Abstract
Objective: This study was designed to observe the expression of platelet-derived growth factor (PDGF) and tumor necrosis factor-α (TNF-α) in 32 patients with primary biliary cholangitis (PBC) and 22 healthy controls. The correlation between these two (PDGF and TNF-α) and PBC was analyzed.
Methods: The expression of PDGF and TNF-α in the serum of PBC patients and healthy controls was detected by ELISA, and a correlation analysis was conducted.
Results: The PDGF serum levels of PDGF was 4.21 ± 2.91 ng/L in the control group, and 1.09 ± 0.43 ng/L in the PBC groups, respectively, and the difference was highly statistically significant (p < 0.001). Furthermore, serum TNF-α level was 126.53 ± 67.79 pg/mL in the control group and 46.13 ± 17.05 pg/mL in the PBC groups, respectively, and the difference was extremely statistically significant (p < 0.001). These variables were compared between the two groups by independent sample t-test. p < 0.05 was considered statistically significant.
Conclusions: The expression of PDGF and TNF-α significantly decreased in the PBC group. PDGF and TNF-α may be important expression regulators in PBC.
Key words
primary biliary cholangitis, platelet derived growth factor, tumor necrosis factor, association
Abbreviations
PDGF: Platelet-Derived Growth Factor; TNF-α: Tumor Necrosis Factor-α; PBC: Primary Biliary Cholangitis; AMA-M2: Anti-Mitochondrial M2 Antibody; ELISA: Enzyme-Linked Immunosorbent Assay
Introduction
Primary biliary cholangitis (PBC, previously called primary biliarycirrhosis) is a chronic cholestatic liver disease pathologically characterized by the destruction of the intrahepaticbile ducts [1]. Early pathological changes of PBC are generally in the reversible or partially reversible stage of clinical treatment, and the curative effect is satisfactory. However, the later or end stages are irreversible stages in clinical treatment, the prognosis is poor, and the consequences are serious. Patients who develop hepatic failure have poor prognosis, with a 5-year survival rate of o50% [2-4]. Since there is presently no ideal marker for PBC, the onset of PBC is insidious, and the progression of its course is slow. In addition, the liver has a strong compensatory ability, which makes it very difficult to discover and diagnose PBC patients in time. At present, patients clinically diagnosed with PBC are often in the middle and late stages of the disease and have lost the best time of treatment. Therefore, it is very important and challenging to completely and accurately distinguish PBC patients from the general population in clinic [5]. At present, anti-mitochondrial M2 antibody (AMA-M2) is very sensitive in the diagnosis of PBC and has been considered a very ideal antibody marker for PBC for a long period of time. However, with the deepening of research on various diseases, it was gradually found that AMA-M2 is not a specific antibody for PBC. It widely exists in many autoimmune diseases, such as Sjogren's syndrome, myositis/dermatomyositis and systemic sclerosis. Furthermore, high titers of AMA-M2 have been found in 5-20% of patients with liver diseases, such as hepatitis C and acute liver functional damage [6]. Indeed, previous studies have demonstrated increased serum levels of pro-inflammatory cytokines (IL-6, TNF-α, and IFN-γ) in PBC patients with fibrosis [7,8]. Therefore, it is very important to study the serum markers of PBC through new methods.
Materials and methods
Specimens
The serum of healthy subjects and PBC patients were collected in the Physical Examination Center of the Second Affiliated Hospital of Harbin Medical University. All serum collected from the PBC group met the criteria for the "Diagnosis and Treatment of Primary Biliary Cholangitis" published in Europe in 2017 [9]. All patients in the PBC group were approved by liver pathology and immunology.
Major reagents
Human platelet-derived growth factor (PDGF) enzyme-linked immunosorbent assay (ELISA) Kit (Beijing Donggeboye Biological Technology Co., Ltd.; Batch no.: 23045673.). Human tumor necrosis factor-α (TNF-α) ELISA Kit (Beijing Donggeboye Biological Technology Co., Ltd.; Batch no.: 23045672).
PBC and PDGF
Serum was detected by ELISA. In the Excel sheet, with the concentration of standards as the abscissa, and the corresponding OD value as the ordinate, the curve of the standards was drawn. The concentration value of each sample was calculated according to the equation of the curve.
PBC and TNF-α
The results were determined by ELISA. In the Excel sheet, with the concentration of standards as the abscissa, and the corresponding OD value as the ordinate, the curve of the standard was drawn. The concentration value of each sample was calculated according to the equation of the curve.
Statistical analysis
All data analyses were conducted using SPSS 19.0. All measurement data were expressed as mean ± standard deviation ( ± SD). The variables were compared between two groups using independent sample t-test, p < 0.05 was considered statistically significant.
Results
ELISA results revealed that PDGF serum levels was 4.21 ± 2.91 ng/L and 1.09 ± 0.43 ng/L in the control and PBC groups, respectively, and the difference was extremely statistically significant (p < 0.001); (Table 1, Figure 1).
Table 1. Comparison of PDGF serum levels in the control and PBC groups.
Group |
Sample number (n) |
PDGF levels (ng/L) |
t value |
p value |
Control group |
22 |
4.21 ± 2.91 |
4.980 |
0.001 |
PBC group |
22 |
1.09 ± 0.43** |
Note: Compared with the normal group, ** represents extremely significant difference, p < 0.001.
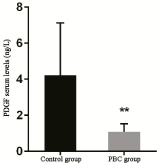
Figure 1. Comparison of PDGF serum levels between the control and PBC group.
Note: Compared with the normal group, ** represents extremely significant difference, p < 0.001.
ELISA results revealed that serum TNF-α level was 126.53 ± 67.79 pg/mL and 46.13 ± 17.05 pg/mL in the control and PBC groups, respectively, and the difference was extremely statistically significant (p < 0.001) (Table 2, Figure 2).
Table 2. Comparison of TNF-α serum levels in the control and PBC groups.
Group |
Sample number (n) |
TNF-α levels (pg/ml) |
t value |
P value |
Control group |
22 |
126.53 ± 67.79 |
5.445 |
0.001 |
PBC group |
32 |
46.13 ± 17.05** |
Note: Compared with the normal group, ** represents extremely significant difference, p < 0.001.
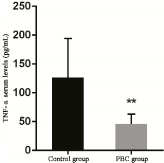
Figure 2. Comparison of TNF-α serum levels between the control and PBC groups.
Note: Compared with the normal group, ** represents extremely significant difference, p < 0.001.
Discussion
PDGF is a strong mitogen and chemical inducer in the body. It can stimulate the division and proliferation of connective tissue cells and is closely correlated to the growth and development of body tissues, wound healing, and the occurrence and development of atherosclerosis and tumors [10]. The results of the present study revealed that PDGF levels in serum were significantly higher in the control group than in the PBC group, and the difference was extremely statistically significant. Inflammation may be the cause of the significant decrease in PDGF level in the PBC group. At present, the specific mechanism of the significant decrease in expression of PDGF in PBC remains unknown.
TNF-α has extensive biological activities, which not only serve as a mediator in the physiological processes of the body, such as the host's defense, immunity and the stability of the internal environment, but also plays an important role in endotoxic shock, inflammation, the occurrence and development of tumors, cytotoxicity, and antiviral and bacterial infection. The results of the present study revealed that serum TNF-α level was significantly higher in the control group than in the PBC group, and the difference was extremely statistically significant. The analysis results revealed that TNF-α level in peripheral blood was lower in PBC patients than in normal subjects [11,12]. However, other reports have revealed a contrary conclusion, in which the level of TNF-α in peripheral blood was higher in PBC patients than in normal subjects, and its level was positively correlated to the patient's condition [13]. The reason may be that the expression of TNF-α in the body is affected by a variety of factors, including heredity, environment, the degree of inflammation in tissues, and course of the disease.
In summary, the serum 2021 Copyright OAT. All rights reservignificantly higher in the control group than in the PBC group. PDGF and TNF-α may be important expression regulators in PBC. However, the clinic practical values of these identified markers should be validated through studies with expanded sample sizes and a range of diseases.
Acknowledgment
This study was supported by Heilongjiang Natural Foundation (Grant No. D201271).
Reference
- Nakanuma Y, Ohta G (1979) Histometric and serial section observations of the intrahepatic bile ducts in primary biliary cirrhosis. Gastroenterology 76: 1326-1332. [Crossref]
- Corpechot C, Carrat F, Bahr A, Chrétien Y, Poupon RE, et al. (2005) The effect of ursodeoxycholic acid therapy on the natural course of primary biliary cirrhosis. Gastroenterology 128: 297-303. [Crossref]
- Corpechot C, Poupon R (2007) Geotherapeutics of primary biliary cirrhosis: bright and sunny around the Mediterranean but still cloudy and foggy in the United Kingdom. Hepatology 46: 963-965. [Crossref]
- Metcalf JV, Mitchison HC, Palmer JM, Jones DE, Bassendine MF, et al. (1996) Natural history of early primary biliary cirrhosis. Lancet 348: 1399-1402. [Crossref]
- Kim WR, Ludwig J, Lindor KD (2000) Variant forms of cholestatic diseases involving small bile ducts in adults. Am J Gastroenterol 95: 1130-1138. [Crossref]
- Leung PS, Rossaro L, Davis PA, Park O, Tanaka A, et al. (2007) Antimitochondrial antibodies in acute liver failure: implications for primary biliary cirrhosis. Hepatology 46: 1436-1442. [Crossref]
- Neuman M, Angulo P, Malkiewicz I, Jorgensen R, Shear N, et al. (2002) Tumor necrosis factor-alphaand transforming growth factor-beta reflect severity of liver damage inprimary biliary cirrhosis. J Gastroenterol Hepatol 17: 196-202. [Crossref]
- Golovanova EV, Il'chenko LIu, Tsaregorodtseva TM, Serova TI, Gudkova RB (2004) Cytokines in primary biliary cirrhosis (diagnostic and prognostic value). Ter Arkh 76: 8-11. [Crossref]
- Hirschfield GM, Beuers U, Corpechot C, Invernizzi P, Jones D, et al. (2017) EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J Hepatol 67: 145-172. [Crossref]
- Allen CL, Bayraktutan U (2008) Differential mechanisms of angiotensin II and PDGF -BB on migration and proliferation of coronary artery smooth muscle cells. J Mol Cell Cardiol 45: 198-208. [Crossref]
- Broomé U, Eriksson LS, Sundin U, Sundqvist KG (1992) Decreased in vitro production of tumor necrosis factor in primary biliary cirrhosis patients. Scand J Gastroenterol 27: 124-128. [Crossref]
- Spengler U, Möller A, Jung MC, Messer G, Zachoval R, et al. (1992) T lymphocytes from patients with primary biliary cirrhosis produce reduced amounts of lymphotoxin, tumor necrosis factor and interferon-gamma upon mitogen stimulation. J Hepatol 15: 129-135. [Crossref]
- Neuman M, Angulo P, Malkiewicz I, Jorgensen R, Shear N, et al. (2002) Tumor necrosis factor-alpha and transforming growth factor-beta reflect severity of liver damage in primary biliary cirrhosis. J Gastroenterol Hepatol l7: 196-202. [Crossref]


