdes P, Pascual R (1987) EEG developmental equations confirmed for Cuban schoolchildren. Electroencephalogr Clin Neurophysiol 67: 330-332.
- Hu S, Ngulugulu A, Bosch-Bayard J, Bringas-Vega ML, Pedro A (2019) Multinational qEEG developmental surfaces. BioRxiv
- Cohen J (1977) Statistical power analysis for the behavioral sciences Ed. Lawrence Erlbaum Associates, Inc.
- Berger H (1929) Uber das Electrenkephalogram des Menschen. Archiv. Fur. Psychiatrie und Neverkrankheiten 87: 527-570.
- Berger H (1932) Uber das Electrenkephalogramm des Menschen. Vierte Mitteilungj. Archiv. Fur. Psychiatrie und Neverkrankheiten 97: 6-26.
- Adey WR, Walter DO, Hendrix CE (1961) Computer techniques in correlation and spectral analyses of cerebral slow waves during discriminative behavior. Exp Neurol 3: 501-524.
- Adey WR (1964) Data acquisition and analysis techniques in a Brain Research Institute. Ann N Y Acad Sci 115: 844-866.
- Adey WR (1964) Biological instrumentation, electrophysiological recording and analytic techniques. Physiologist 72: 65-68.
- Matousek M, Petersen I (1973) Automatic evaluation of background activity by means of agee-dependent EEG quotients. EEG & Clin Neurophysiol 35: 603-612.
- Matousek M, Petersen I (1973) Frequency analysis of the EEG background activity by means of age dependent EEG quotients. In P. Kellaway & I. Petersen (Eds.) Automation of clinical electroencephalography. New York: Raven Press 75-102.
- John ER (1977) Functional Neuroscience, Vol. II: Neurometrics: Quantitative Electrophysiological Analyses. E.R. John and R.W. Thatcher, Editors. L. Erlbaum Assoc, N.J.
- John ER, Karmel B, Corning W (1987) Neurometrics: Numerical taxonomy identifies different profiles of brain functions within groups of behaviorally similar people. Science 196: 1393-1409.
- John ER (1987) Normative data banks and neurometrics: Basic concepts, methods and results of norm construction. In A. Remond (Ed.), Amsterdam: Elsevier. Handbook of electroencephalography and clinical neurophysiology: Vol. III. Computer analysis of the EEG and other neurophysiological signals 449-495.
- Hughes JR, John ER (1999) Conventional and quantitative electroencephalography in psychiatry. Neuropsychiatry 11: 190-208.
- Duffy F, Hughes JR (1994) Status of quantitative EEG (QEEG) in clinical practice. Clinical. Electroencephalography 25.
- Daubert V (1993) Merrell Dow Pharmaceuticals (Daubert), 61 U.S.L.W 4805 U.S.
- Kumho Tire Company Ltd (1999) Carmichael, 526 U.S. 137, 119 S.Ct 1176, 143 L.Ed. 2d 238.
- Frye V (2001). U.S. (Frye), 293 F. 1013, 1014 (D.C. Cir. 1923). 5 ñ Mahle, S. Daubert and the Law and Science of Expert Testimony in Business Litigation ìBusiness Litigation in Florida," 4th ed.
- Mahle S (2001) Daubert and the Law and science of expert testimony in business litigation ìBusiness Litigation in Florida," 4th ed.
- Thatcher RW, Walker RA, Giudice S (1987) Human cerebral hemispheres develop at different rates and ages. Science 29: 1110-1113.
- Thatcher RW (1998) EEG normative databases and EEG biofeedback. Journal of Neurotherapy 2: 8-39.
- Thatcher RW, Camacho M, Salazar A (1997) Quantitative MRI of the gray-white matter distribution in traumatic brain injury. J Neurotrauma 14: 1-14.
- Thatcher RW, Biver C, McAlaster R, Salazar AM (1998) Biophysical linkage between MRI and EEG coherence in traumatic brain injury. NeuroImage 8: 307-326.
- Thatcher RW, Walker RA, Biver C, North D, Curtin R (2003) Quantitative EEG Normative databases: Validation and Clinical Correlation. J Neurotherapy 7: 87-122.
- Winer BJ (1971) Statistical Principles in Experimental Design. McGraw-Hill, New York.
- Hayes WL (1973) Statistics for the social sciences. New York: Holt, Rhinehart and Winston.
- Thatcher RW, North D, Biver C (2005) EEG inverse solutions and parametric vs. non-parametric statistics of Low-Resolution Electromagnetic Tomography (LORETA). Clin EEG and Neuroscience 36: 1-9.
- Thatcher RW, North D, Biver C (2005) Evaluation and Validity of a LORETA normative EEG database. Clin EEG and Neuroscience 36: 116-122.
- Tyner F, Knott J (1983) Fundamentals of EEG technology. New York: Raven Press.
- J Yao D, Qin Y, Hu S, Dong L, Bringas Vega ML (2019) Which Reference Should We Use for EEG and ERP practice? Brain Topogr 32: 530-549.
- Scherg M, Ille N, Bornfleth H, Berg P (2002) Advanced tools for digital EEG review: Virtual source montages, whole-head mapping, correlation, and phase analysis. Journal of Clinical Neurophysiology 19: 91-112.
- https://www.acns.org/practice/guidelines
- https://www.accessdata.fda.gov/cdrh_docs/pdf11/K112711.pdf
- https://www.accessdata.fda.gov/scripts/cdrh/cfdocs/cfpcd/classification.cfm?id=3865
- https://www.accessdata.fda.gov/cdrh_docs/pdf4/K041263.pdf
- John ER, Prichep LS, Fridman J, Easton P (1988) Neurometrics: Computer assisted differential diagnosis of brain dysfunctions. Science 93: 162-169.
- Thatcher RW, Biver C, Camacho M, McAlaster R, Salazar AM (1998) Biophysical linkage between MRI and EEG amplitude in traumatic brain injury. NeuroImage 7: 352-367.
- Thatcher R, Collura TF (2006) Z-Score EEG Biofeedback. Int. Soc. for Neuronal Regulation, Atlanta, GA.
- Thatcher RW, Biver CJ, North D (2007) Spatial-temporal current source correlations and cortical connectivity. Clin EEG and Neuroscience 38: 35-48.
- Thatcher RW, North D, Biver C (2007) Self-organized criticality and the development of EEG phase reset. Human Brain Mapping.
- Arruda JE, Weiler MD, Valentino D, Willis WG, Rossi JS (1996) A guide for applying principal-components analysis and confirmatory factor analysis to quantitative electroencephalogram data. Int J Psychophysiol 23: 63-81.
- Burgess A, and Gruzelier J (1993) Individual reliability of amplitude distribution in topographical mapping of EEG. Electroencephalogr Clin Neurophysiol 86: 219-223.
- Corsi-Cabrera M, Solis-Ortiz S, Guevara MA (1997) Stability of EEG inter- and intrahemispheric correlation in women. Electroencephalogr Clin Neurophysiol 102: 248-255.
- Gasser T, Sroka L, Möcks J (1985) The transfer of EOG activity into the EEG for eyes open and closed. Electroencephalogr Clin Neurophysiol 61: 181-193.
- Hamilton-Bruce MA, Boundy KL, Purdie GH (1991) Interoperator variability in quantitative electroencephalography., Clin Exp Neurol 28: 219-224.
- Harmony T, Fernandez T, Rodriguez M, Reyes A, Marosi E (1993) Test-retest reliability of EEG spectral parameters during cognitive tasks: II. Coherence. Int J Neurosci 68: 263-271.
- Lund TR, Sponheim SR, Iacono WG, Clementz BA (1995) Internal consistency reliability of resting EEG power spectra in schizophrenic and normal subjects. Psychophysiology 32: 66-71.
- Salinsky MC, Oken BS, Morehead L (1991) Test-retest reliability in EEG frequency analysis. Electroencephalogr Clin Neurophysiol 79: 382-392.
- Pollock VE, Schneider LS, Lyness SA (1991) Reliability of topographic quantitative EEG amplitude in healthy late-middle-aged and elderly subjects. Electroencephalogr Clin Neurophysiol 79: 20-26.
- Thatcher RW, John ER (1977) Functional Neuroscience, Vol. 1: Foundations of Cognitive Processes, Thatcher (Ed.) L. Erlbaum Assoc N.J.
- Thatcher RW, McAlaster R, Lester ML, Horst, RL, Cantor DS (1983) Hemispheric EEG Asymmetries Related to Cognitive Functioning in Children. In: Cognitive Processing in the Right Hemisphere, A. Perecuman (Ed.) New York: Academic Press.
- Thatcher RW, Krause P, Hrybyk M (1986) Corticocortical Association Fibers and EEG Coherence: A Two Compartmental Model. Electroencephalog Clinical Neurophysiol 64: 123-143.
- Thatcher RW, Walker RA, Gerson I, Geisler F (1989) EEG discriminant analyses of mild head trauma. EEG and Clin Neurophysiol 73: 93-106.
- Thatcher RW, Cantor DS, McAlaster R, Geisler F, Krause P (1991) Comprehensive predictions of outcome in closed head injury: The development of prognostic equations. Annals New York Academy of Sciences 620: 82-104.
- Thatcher R, Wang B, Toro C, Hallett M (1994) Human neural network dynamics using multimodal registration of EEG, PET and MRI. In: R. Thatcher, M. Hallett, T. Zeffiro, E. John and M. Huerta (Eds.) Functional Neuroimaging: Technical Foundations, Academic Press: New York.
- Thatcher RW (1995) Tomographic EEG/MEG. Journal of Neuroimaging 5: 35-45.
- Thatcher RW (2000) EEG Operant Conditioning (Biofeedback) and Traumatic Brain Injury. Clinical EEG 31: 38-44.
- Thatcher RW (2000) "An EEG Least Action Model of Biofeedback" 8th Annual ISNR conference, St. Paul, MN.
- Thatcher RW, North D, Curtin R, Walker RA, Biver C, et al. (2001) An EEG Severity Index of Traumatic Brain Injury. J Neuropsychiatry and Clinical Neuroscience 13: 77-87.
- Thatcher RW, Biver CL, Gomez-Molina JF, North D, Curtin R, et al. (2001) Estimation of the EEG Power Spectrum by MRI T2 relaxation time in traumatic brain injury. Clinical Neurophysiology 112: 1729-1745.
- Thatcher RW, North D, Biver C (2005) EEG and Intelligence: Univariate and multivariate comparisons between EEG coherence, EEG phase delay and power. Clinical Neurophysiology 116: 2129-2141.
- Gasser T, Verleger R, Bacher P, Sroka L (1988) Development of the EEG of school-age children and adolescents. I. Analysis of band power. Electroencephalography and Clinical Neurophysiology 69: 91-99.
- Gasser T, Jennen-Steinmetz C, Sroka L, Verleger R, Mocks J (1988) Development of the EEG of school-age children and adolescents. II: Topography. Electroencephalography Clinical Neurophysiology 69: 100-109.
- Cooley WW, Lohnes PR (1971) Multivariate Data Analysis. New York: Wiley.
- Nunnally JC (1978) Psychometric theory. New York: McGraw-Hill.
- Prichep LS, Mas F, Hollander E, Liebowitz M, John ER (1993) Quantitative electroencephalographic subtyping of obsessive-compulsive disorder. Psychiatry Res 50: 25-32.
- Bolwig TG, Hansen ES, Hansen A, Merkin H, Prichep LS (2007) Toward a better understanding of the pathophysiology of OCD SSRI responders: QEEG source localization. Acta Psychiatr Scand 115: 237-242.
- Sari Gokten E, Tulay EE, Beser B, Elagoz Yuksel M, Arikan K (2019) Predictive value of slow and fast eeg oscillations for methylphenidate response in ADHD. Clin EEG Neurosci 50: 332-338.
- Arns M, Vollebregt MA, Palmer D, Spooner C, Gordon E (2018) Electroencephalographic biomarkers as predictors of methylphenidate response in attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 28: 881-891.
- Kanda PAM, Anghinah R, Smidth MT, Silva JM (2009) The clinical use of quantitative EEG in cognitive disorders. Dement Neuropsychol 3: 195-203.
- Ogrim G, Kropotov J, Hestad K (2012) The quantitative EEG theta/beta ratio in attention deficit/hyperactivity disorder and normal controls: sensitivity, specificity, and behavioral correlates. Psychiatry Res 15: 482-488.
- Blue Cross Blue Shield Asssociation (2014) Quantitative electroencephalography as a diagnostic aid for attention-deficit/hyperactivity disorder in children. Technol Eval Cent Assess Program Exec Summ 29: 1-6. [Crossref]
- Lehmann C, Koenig T, Jelic V, Prichep L, John RE, et al. (2007) Application and comparison of classification algorithms for recognition of Alzheimer’s disease in electrical brain activity (EEG). J Neurosci Methods 161: 342-50.
- Stylianou M, Murphy N, Peraza LR (2018) Quantitative electroencephalography as a marker of cognitive fluctuations in dementia with Lewy bodies and an aid to differential diagnosis. Clin Neurophysiol 129: 1209-1220.
- Anghinah R, Kanda PA, Lopes HF, Basile LF, Machado S (2011) Alzheimer's disease qEEG: spectral analysis versus coherence. Which is the best measurement? Arq Neuropsiquiatr 69: 871-87 4.
- Houmani N, Vialatte F, Gallego-Jutglà E, Dreyfus G, Nguyen-Michel VH (2013) Diagnosis of Alzheimer's disease with Electroencephalography in a differential framework. PLoS One 13: e0193607.
- Koenig T, Prichep L, Lehmann D, Sosa PV, Braeker E (2002) Millisecond by millisecond, year by year: normative EEG microstates and developmental stages. Neuroimage 16: 41-48.
- Prichep LS (2005) Use of normative databases and statistical methods in demonstrating clinical utility of QEEG: importance and cautions. Clin EEG Neurosci 36: 82-87.
- Goenka A, Boro A, Yozawitz E (2018) Comparative sensitivity of quantitative EEG (QEEG) spectrograms for detecting seizure subtypes. Seizure 55: 70-75.
- Jobert M, Wilson J, Ruigt GSF, Prichep LS (2012) Guidelines for the recording and evaluation of pharmaco-EEG data in man: the international Pharmaco-EEG Society (IPEG). Neuropsychobiology 66: 201-220.
- Höller Y, Helmstaedter C, Lehnertz K (2018) Quantitative Pharmaco-Electroencephalography in Antiepileptic Drug Research. CNS Drugs 32: 839-848.
- Rosadini G, Sannita WG (1978) Quantitative EEG in relation to plasma concentration during treatment with antiepileptic drugs. Proceedings of the 11th Congress of the Collegium Internationale Neuro-Psychopharmacologicum, Viena.
- Tedrus GM, Negreiros LM, Ballarim RS, Marques TA, Fonseca LC (2018) Correlations between cognitive aspects and quantitative EEG in adults with epilepsy. Clinical EEG and Neuroscience 50: 348-353. [Crossref]
- Hanley D, Prichep LS, Badjatia N, Bazarian J, Chiacchierini R, et al. (2018) A brain electrical activity electroencephalographic-based biomarker of functional impairment in traumatic brain injury: a multi-site validation trial. J Neurotrauma 35: 41-47.
- Nardi Cesarini E, Babiloni C, Salvadori N, Farotti L, Del Percio C, et al. (2020) Late-onset epilepsy with unknown etiology: A pilot study on neuropsychological profile, cerebrospinal fluid biomarkers, and quantitative eeg characteristics. Front Neurol 11: 199.
- Sansevere AJ, Hahn CD, Abend NS (2019) Conventional and quantitative EEG in status epilepticus. Seizure 68: 38-45.
- Hanley D, Prichep LS, Badjatia N, Bazarian J, Chiacchierini R, et al. (2018) A brain electrical activity electroencephalographic-based biomarker of functional impairment in traumatic brain injury: a multi-site validation trial. J Neurotrauma 35: 41-47.
- Thatcher RW, Walker RA, Gerson I, Geisler FH (1989) EEG discriminant analyses of mild head trauma. Electroencephalogr Clin Neurophysiol 73: 94-106.
- Thatcher RW, North DM, Richard Curtin MT, Walker BA, Biver BJ, et al. (2001) An EEG severity index of traumatic brain injury. J Neuropsychiatry Clin Neurosci 13: 77-87.
- Ducker TB, Cantor DL, Meyer W, Mc Alaster R (1982) Comprehensive assessment of coma in neurotrauma patients. 32nd Annu. Int. Congr. Neurol. Surg. Toronto.
- Ianof JN, Anghinah R (2017) Traumatic brain injury: An EEG point of view. Dement Neuropsychol. 11: 3-5.
- Gosselin N, Lassonde M, Petit D, Leclerc S, Mongrain V, et al. (2009) Sleep following sport-related concussions. Sleep Med 10: 35-46.
- Tebano MT, Cameroni M, Gallozzi G, Loizzo A, Palazzino G, et al. (1988) EEG spectral analysis after minor head injury in man. Electroencephalogr Clin Neurophysiol 70: 185-189.
- Chen XP, Tao LY, Chen CAN (2006) Electroencephalogram and evoked potential parameters examined in Chinese mild head injury patients for forensic medicine. Neurosci Bull 22: 165-170.
- Fenton GW (1996) The postconcussional syndrome reappraised. Clin Electroencephalogr 27: 174-182.
- McClelland RJ, Fenton GW, Rutherford W (1994) The postconcussional syndrome revisited. Journal of The Royal Society of Medicine 87: 508-510.
- Watson MR, Fenton GW, McClelland RJ, Lumsden J, Headley M (1995) The post-concussional state: neurophysiological aspects. Br J Psychiatry 167: 514-521.
- Meyer JS, Denny-Brown D (1955) Studies of cerebral circulation in brain injury. II. Cerebral concussion. Electroencephalogr Clin Neurophysiol 7: 529-544.
- Hayes RL, Katayama Y, Young HF, Dunbar JG (1988) Coma associated with flaccidity produced by fluid-percussion concussion in the cat. I: Is it due to depression of activity within the brainstem reticular formation? Brain Injury 2: 31-49.
- Nuwer MR, Hovda DA, Schrader LM, Vespa PM (2005) Routine and quantitative EEG in mild traumatic brain injury. Clin Neurophysiol 116: 2001-2025.
- Koufen H, Dichgans J (1978) Frequency and course of posttraumatic EEG-abnormalities and their correlations with clinical symptoms: a systematic follow up study in 344 adults. Fortschr Neurol Psychiatr Grenzgeb 46: 165-177.
- Lewine JD, Davis JT, Bigler ED, Thoma R, Hill D, et al. (2007) Objective documentation of traumatic brain injury subsequent to mild head trauma. J Head Trauma Rehabil 22: 141-155.
- Afonso De Medeiros Kanda P, Anghinah R, Smidth MT, Silva JM (2009) The clinical use of quantitative EEG in cognitive disorders. Dementia & Neuropsychologia 3: 195-203.
- Luccas FJ, Anghinah R, Braga NI, Fonseca LC, Frochtengarten ML (1999) Guidelines for recording/analyzing quantitative EEG and evoked potentials. Part II: Clinical aspects. Arq Neuropsiquiatr 57: 132-146.
- Jordan KG (1999) Continuous EEG monitoring in the neuroscience intensive care unit and emergency department. J Clin Neurophysiol 16: 14-39.
- Hughes JR, John ER (1999) Conventional and quantitative electroencephalography in psychiatry. J Neuropsychiatry Clin Neurosci 11: 190-208.
- Chabot RJ, di Michele F, Prichep L (2005) The role of quantitative electroencephalography in child and adolescent psychiatric disorders. Child Adolesc Psychiatr Clin N Am 14: 21-53.
- Coburn KL, Lauterbach EC, Boutros NN, Black KJ, Arciniegas DB (2006) The value of quantitative electroencephalography in clinical psychiatry: a report by the committee on research of the american neuropsychiatric association. J Neuropsychiatry Clin Neurosci 18: 460-500.
- John ER (2002) The neurophysics of consciousness. Brain Res Brain Res Rev 39: 1-28.
- No authors listed (2000) Clinical practice guideline: diagnosis and evaluation of the child with attention-deficit/hyperactivity disorder. American Academy of Pediatrics. Pediatrics 105: 1158-1170. [Crossref]
- Bresnahan SM, Anderson JW, Barry RJ (1999) Age-related changes in quantitative EEG in attention-deficit/hyperactivity disorder. Biol Psychiatry 46: 1690-1697.
- Bresnahan SM, Barry RJ (2002) Specificity of quantitative EEG analysis in adults with attention deficit hyperactivity disorder. Psychiatry Res 112: 133-144.
- Bashiri A, Shahmoradi L, Beigy H, Savareh BA, Nosratabadi M (2018) Quantitative EEG features selection in the classification of attention and response control in the children and adolescents with attention deficit hyperactivity disorder. Futur Sci OA 4: FSO292. [Crossref]
- Tas C, Cebi M, Tan O, Hızlı-Sayar G, Tarhan N (2015) EEG power, cordance and coherence differences between unipolar and bipolar depression. J Affect Disord 172: 184-190.
- Hunter AM, Cook IA, Leuchter AF (2007) The pomise of the Quantitative Electroencephalogram as a predictor of antidepressant treatment outcomes in major depressive disorder. Psychiatr Clin North Am 30: 105-124.
- Spronk D, Arns M, Bootsma A, van Ruth R, Fitzgerald PB (2008) Long term effects of left frontal rTMS on EEG and ERPs in patients with depression. Clin EEG Neurosci 39: 118-124.
- Pozzi D, Golimstock A, Petracchi M, García H, Starkstein S (1995) Quantified electroencephalographic changes in depressed patients with and without dementia. Biol Psychiatry 38: 677-683.
- Morgan ML, Cook IA, Rapkin AJ, Leuchter AF (2007) Neurophysiologic changes during estrogen augmentation in perimenopausal depression. Maturitas 56: 54-60.
- Morgan ML, Witte EA, Cook IA, Leuchter AF, Abrams M (2005) Influence of age, gender, health status and depression on quantitative EEG. Neuropsychobiology 52: 71-76.
- Koek RJ, Yerevanian BI, Tachiki KH, Smith JC, Alcock J (1999) Hemispheric asymmetry in depression and mania. A longitudinal QEEG study in bipolar disorder. J Affect Disord 53: 109-122.
- Leuchter AF, Cook IA, Uijtdehaage SH, Dunkin J, Lufkin RB, et al. (1997) Brain structure and function and the outcomes of treatment for depression. J Clin Psychiatry 16: 22-31.
- Lieber AL (1988) Diagnosis and subtyping of depressive disorders by quantitative electroencephalography: II. Interhemispheric measures are abnormal in major depressives and frequency analysis may discriminate certain subtypes. Hillside J Clin Psychiatry 10: 84-97.
- Kwon JS, Youn T, Jung HY (1996) Right hemisphere abnormalities in major depression: quantitative electroencephalographic findings before and after treatment. J Affect Disord 40: 169-173.
- Pozzi D, Golimstock A, Migliorelli R, Tesón A, García H (1993) Quantified electroencephalographic correlates of depression in Alzheimer’s disease. Biol Psychiatry 34: 386-391.
- Lieber AL, Prichep LS (1988) Diagnosis and subtyping of depressive disorders by quantitative electroencephalography: I. Discriminant analysis of selected variables in untreated depressives. Hillside J Clin Psychiatry 10: 71-83.
- Haneef Z, Levin HS, Frost JD, Mizrahi EM, Mizrahi EM (2013) Electroencephalography and quantitative electroencephalography in mild traumatic brain injury. J Neurotrauma 30: 653-656.
- Heller W, Nitschke JB, Etienne MA, Miller GA (1997) Patterns of regional brain activity differentiate types of anxiety. J Abnorm Psychol 106: 376-385.
- Davidson RJ, Marshall JR, Tomarken AJ, Henriques JB (2000) While a phobic waits: regional brain electrical and autonomic activity in social phobics during anticipation of public speaking. Biol Psychiatry 47: 85-95.
- Wiedemann G, Pauli P, Dengler W, Lutzenberger W, Birbaumer N, et al. (1999) Frontal brain asymmetry as a biological substrate of emotions in patients with panic disorders. Arch Gen Psychiatry 56: 78-84.
- Ribas VR, Ribas RG, Nóbrega JA, da Nóbrega MV, Espécie JAA (2018) Pattern of anxiety, insecurity, fear, panic and/or phobia observed by quantitative electroencephalography (QEEG). Dement Neuropsychol 12: 264-271.
- Bong SH, Choi TY, Kim KM, Lee J, Kim JW (2020) Correlation between executive function and quantitative EEG in patients with anxiety by the Research Domain Criteria (RDoC) framework. Sci Rep 10: 18578.
- Nuwer MR, Comi G, Emerson R, Fuglsang-Frederiksen A, Guérit JM (1998) IFCN standards for digital recording of clinical EEG. International Federation of Clinical Neurophysiology. Electroencephalogr Clin Neurophysiol 106: 259-261.
- Klass DW, Brenner RP (1995) Electroencephalography of the elderly. J Clin Neurophysiol 12: 116-131.
- Duffy FH, Burchfiel JL, Lombroso CT (1979) Brain electrical activity mapping (BEAM): A method for extending the clinical utility of EEG and evoked potential data. Ann Neurol 5: 309-321.
- Martin-Loeches M, Gil P, Jimenez F, Exposito FJ, Miguel F (1991) Topographic maps of brain electrical activity in primary degenerative dementia of the Alzheimer type and multiinfarct dementia. Biol Psychiatry 29: 211-223.
- Saletu B, Paulus E, Grunbergerer J, Maurer K (1993) Correlation maps: on the relation of electroencephalographic slow wave activity to computerized tomography and psycopathometric measurements in dementia in Maurer K. Imaging of Brain in Psychiatry and Related Fieldsed 263-265.
- Claus JJ, Strijers RL, Jonkman EJ, Ongerboer de Visser BW, Jonker C, et al. (1999) The diagnostic value of electroencephalography in mild senile Alzheimer’s disease. Clin Neurophysiol 110: 825-832.
- Nielsen T, Montplaisir J, Lassonde M (1993) Decreased interhemispheric EEG coherence during sleep in agenesis of the corpus callosum. Eur Neurol 33: 173-176.
- Leuchter AF, Spar JE, Walter DO, Weiner H (1987) Electroencephalographic spectra and coherence in the diagnosis of Alzheimer’s-type and multi-infarct dementia. A pilot study. Arch Gen Psychiatry 44: 993-998.
- Yuvaraj R, Murugappan M, Mohamed Ibrahim N, Iqbal M, Sundaraj K, et al. (2014) On the analysis of EEG power, frequency and asymmetry in Parkinson’s disease during emotion processing. Behav Brain Funct 10: 12.
- Cozac VV, Gschwandtner U, Hatz F, Hardmeier M, Rüegg S, et al. (2016) Quantitative EEG and Cognitive Decline in Parkinson's Disease. Parkinsons Dis 9060649.
- Hunter A, Crouch B, Webster N, Platt B (2020) Delirium screening in the intensive care unit using emerging QEEG techniques: A pilot study. AIMS Neurosci 7: 1-16.
- Collura T, Thatcher R, Smith ML, Lambos W, Stark C (2009) EEG biofeedback training using live z-scores and a normative database. Ed. 2nd Philadelphia, PA: Elsevier.
- Thatcher RW (2010) Z-score biofeedback. NeuroConnections 4-17.
- Thatcher RW (2012) Latest developments in live z-score training: Symptom check list, phase reset, and LORETA z-score biofeedback. Journal of Neurotherapy 17: 69-87.
- Thatcher RW, North DM, Biver CJ (2014) Technical foundations of z score neurofeedback. In R. W. Thatcher & J. F. Lubar Z Score Neurofeedback: Clinical Applications. San Diego, CA: Academic Press.
- Sterman M B, Kaiser D (2001) Comodulation: A new qEEG analysis metric for assessment of structural and functional disorders of the central nervous system. Journal of Neurotherapy 4: 73-83
- Gordon E, Cooper N, Rennie C, Hermens D, Williams LM (2005) Integrative neuroscience: the role of a standardized database. Clin EEG Neurosci 36: 64-75.
- Chicurel M (2000) Databasing the Brain. Nature 406: 822-825.
- Tamara D (2003) Quantitative EEG Normative Databases: A comparative investigation. Journal of Neurotherapy 7: 3-4.
- Hernandez-Gonzalez G, Bringas-Vega ML, Galán-Garcia L (2011) Cuban human brain mapping project (CHBMP). Multimodal quantitative neuroimaging databases and methods: the Cuban Human Brain Mapping Project. Clin EEG Neurosci 42: 149-159.
- Duffy FH, Burchfiel JL, Lombroso CT (1979) Brain electrical activity mapping (BEAM): A method for extending the clinical utility of EEG and evoked potential data. Annals of Neurology 5: 309-321.





