Backgroung & purpose: Necrotizing enterocolitis (NEC) with high morbidity and mortality rates is a frequent gastrointestinal disease among preterm infants. This study was conducted to evaluate any relationships between maternal/ neonatal serum vitamin D concentrations and the incidence of necrotizing enterocolitis in preterm newborns.
Methods: A prospective case-control study was carried out in an Iranian hospital in 2018.
Patients and methods: Thirty-two NICU hospitalized neonates due to NEC and 32 hospitalized neonates due to prematurity with their mothers were considered as the case and control groups. Immediately after delivery, 5 ml of the mother’s blood was collected and sent to the laboratory. Two ml of neonate's blood was also collected in the time of admission and sent to the laboratory. Our primary objective was to assess the association between maternal/neonatal vitamin D serum concentrations and the risk of NEC.
Results: The means of maternal and neonatal serum vitamin D were 35.00 ± 15.94 and 33.29 ± 14.96. There was a significant positive correlation between maternal and neonatal vitamin D status (p=0.0001). There were significant associations between NEC and some neonatal factors including neonate's low birth weight (p=0.01), head circumference (p=0.02), and height (p=0.03), as well as low Apgar score at first minute (p=0.04). No significant associations were observed between NEC with maternal and neonatal levels of vitamin D status.
Conclusion: Our results showed a significant positive correlation between maternal and neonatal vitamin D status. Although some neonatal characteristics were significantly correlated to NEC, this significant association was not observed with maternal/neonatal levels of vitamin D.
vitamin D, enterocolitis, necrotizing, preterm birth, mothers
Necrotizing enterocolitis (NEC) is a frequent gastrointestinal inflammatory disease among preterm infants and characterized by bowel wall necrosis. The incidence of NEC and its related mortality rate in preterm infants with very low birth weight were reported 5–10% and 15-30%, respectively [1-3]. The pathophysiology of NEC is unclear; however, some multifactorial risk factors have been reported. Genetic predispositions, preterm birth, intestinal immaturity, hemodynamic instability, intestinal microbial ecology, non-breastfeeding nutritions, microbial abnormalities in the digestive system, and exaggerated responses of the immune system are some of them [4-6].
Recently some investigations have focused on the influences of 1, 25-Dihydroxy vitamin D3 on inflammatory bowel diseases. Vitamin D as a key modulator of the immune system influences cells' adaptive and innate responses. Inhibition of T helper cells proliferation, a decrease of interleukin, interferon-g and tumor necrosis factor productions were demonstrated by vitamin D. Moreover, vitamin D receptors (VDR) are expressed in both colonic mucosa and immune cells [7-9].
Preserving the lives of preterm neonates with the ever-increasing improvement of neonatal intensive care is now possible; however, some serious complications like NEC threaten them. Regarding such severe diseases, finding possible associated risk factors, early diagnosis and treatment can improve the neonatal outcome [10]. Vitamin D deficiency is a worldwide complication with the highest prevalence rate by 60-80% among high-risk population including pregnant women, low dietary vitamin D intake, and limited sun exposure [11,12]. There are very few studies that assessed correlations between maternal serum vitamin D status and prevalence of NEC [13,14]. On the other hand, it seems more approaches are needed because of diversities in the population's ethnicity, type of clothing and dietary intake in different geographic areas. So this study was conducted to evaluate the relationship between maternal & neonatal serum vitamin D concentrations and necrotizing enterocolitis in NICU hospitalized newborns. Our results can provide informative data related such correlation among Iranian mothers with highly prevalent vitamin D deficiency and the possible preventing role of vitamin D supplementation during pregnancy against neonatal NEC prevalence.
Study design
A prospective case-control study was carried out in the NICU of Yas women Hospital affiliated to Tehran University of Medical Sciences (Tehran-Iran) in 2018. Population study was sixty-four singletone preterm neonates (gestational age<36 weeks) and their mothers. The case group consisted of 32 NICU hospitalized neonates with NEC diagnosis. NEC diagnosis and its staging were considered based on clinical and radiographic findings [15]. Thirty-two other NICU hospitalized newborns due to prematurity were also considered as the controls. Both case and control groups were matched regarding their age and gestational ages.
Exclusion criteria were congenital anomalies, spontaneous intestinal perforation and neonates without parents' written consent.
Immediately after delivery, 5 ml of the mother’s blood was collected, labeled, and sent to the laboratory to assay serum vitamin D level by 25-Hydroxy Vitamin D Elisa method. Vitamin D insufficiency and deficiency were defined as serum concentrations of vitamin D 20 to 30 ng/ml and concentration < 20 ng/ml, respectively [16].
Determining neonatal vitamin D status, 2 ml of the neonate's blood was collected in the time of admission. Blood samples were labeled and sent to the laboratory to assay serum 25- (OH)-vitamin D level. Detailed demographic and clinical data related neonates and mothers including maternal age, perinatal complications, history of using vitamin D supplement during pregnancy, twin pregnancy, type of delivery, corticosteroid administration, neonatal birth weight, gestational age, sex, first- & fifth-minutes Apgar scores and the number of neonatal deaths during hospitalization were recorded in checklists.
Based on an investigation by certinkaya et al., [14] the mean vitamin D serum concentrations among NEC suspected and unsuspected newborns were 15.78 ± 5.5 and 22.91 ± 10.3. With using formula, the proposed sample size of 32 for each group, our study had a power of 80% and an alpha error of 0.05.
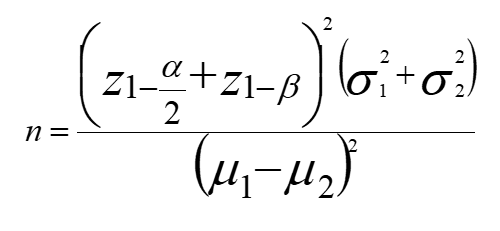
alfa=0.05
Beta=0.2
μ1=15.78
μ2=22.91
SD1=5.5
SD2=10.3
Z1-a/2=1.96
Z1-B=0.84
n=32
Primary/ Secondary outcomes
Our primary objective was to assess the association between maternal-neonatal 1,25 dihydroxy vitamin D serum concentrations and the risk of NEC. The secondary objective was assessment of correlations between neonatal NEC with some factors like mother's age, type of delivery and vitamin D supplement usage during pregnancy.
Ethical considerations
The present study was taken from the medical student thesis with ID; 9611165006. Ethics approval was obtained from the institutional review board of Tran University of Medical Sciences according to Helsinki declaration (IR.TUMS.VCR.REC.1397.875]. All participants' parents gave written consent before enrollment. Moreover, no extra cost was imposed on our participants.
Data analysis
Analyses were statistically performed by using software package
SPSS
version 16. Quantitative and qualitative variables were reported by mean ± SD and percent, respectively. Normal distribution related all variables have been shown by Kolmogorov-Smirnov test. Independent Student t test was used for comparing quantitative data and Chi square was used to analyze the correlations between qualitative variables. Linear Logistic regression analyses were also performed to analyze the correlations between variables and omit the effects of confounding factors. Also Bootstrapping analysis was used because of small sample size. The level of significance was considered as P < 0.05, Power = 80%.
Sixty-four neonates with mean gestational age 31.03 ± 1.62 weeks, 40.6% males and 50% females entered the study. The mean age of mothers was 30.18 ± 5.25518 years. Four mothers (6.8%) had normal vaginal delivery while 55 (93.2%) had cesarean section. Of all mothers, 28 subjects (43.8%) had twin and 5 mothers (7.8%) had triplet gestations. Thity-nine mothers (60.9%) had no complications however, 25 mothers (39.1%) had a history of diabetes, chronic hypertension, hypothyroidism disorder. Of all mothers, 58 (90.6%) did not receive vitamin D supplements during pregnancy while 6 mothers (9.4%) consumed vitamin D regularly. Moreover, three mothers (4.7%) had recived corticosteroid in third trimester. Of all, six neonates (10.2%) died during NICU hospitalization. Some maternal and neonatal characteristics are shown in table 1.
Table 1. Neonatal-maternal demographic and clinical data
Variables |
|
Neonatal Characteristics |
|
Birth weight (gram; Mean ± SD) |
1516.32 ± 463.17 |
Height (Cm; Mean ± SD) |
40.79 ± 4.42 |
Head circumference (Mean ± SD) |
28.82 ± 2.74 |
First minute Apgar score (Mean ± SD) |
6.65 ± 2.16 |
Fifth minute Apgar score (Mean ± SD |
8.61 ± 1.53 |
Maternal Characteristics |
|
Gravida (Mean ± SD) |
2.10 ± 1.37 |
Abortion (Mean ± SD) |
0.55 ± 1.03 |
Gestational hypertension (n%) |
1 (1.6) |
Fetal heart rate disorder (n%) |
6 (9.4) |
Gestational diabetes (n%) |
2 (3.1) |
Premature rupture of membrane (n%) |
6 (9.4) |
Preeclampsia (n%) |
10 (15.6) |
Placenta abruption (n%) |
1 (1.6) |
Mixed prenatal disorders (n%) |
8 (12.6) |
Diabetes mellitus (n%) |
4 (6.3) |
Chronic hypertension (n%) |
12 (18.8) |
Thyroid disorders (n%) |
7 (10.9) |
Chronic hypertension & hypothyroidism (n%) |
1 (1.6) |
Chronic hypertension & diabetes mellitus (n%) |
1 (1.6) |
Other diseases (n%) |
13 (20.3) |
The mean maternal and neonatal serum vitamin D were 35.00 ± 15.94 (Min=8.90, Max=74.20) and 33.29 ± 14.96 (Min=2.51, Max=79.30), respectively. There was a significant positive correlation between maternal and neonatal vit D status (p=0.0001; β=0.609; 95% CI:0 .398,0.782); in which every one unit increase of maternal vit D could increase neonatal vit D level by 0.6 unit.
Based on the results, the mean of gestational age in the case group was significantly lower than the control group (p<0.001). The means of birth weight, height and head circumference in the case subjects were also lower than in the counterpart group (p<0.001). There was a significant difference between case and control groups regarding the type of delivery (p=0.049). Moreover, more neonates in the case group were female while more participants in the control group were male (p=0.039). On the other hand, there were no significant differences between groups with respect to levels of maternal and neonatal vitamin D statuses (p>0.05). The frequencies of maternal and neonatal vit D deficiency or insufficiency were not also different between the two NEC and control groups (p=0.798 & p=0.595). Furthermore, receiving vitamin D as well as corticosteroid during pregnancy could not affect the prevalence of neonatal NEC (p>0.05) (Table 2).
Table 2. Comparison of neonatal and maternal varibles between case and control groups
p value |
Case
n=32 |
Control
n=32 |
Variables |
<0.001 |
29.97 ± 2.09 |
32.09 ± 1.62 |
Gestational age (week; Mean ± SD) |
0.482 |
30.66 ± 4.42 |
29.70 ± 5.98 |
Mother's age (year; Mean ± SD) |
<0.001 |
1292.81 ± 422.76 |
1739.84 ± 392.56 |
Birth weight (gram; Mean ± SD) |
<0.001 |
38.57 ± 4.83 |
43.16 ± 2.24 |
Height (Cm; Mean ± SD) |
<0.001 |
27.29 ± 2.36186 |
30.45 ± 2.15 |
Head circumference (Cm; Mean ± SD) |
0.270 |
1.88 ± 1.18 |
2.29 ± 1.50 |
Gravida (Mean ± SD) |
0.158 |
6.25 ± 2.44 |
7.03 ± 1.80 |
First minute Apgar score (Mean ± SD) |
0.604 |
8.50 ± 1.71 |
8.70 ± 1.37 |
5th minute Apgar score (Mean ± SD) |
0.069 |
38.62 ± 18.34 |
31.38 ± 12.38 |
Mothers' Vitamin D (ng/ml; Mean ± SD) |
0.907 |
33.07 ± 17.43 |
33.51 ± 12.28 |
Neonates' Vitamin D (ng/ml; Mean ± SD) |
0.586 |
2 (12.9) |
4 (7.1) |
Neonatal death (n%) |
0.039 |
10 (32.3)
22 (67.7) |
19 (59.3)
13 (40.7) |
Neonats' gender (n%)
Male
Female |
0.391 |
4 (12.5)
28 (87.5) |
2 (6.3)
30 (93.8) |
Reciving Vitamin D suplemnt (n%)
Yes
No |
0.798 |
4 (12.5%)
9 (28.1%)
19 (59.4%) |
10 (31.3%)
2 (6.3%)
20 (62.5%) |
Maternal Vitamin D deficiency (n%) deficient
Insufficient
sufficient |
0.595 |
1 (3.1%%)
13 (40.6%)
18 (56.3%) |
0 (0%)
13 (40.6%)
19 (59.4%) |
Neonatal Vitamin D status
deficient
Insufficient
sufficient |
0.554 |
2 (6.3)
30 (93.8) |
1(3.1)
31 (96.9) |
Corticosteroid administration (n%)
Yes
No |
0.049 |
27 (87.1)
4 (12.9) |
28 (100)
0 (0) |
Type of delivery
Cesarean section
Vaginal delivery |
Considering some confounding factors, more analyses were used. Due to the small sample size, Bootstrapping in Binary logistic regression test was used. The results demonstrated significant associations between NEC and some neonatal factors including neonate's low birth weight (p=0.01), Head circumference (p=0.02), and height (p=0.03), as well as low Apgar score at first minute (p=0.04). Detailed data are shown in table 3.
Table 3: Correlations between maternal/neonatal factors with necrotizing enterocolitis in NICU hospitalized preterm neonates
95% Confidence Interval |
P value |
B |
Variables |
Upper |
Lower |
18.074 |
-15.211 |
0.210 |
0.024 |
Neonate's Vit D |
0.126 |
-4.294 |
0.010 |
-0.006 |
Birth Weight |
150.882 |
-616.412 |
0.060 |
-1.568 |
sex |
27.843 |
-26.098 |
0.270 |
-0.047 |
Mother's age |
215.647 |
-32.191 |
0.070 |
0.490 |
Gestational age |
243.747 |
-29.622 |
0.030 |
0.594 |
Height |
614.561 |
-22.212 |
0.020 |
0.799 |
Head circumference |
953.455 |
-437.616 |
0.440 |
-18.751 |
Type of delivery |
257.906 |
-112.963 |
0.040 |
0.487 |
First minute Apgar score |
209.761 |
-330.163 |
0.230 |
-0.252 |
5th minute Apgar score |
752.885 |
-299.452 |
0.050 |
0.814 |
Vit D suplment |
1936.845 |
-357.223 |
0.570 |
-17.314 |
Corticosteroid |
The pathogenesis of NEC is not clearly identified; however, increased inflammatory responses including elevated plasma and intestinal cytokine levels suggest NEC as an imflammatory disease [4-6]. Former studies have also indicated the immunoregulatory and anti-inflammatory effects of vitamin D with its steroid hormone structure [7-9]. In the present study, we evaluated any associations between the incidence of NEC with maternal and neonatal serum vitamin D levels among NICU hospitalized preterm neonates.
Results of the present study have indicated a significant positive correlation between maternal and neonatal vit D status. This strong correlation of neonatal serum vitamin D level with maternal vitamin D level has been revealed by other studies [17-19]. Sathish et al., have shown that the fetus for the supply of vitamin D is absolutely dependent on his mother [17].
According to the results, maternal and neonatal low levels of serum vitamin D were not significant risk factors for NEC. Moreovere, the frequencies of maternal and neonatal vit D deficiency or insufficiency were not significantly different between the two NEC and control groups. It is supposed that other important risk factors like low birth weight, low gestational age, being small for gestational age, sepsis, hypotension, severe respiratory distress syndrome and assisted ventilation may mask the role of vit D deficiency in pathogenesis of NEC [20]. As there are very few investigations evaluating the association between vitamin D level and neonatal NEC, we could not find other studies that confirm our results. It shows additional studies are required. On the contrary to our findings, Cetinkaya et al. demonstrated that only maternal but not neonatal vitamin D status could significantly predict neonatal NEC. They also showed that every 1 ng/ ml increase of maternal serum vitamin D level could decrease the risk of NEC by 0.86 times [14]. Yang et al. have shown a significant difference between two groups preterm neonates' with and without NEC regarding their mothers' serum vitamin D levels. They concluded that the serum vitamin D levels of preterm infants' mothers may be correlated to the development of neonatal NEC. They also indicated a significant difference between the NEC and non-NEC groups with respect to the frequency of neonatal vitamin D deficiency [13].
Based on the results, the mean of gestational age, birth weight, height and head circumference in NEC group was significantly lower than the control group. Consistent to our results, Samuels et al. in a systematic review showed that low birth weight and low gestational age were the main and the most frequent risk factors for necrotizing enterocolitis in neonates [2]. Markel et al. have noted that about 85% of NEC subjects have birth weight <1500 grams or gestational age<32 weeks of gestations [20]. Lodha et al. also demonstrated that infants with NEC had lower growth indices including body weight, height, and head circumference compared with their control counterparts (p < 0.05) [21]. But inconsistent to our findings, Salhab et al. have shown no significant differences between extremely low birth weight neonates with and without NEC regarding to neonates' weight, gestational age, length and head circumference at birth [22].
There was a significant difference between NEC and control groups with regard to the type of delivery; the number of normal vaginal delivery in the NEC group was significantly higher than the control group. Other studies have also confirmed the protective effect of cesarean section against the risk of NEC because of less stress during delivery [2,20,23].
Moreover, our results have indicated that more neonates in the NEC group were female while more participants in the control group were male. Compatible to this result, Cetinkaya et al. have demonstrated that more neonates in the NEC group were female in comparison with the control group; however, this difference between groups was not significant (57.7% vs. 46.2; p= 0.33) [14]. In contrast to our results, Qi et al. showed more neonates in the NEC group were male; however, they could not find any significant association between NEC and participants' gender [24]. Carter et al., also did not indicate any significant relationship between gender and NEC [25].
Results of the present study have shown a significant association between the incidence of NEC and low Apgar score at the first minute. In accordance with this finding, Acunas et al. have shown a statistically significant lower first minute Apgar scores in 70 preterm neonates with NEC compared to 135 NEC negative subjects (p<0.01) [26]. On the other hand, other studies by Cetinkaya et al., Ahle et al., and Jayasree et al. did not support our finding [14,27,28].
Our study had some limitations; we did not include other risk factors like chorioamnionitis, formula feeding, neonatal sepsis, mechanical ventilation and so on. Moreover, further studies with larger sample sizes are strongly suggested.
Our results showed a significant positive correlation between maternal and neonatal vit D status. Some neonatal factors including neonate's low birth weight, head circumference, and height, as well as low Apgar score at first minute were significant risk factors for necrotizing enterocolitis among NICU hospitalized preterm neonates. However, this significant associations were not observed with maternal or neonatal low levels of serum vitamin D. These results should be confirmed by further studies with larger sample sizes.
Ethics and consent to participate
Our study was approved by the institutional review board of Tehran University of Medical Sciences and according to Helsinki declaration. All participants signed an informed consent. Moreover, subjects were assured of their right to discontinue the study course.
Availability of data and materials
The datasets related our study is available from the corresponding author on reasonable request.
Competing interests
The authors declare that there is no conflict of interests.
Funding
This research has been supported by Tehran University of Medical Sciences and health service as well as Maternal Fetal and Neonatal Research Center.
Acknowledgment
This study was supported by Tehran University of medical sciences (TUMS) and Maternal Fetal and Neonatal Research Center. We acknowledge their kindly supports in this study.
- Ladegaard PB, Rasmussen L, Zachariassen G (2018) Intrauterine necrotizing enterocolitis in premature newborns. Ugeskr Laeger 180: 4170340. [Crossref]
- Samuels N, van de Graaf RA, de Jonge R, Reiss I, Vermeulen M (2017) Risk factors for necrotizing enterocolitis in neonates: a systematic review of prognostic studies. BMC Pediatr 17: 105. [Crossref]
- AlFaleh K, Anabrees J (2014) Probiotics for prevention of necrotizing enterocolitis in preterm infants (Review). Cochrane Database Syst Rev 9: 584-671. [Crossref]
- Jean-Christophe R, Pierre-Yves A, Patricia L, Laetitia M, Al Nabhani Z, et al. (2017) Nutritional strategies and gut microbiota composition as risk factors for necrotizing enterocolitis in very-preterm infants. Am J Clin Nutr 106: 821-830. [Crossref]
- Gan X, Li J (2018) Research advances in necrotizing enterocolitis in neonates. Zhongguo Dang Dai Er Ke Za Zhi 20: 164-168. [Crossref]
- Bein A, Eventov-Friedman S, Arbell D, Schwartz B (2017) Intestinal tight junctions are severely altered in NEC preterm neonates. Pediatr Neonatol 59: 464-473. [Crossref]
- Ardizzone S, Cassinotti A, Trabattoni D, Manzionna G, Rainone V, et al. (2009) Immunomodulatory effects of 1,25- dihydroxyvitamin D3 on TH1/TH2 cytokines in inflammatory bowel disease: an in vitro study. Int J Immunopathol Pharmacol 22: 63-71. [Crossref]
- Di Rosa M, Malaguarnera G, De Gregorio C, Palumbo M, Nunnari G, et al. (2012) Immuno-modulatory effects of vitamin D3 in human monocyte and macrophages. Cell Immunol 280: 36-43. [Crossref]
- Cantorna MT, Munsick C, Bemiss C, Mahon BD (2000) 1,25-Dihydroxycholecalciferol prevents and ameliorates symptoms of experimental murine inflammatory bowel disease. J Nutr 130: 2648-2652. [Crossref]
- Nayeri F, Asghari F, Baser A, et al. (2017) Views and Decisions of Physicians in Encountering Neonates with Poor Prognosis. Arch Iran Med 20: 172-177. [Crossref]
- Sasan B, Zandvakili F, Soufizadeh N (2017) Effects of Vitamin D Supplement on Prevention of Recurrence of Preeclampsia in Pregnant Women with a History of Preeclampsia. Obstet Gynecol Int 249264. [Crossref]
- Rostami M, Ramezani Tehrani F, Simbar M (2017) Rationale and Design of Khuzestan Vitamin D Deficiency Screening Program in Pregnancy: A Stratified Randomized Vitamin D Supplementation Controlled Trial. JMIR Res Protoc 6: 54. [Crossref]
- Yang LR, Li H, Zhang T, Zhao RC (2018) Relationship between vitamin D deficiency and necrotizing enterocolitis in preterm infants. Zhongguo Dang Dai Er Ke Za Zhi 20: 178-183. [Crossref]
- Cetinkaya M, Erener-Ercan T, Kalayci-Oral T, Babayiğit A, Cebeci B, et al. (2017) Maternal/neonatal vitamin D deficiency: a new risk factor for necrotizing enterocolitis in preterm infants? Journal of Perinatology 1-6. [Crossref]
- Walsh MC, Kliegman RM (1986) Necrotizing enterocolitis: treatment based on staging criteria. Pediatr Clin North Am 33: 179-201. [Crossref]
- Yu X, Wang W, Wei Z (2014) Vitamin D Status and Related Factors in Newborns in Shanghai. China. Nutrients 6: 5600-5610. [Crossref]
- Sathish P, Raveendran S, Padma R, Balakrishnan D, Muthusami M (2016) Correlation between maternal and neonatal blood vitamin D levels and its effect on the newborn anthropometry. Int J Reprod Contracept Obstet Gynecol 9: 2983-2988.
- Bassir M, Laborie S, Lapillonne A, Claris O, Chappuis MC, et al. (2001) Vitamin D deficiency in Iranian mothers and their neonates: a pilot study. Acta Paediatr 90: 577-579. [Crossref]
- Kazemi A, Sharifi F, Jafari N, Mousavinasab N (2009) High prevalence of vitamin D deficiency among pregnant women and their newborns in an Iranian population. J Womens Health 18: 835-839. [Crossref]
- Troy AM, Holly E, Brenda B (2014) Predicting Disease Severity of Necrotizing Enterocolitis: How to Identify Infants for Future Novel Therapies. J Clin Neonatol 3: 1-9. [Crossref]
- Lodha A, Asztalos E, Moore AM (2010) Cytokine levels in neonatal necrotizing enterocolitis and long-term growth and neurodevelopment. Acta Paediatr 99: 338-343. [Crossref]
- Salhab WA, Perlman JM, Silver L, Sue Broyles R (2004) Necrotizing enterocolitis and neurodevelopmental outcome in extremely low birth weight infants <1000 g. J Perinatol 24: 534-540. [Crossref]
- Guthrie SO, Gordon PV, Thomas V, Thorp JA, Peabody J, et al. (2003) Necrotizing enterocolitis among neonates in the United States. J Perinatol 23: 278-285. [Crossref]
- Qi L, Shupeng Ch, Min ZH (2017) Risk Factors for Necrotizing Enterocolitis in Neonates: A Retrospective Case-Control Study. Pediatr Neonatol 58: 165-170. [Crossref]
- Carter BM, Holditch-Davis D (2008) Risk factors for necrotizing enterocolitis in preterm infants: how race, gender, and health status contribute. Adv Neonatal Care 8: 285-290. [Crossref]
- Acunas B, Vatansever U, Duran R, Aladag N (2005) Risk Factors for Necrotizing Enterocolitis in Very Low Birth Weight Infants. Pediatr Res 58: 421. [Crossref]
- Jayasree N, Rachel L, Satyan L (2018) Necrotizing Enterocolitis in Moderate Preterm Infants. BioMed Res Int 1- 6. [Crossref]
- Ahle M, Drott P, Elfvin A, Andersson RE (2018) Maternal, fetal and perinatal factors associated with necrotizing enterocolitis in Sweden. A national case-control study. PLOS ONE 1-13. [Crossref]
Editorial Information
Editor-in-Chief
Michael A. Portman
University of Washington, USA
Article type
Research Article
Publication History
Received: July 03, 2020
Accepted: July 17, 2020
Published: July 23, 2020
Copyright
©2020 Kamrani K. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Kamrani K, Mardani M, Zarkesh MR (2020) The association between vitamin D levels and necrotizing enterocolitis in preterm neonates. Pediatr Dimensions 5: DOI: 10.15761/PD.1000208.
