Abstract
Telocyte is a type of communicating interstitial cells related to various types of cells and tissues. The current study aimed to investigate the role of telocytes in nephrogenesis. Samples of mesonephros of quail embryos were processed for light microscope and TEM examinations. Mesonephros had a well-organized nephrogenic structure except for the peripheral nephrogenic zone where mesonephric mesenchyme developed new nephrons. Stromal cells were identified by Methylene blue, PAS, Safranin O, Marsland silver stain, Grimelius’s silver nitrate method, Mallory trichrome. By TEM, abundant telocytes were identified in the peripheral nephrogenic zone of the mesonephros where the epithelioid cells aggregated and established closed contact. While, the central nephrogenic zone had a scant number of telocytes where they interspersed between renal tubules. Telocytes released the secretory vesicles in the vicinity to the epitheliod cells. In conclusion, telocytes prevailed the embryonic tissue during the development of mesonephros of quail. Further researchers should consider expression of nephrogenic factors by telocytes during renal development. This may provide further insights into the pathogenesis of renal congenital anomalies.
Key words
telocytes, mesonephric kidney, progenitor cells, quail, embryos
Introduction
Telocytes are a special type of communicating cells, which are related to a wide range of cells and structures. Functional diversity of telocytes depends on the type of target cells [1]. Telocytes have unique characteristics features. They have the cell body and several cell prolongations; telopodes which may extend to hundreds of microns. Telopodes establish cellular connections with different types of cells to form a complex communicating network. Telopodes have thin segments; podomers and interval expansions; podoms which are rich in calcium release units; mitochondria, endoplasmic reticulum, and caveolae. Telocytes establish a synaptic junction connecting to immunoreactive cells such as eosinophil [2].
Telocytes influence target cells either by establishing cellular contact or through paracrine mode. Two types of cellular contact are described for telocytes; homocellular and heterocellular contact. Homocellular contact is formed between two telocytes while, heterocellular type communicates between telocytes and other stromal cells either fixed or the free cells. Different types of cellular contacts are documented for telocytes including direct apposition of the cell membrane, adherence, and gap junction. Gap junction formed a communicating channels for transmission of intercellular signaling pathway [3]. Paracrine signaling is achieved by delivering the microvesicles, and macromolecules such as proteins or RNAs, microRNA to the target cells. Telocytes also secrete exosomes, ectosomes and multivesicular vesicles [2, 4, 5].
Telocytes serve many functions. based on detection of gap junction between telocytes and muscles cells, telocytes have been implicated in generation and transmission of signaling to muscle cells via gap junction [6-10]. Connection of telocytes with different types of immune cells such as eosinophil [5], mast cell, and macrophage [11] indicate their potential role in immune defense. Relations between telocytes and stem or progenitor cells have been widely investigated and raise the suggestion that telocytes have a significant role in repairing of different organs such as heart, lung, skeletal muscle, skin, meninges and choroid plexus, eye, liver, uterus, urinary system [12].
Kidney develops through three sequential stages. They arise from the intermediate mesoderm (nephrogenic cord) as series of transitional structures in a craniocaudal manner; pronephros and mesonephros. While, metanephros remains as the permanent excretory organ in mammals. Pronephros is considered the primitive kidney. The mesenchyme of nephrogenic cord develops the pronephric tubules and duct. Pronephros is functioning in amphibian and fish while in mammals, pronephros degenerate during fetal development. Mesonephric duct develops as a continuation of the pronephric duct. Pronephros is replaced by mesonephros in adult amphibian and fish. Mesonephric tubules and duct are also differentiated form nephrogenic cord mesenchyme. The Mesonephric duct gives rise to an outgrowth (ureteric bud); which is composed of condensed nephrogenic mesenchyme (metanephric duct). Metanephric duct grows toward the mesodermal tissue of the metanephrogenic blastema. Metanephric duct forms the collecting duct system, while metanephric blastema develop into the nephrons [13-15].
On the basis that telocytes serve in stem cell differentiation. The current hypothesis is that telocytes could be identified during differentiation of embryonic stem cells. The current study used quail embryos to investigate existence of telocytes during development of mesonephros.
Materials and methods
Sampling
The study used fertile quail (Coturnix coturnix japonica) eggs obtained from the Research Quail Farm connected to the Department of Histology, Faculty of Veterinary Medicine, South Valley University in, Qena, Egypt. The fertilized eggs were incubated in a c10 “POULTRY TECHNICAL OFFICE, Alexandria, Egypt” at 37.5°C with a relative humidity of 65%. The eggs were rotated automatically every 6 hours after the 3rd day of incubation. We collected 21 quail embryos at day 8, 10, 12, and 15 of incubations. Eggshells were opened at the broad end, and apparently healthy embryos were carefully excised from their shells. Embryos were euthanized by decapitation, Mesonephros were carefully dissected and were immediately fixed in 10% buffered formalin for 3 days for preparation of paraffin embedding specimens. Other samples were fixed in glutaraldehyde (10 mL of 2.5% glutaraldehyde and 90 mL 0.1 M Na-phosphate buffered formalin) for preparation of resin embedding specimens.
Preparation of paraffin embedding specimens
Tewelve embryos were used for light microscpic examination. Formalin-Fixed samples were dehydrated in ascending grades of alcohols at 70%, 80%, 90% and 100% for 90 minutes at each concentration. The sampled were cleared using methyl benzoate. Dehydrated samples were then impregnated and embedded in Paraplast (Sigma Aldrich). Serial sections of 3-5µm were cut using a Richert Leica RM 2125 Microtome, Germany and mounted on glass slides. Sections were kept in an incubator at 40°C for dryness and stained with H&E [16]. Stained sections were examined using DMLS light microscope (Leica, Germany) outfitted with MC120 HD camera (Leica, Germany).
Histochemical investigations
The sections were stained by Periodic acid-Schiff reaction [17] for detection of neutral polysaccharides. Safranin O was used for detection glycosaminoglycans [18]. Other histochemical stains were used including Mallory trichrome [19] and Marsland [20], Grimelius’s silver nitrate method [21], methylene blue [22]. All staining was cited in Bancroft’s theory and practice of histological [22]. Stained sections were examined by Leitz Dialux 20 Microscope. Photos were taken using a Canon digital camera (Canon Powershot A95).
Preparation of resin embedding specimens for semi-thin and ultra-thin sectioning
Five embryod were processed for resin embedding . Glutaraldehyde-fixed samples of mesonephros were cut into small pieces. They were washed 4 times for 15 minutes in 0.1 M sodium phosphate buffer (pH 7.2) then were post-fixed in 1% osmic acid in 0.1 M Na-phosphate buffer at 4°C for 2 hours. The osmicated samples were washed 3 times for 20 minutes in 0.1 M phosphate buffer (pH 7.2). Dehydration was performed through graded action (70, 80, 90, 100%), 10 minutes for each concentration. The dehydrated samples were immersed in a mixture of acetone/resin. The specimens were embedded in the resin at 60 °C for 3 days. Polymerized samples were cut to semi-thin sections by using a ultramicrotome Ultracut E (Reichert-Leica, Germany) and stained with toluidine blue [23].
Ultrathin sections were obtained by a Reichert ultra-microtome. The sections (70 nm) were stained with uranyl acetate and lead citrate (Reynolds, 1963) and examined by JEOL100CX II transmission electron microscope (TEM) at the CENTRAL LABORATORY UNIT of South Valley University.
Sample preparation for SEM
Four embryos were used for SEM. Specimens were washed with 0.1 M Na-phosphate buffer. Then they were fixed in were fixed in Karnovsky fixative [24] for 4 hours at 4 ◦C. Thereafter, they were washed in the same buffer used in fixation 5 minutes x 4 times and post-fixed in 1% osmic acid in 0.1 M Na-phosphate buffer for further 2 hours at room temperature. They were washed by 0.1 M Na- phosphate buffer 15 minutes x 4 times. The samples were dehydrated by alcohol 50%, 70%, 90% for 30 min in each concentration and 100% for 2 days with changes many times followed by isoamyl acetate for 2 days and then subjected to critical point drying method with a polaron apparatus. Finally, they were coated with gold using JEOL -1100 E ion sputtering Device and observed with JEOL scanning electron microscope (JSM – 5400 LV) at KV10.
Coloring images
Transmission electron microscopy images were colored using photo filter 6.3.2 program. Coloring images required to change the color balance, using the stamp tool to color the objective cells.
Results
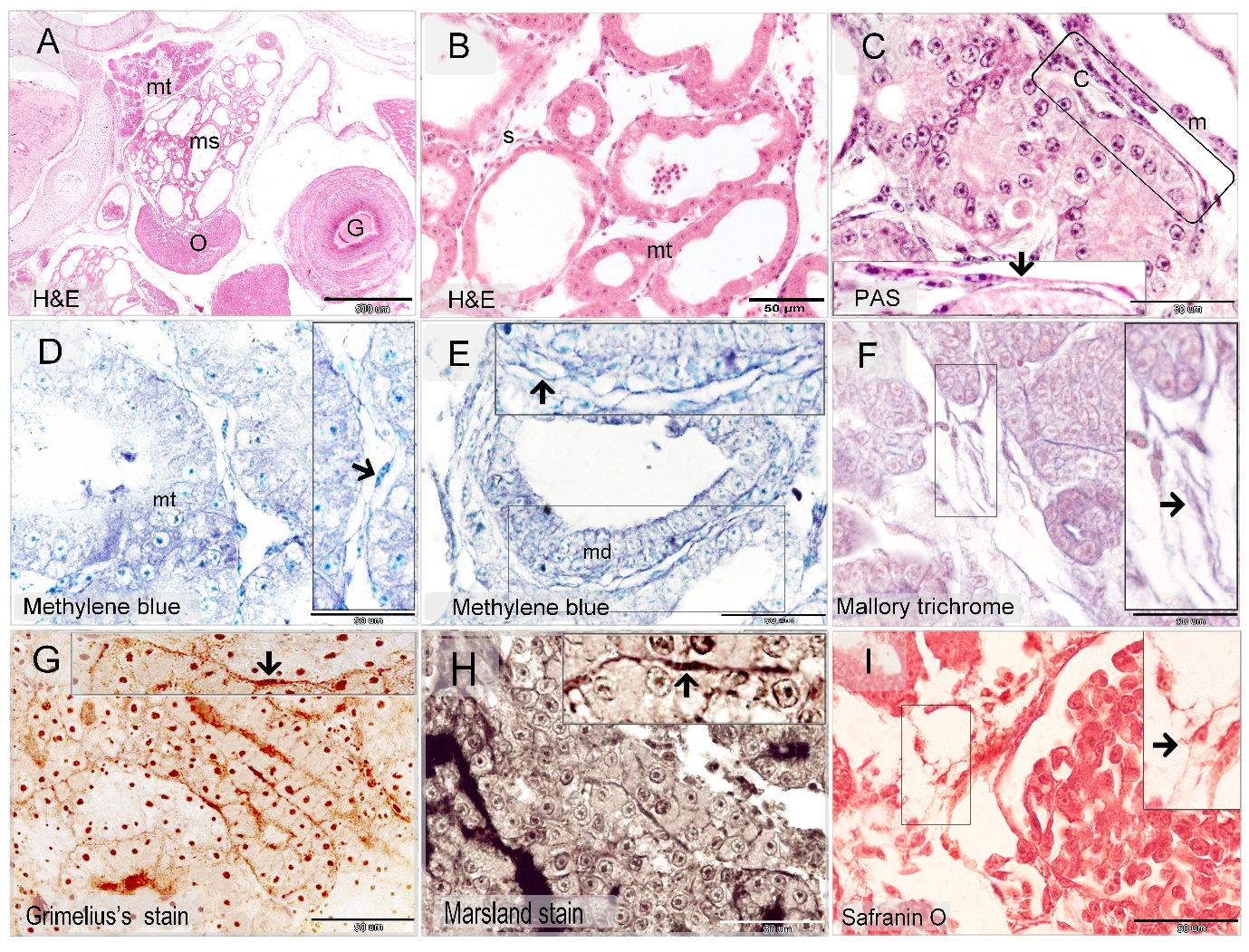
The current study investigated telocytes during the development of mesonephric kidney in quail embryos and their relation to nephrogenic progenitor cells. Stromal cells were identified between the renal tubules of the mesonephric kidney by using H&E (Figures 1A, B) Methylene blue (Figures 1D, E), PAS (Figure 1C), Safranin O (Figure 1I), Marsland silver stain (Figure 1H), Grimelius’s silver nitrate method (Figure 1G), Mallory trichrome (Figure 1F).
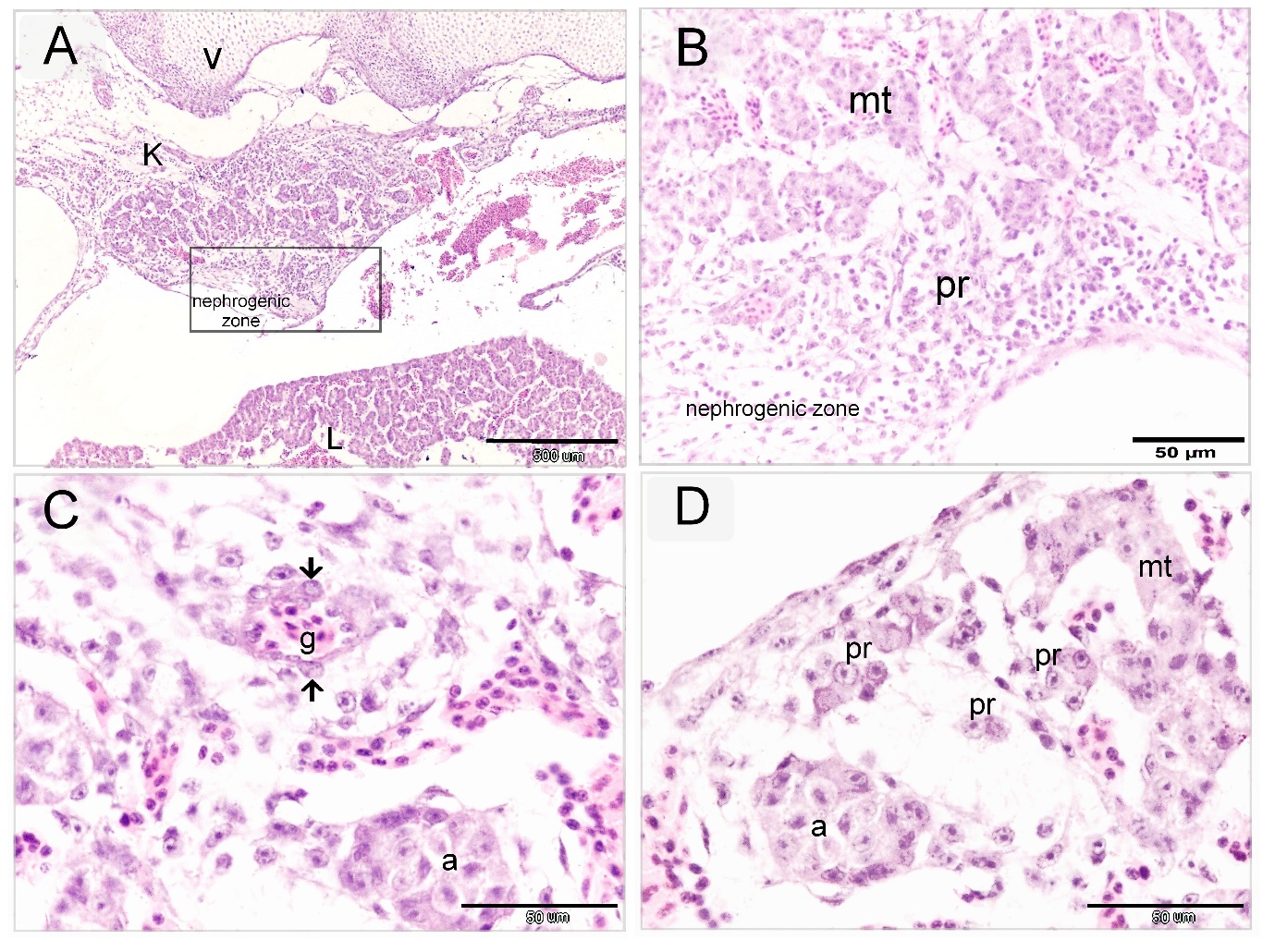
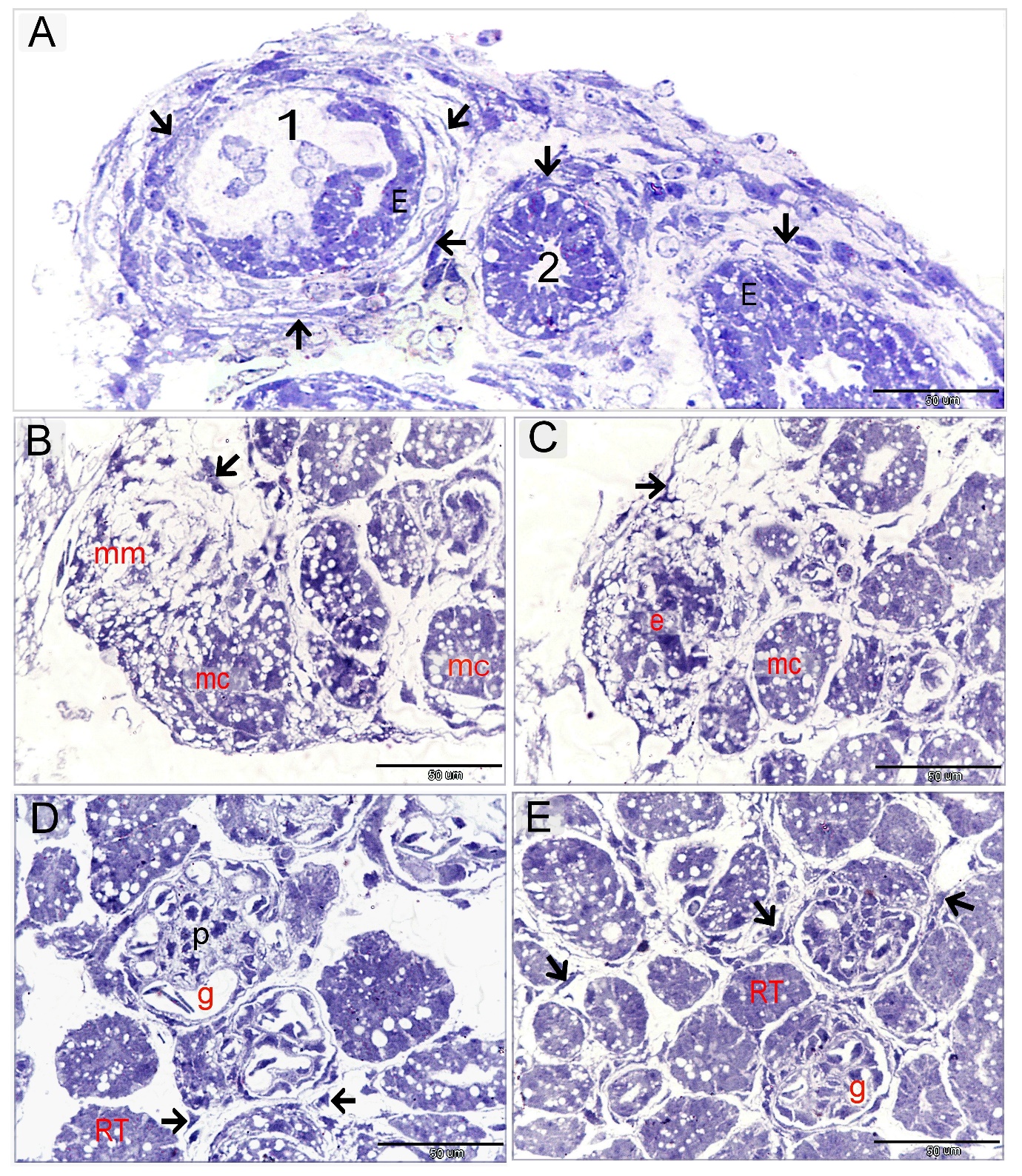
Mesonephros was distinguished into two regions; the central zone had well-developed nephrons (Figures 2A, B, Fig 3E) and the peripheral nephrogenic zone which was rich in nephrogenic progenitor cells which transformed into mesonephric epithelial cells. The epithelioid cells aggregated to develop the mesonephric cords (Figures 2 B-D, Figures 3A-D). By using paraffin and semi-thin sections, mesonephric tubules were seem to develop by differentiation of the mesonephric mesenchyme into epithelioid masses or aggregates of mesonephric cells which established cellular contact and formed the epithelial sheath of the mesonephric cords (Figure 2D, Figures 3A-C). Telocytes were distinguished in both peripheral and central nephrogenic zones. Abundant telocytes were observed around the developing mesonephric cord (Figure 3A).
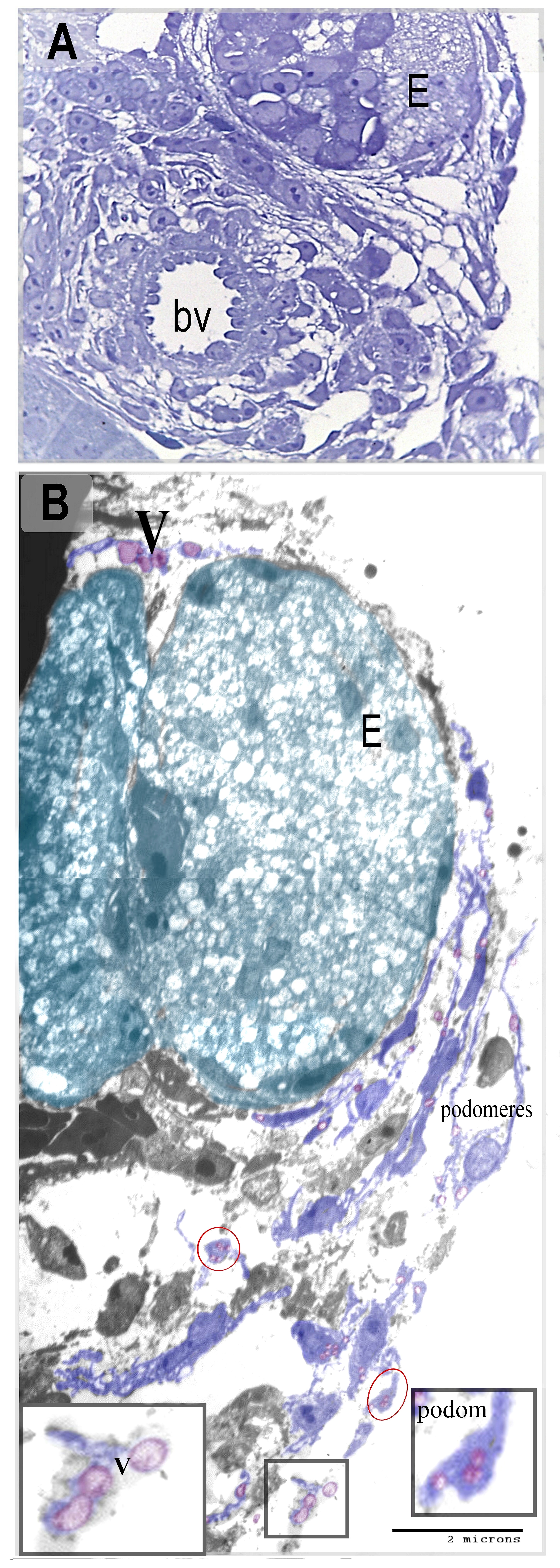
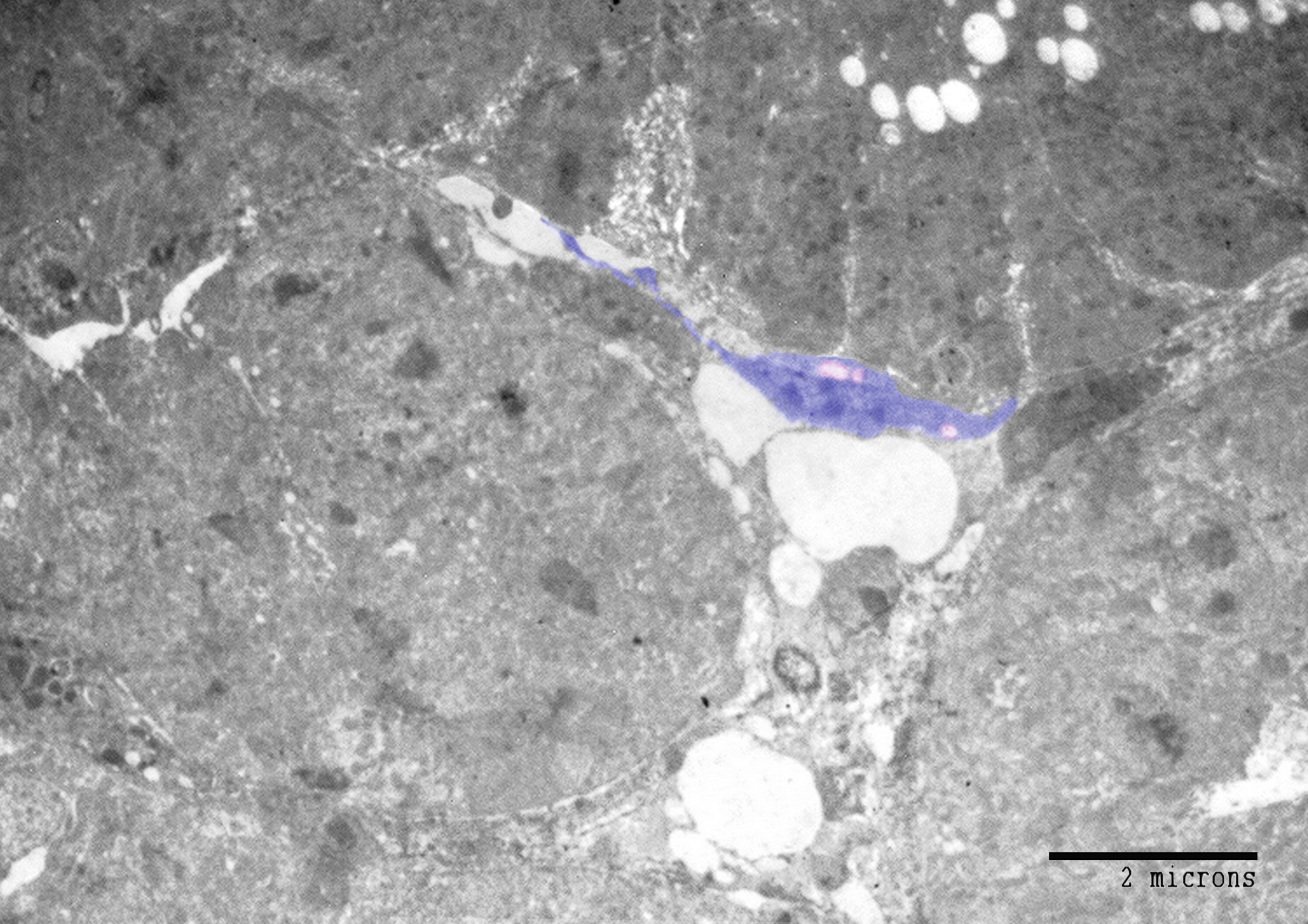
By TEM and SEM, a large number of telocytes were seen to be located in the peripheral nephrogenic zone of the mesonephros where the epithelioid cells aggregated. (Figure 4A, B, Figure 6). While the central nephrogenic zone had a scant number of telocytes where they interspersed between renal tubules (Figure 5). Telocytes shed the secretory vesicles closed to the mesonephric cords (Figure 4B).
Discussion
The current study investigated telocytes during the development of the mesonephros in quail embryos by light microscopic examination using consequential histological staining, ESM and TEM.
Using light and TEM, mesonephros had a well-organized nephrogenic structure except for the peripheral nephrogenic zone in which different stages of the developing nephrons were observed. Mesonephric mesenchyme differentiated into epithelioid masses which established cellular contact and formed the epithelial sheath of the mesonephric cords. The developmental events of the avian mesonephros is similar to human. The intermediate mesoderm forms the nephrogenic cord which develop the mesonephric tubules [25].
Stromal cells were identified between the renal tubules of the mesonephric kidney by using Methylene blue, Grimelius’s silver nitrate method, Marsland sliver stain, Mallory trichrome, PAS, Safranin O, toluidine blue. Telocytes were distinguished by TEM. The peripheral nephrogenic zone was rich in telocytes which surrounded the epithelioid cells of nephrogenic cords. Telocytes shed the secretory vesicles to the extracellular matrix. Telocytes in the central zone reduced in number and were interspersed between the renal tubules. Thus, the current study recognized telocytes during development of mesonephros of quail embryos. Pervious researches identified telocytes in the sub-capsular space [26], interstitium of the cortex surrounding renal tubules and vascular walls in the human kidney [27]. Telocytes have been investigated in improving renal injury. Transplantation of telocytes promote renal repair and regeneration after ischaemia–reperfusion injury. The authors concluded that telocytes have the protective effect against ischaemia–reperfusion injury through inflammation-independent mode [28].
In conclusion, telocytes distinguished in mesonephros of quail embryos. Future investigations should concern in whether telocytes express nephrogenic factors or not.
References
- Varga I, Danisovic L, Kyselovic J, Gazova A, Musil P, Miko M, et al. (2016) The functional morphology and role of cardiac telocytes in myocardium regeneration. Can J Physiol Pharmacol: 1-5. [Crossref]
- Popescu LM, MS Faussone-Pellegrini (2010) Telocytes - a case of serendipity: the winding way from Interstitial Cells of Cajal (ICC), via Interstitial Cajal-Like Cells (ICLC) to telocytes. J Cell Mol Med 14: 729-740. [Crossref]
- Mirancea N (2016) Telocyte-a particular cell phenotype. Infrastructure, relationships and putative functions. Rom J Morphol Embryol 57: 7-21. [Crossref]
- Popescu LM, Gherghiceanu M, Cretoiu D, Radu E (2005) The connective connection: interstitial cells of Cajal (ICC) and ICC-like cells establish synapses with immunoreactive cells. Electron microscope study in situ. J Cell Mol Med 9: 714-730. [Crossref]
- Cantarero Carmona I, MJ Luesma Bartolome, Junquera Escribano (2011) Identification of telocytes in the lamina propria of rat duodenum: transmission electron microscopy. J Cell Mol Med 15: 26-30. [Crossref]
- Takaki M (2003) Gut pacemaker cells: the interstitial cells of Cajal (ICC). J Smooth Muscle Res 39: 137-161. [Crossref]
- Hutchings G, Williams O, Cretoiu D, Ciontea SM (2009) Myometrial interstitial cells and the coordination of myometrial contractility. J Cell Mol Med 13: 4268-42682. [Crossref]
- Gandahi JA, Chen SF, Yang P, Bian XG, Chen QS (2012) Ultrastructural identification of interstitial cells of Cajal in hen oviduct. Poult Sci 91: 1410-1417. [Crossref]
- Drumm BT, Koh SD, Andersson KE, Ward SM (2014) Calcium signalling in Cajal-like interstitial cells of the lower urinary tract. Nat Rev Urol 11: 555-564. [Crossref]
- Smythies J, Edelstein (2014) Telocytes, exosomes, gap junctions and the cytoskeleton: the makings of a primitive nervous system? Front Cell Neurosci 7: 278. [Crossref]
- Gherghiceanu M, Popescu L (2012) Cardiac telocytes - their junctions and functional implications. Cell Tissue Res 348: 265-279. [Crossref]
- Bei Y, Wang F, Yang C, Xiao J (2015) Telocytes in regenerative medicine. J Cell Mol Med 19: 1441-1454. [Crossref]
- Brown DL, Walling BE, Mattrix ME (2016) Atlas of Histology of the Juvenile Rat, in Chapter 13: The Urinary System, Parker GA and Picut CA (Eds) Academic Press, USA. pp: 395-396.
- Ruchelli ED, Huff DS (2011) Color Atlas of Fetal and Neonatal Histology, in chapter 7: Kidney, Ernst LM, Ruchell ED and Huff DS (Eds) Springer Science & Business Media, Germany. pp: 107-108.
- Bellairs R, Osmond M (2014) Atlas of Chick Development. Academic Press, USA.
- Harris HF (1898) A new method of ripening hematoxylin. In: Mikroskopische Technik. Romeis in, Oldenburg, München.
- McMANUS JF (1948) Histological and histochemical uses of periodic acid. Stain Technol 23: 99-108. [Crossref]
- Tran D, Golick M, Rabinovitz H, Rivlin D, Elgart G, et al. (2000) Hematoxylin and safranin O staining of frozen sections. Dermatol Surg 26: 197-199. [Crossref]
- Mallory (1936) Stain technology 11: 101.
- Marsland TA, Glees P, Erikson LB (1954) Modification of the Glees silver impregnation for paraffin sections. J Neuropathol Exp Neurol 13: 587-591. [Crossref]
- Grimelius L (1968) A silver nitrate stain for alpha-2 cells in human pancreatic islets. Acta Soc Med Ups 243-270. [Crossref]
- Bancroft JD, Layton C, Suvarna SK (2013) Bancroft's Theory and Practice of Histological Techniques. London, Churchill Livingstone, 7th (edn), United Kingdom.
- Bancroft JD, Layton C, Suvarna SK, (2013) Bancroft's Theory and Practice of Histological Techniques, London, Churchill Livingstone, United Kingdom.
- Karnovsky MJ (1965) A Formaldehyde-Glutaraldehyde Fixative of High Osmolarity for use Electron Microscopy. Cell Biol 27(137 A).
- Abdul Hamid R (1998) Human Embryology Made Easy. Florida CRC Press. USA pp: 224.
- Zheng Y, Zhu T, Lin M, Wu D, Wang X (2012) Telocytes in the urinary system. J Transl Med 10: 188. [Crossref]
- Qi G, Lin M, Xu M, Manole CG, Wang X, et al. (2012) Telocytes in the human kidney cortex. J Cell Mol Med 16: 3116-3122. [Crossref]
2021 Copyright OAT. All rights reserv
- Li L, Lin M, Li L, Wang R, Zhang C, et al. (2014) Renal telocytes contribute to the repair of ischemically injured renal tubules. J Cell Mol Med 18: 1144-1156. [Crossref]