It is a sad situation when science and technology are being subjected to an almost religious dogma as easy explanations are not sought. Instead the opinions and statements of perhaps biased individuals are taken as ‘gospel truth’. All this without verification. Such complacency in Science and technology has resulted in no attempts being made to introduce a standard for silver as a biocide. Since production and trials were initiated during the early 1900 s little progress has been recorded. Neither have any official clinical trials been done ‘in vivo’. Today little more is known than the initial findings conducted decades ago. This paper will demonstrate that finding answers, setting standards and the creation of a proper nomenclature as well as scientific description of this material must be of the highest priority. No longer must vague identification and descriptions such as AGNPs, nano particles etc. be tolerated in any scientific papers. The names and descriptions given to the material so far are technically and scientifically meaningless. In addition, the HYPE and erroneous claims of ‘a cure for all’ for nano metre sized silver clusters should be avoided. Simultaneously increased attention must be paid to appropriate technical and scientific testing for hazardous contamination of both the silver and the water used and a thorough quality control exercised. Such strategies are seriously missing at present.
Key words: pico and nano metre sized, ohm’s law, parallel resistance factor, cations and anions
So-called colloidal silver particles are neither a colloid nor consisting of particles. Instead a more apt and technical description would be Pico or Nano metre sized atomic silver clusters, static electrically suspended in water by a mutually repelling force. The reason for the word Pico being used is necessary to indicate that the smallest stable atomic cluster consisting of just two atoms is just around 600 pico metres (0.6 nano metre) in size. It goes by the name of a “Dimer” and most likely the start of a large atomic cluster of what is referred to as re-constituted silver, valence bonded and inherently stable with no ionic silver present. At sizes at 10 nm and below, it is subjected to Quantum effects and is no longer in its usual metallic state. In addition, it must be said that by scientific definition that either bulk metallic silver as well as the reconstituted Quantum silver do have the characteristics of a free radical. This is evidenced by its indiscriminate killing factor, the Oligo-dynamic Effect [1,2].
Silver has that in common with the metals Copper and Gold. Presently, so called “colloidal silver” is in the doldrums brought about by lack of scientific explanation, use of meaningless ‘buzzwords’ and frowned on by Governments due to fraudulent claims made through ignorance, resulting in legislation being passed, banning the material for use in medical applications. Rightly so, for all of the ‘colloidal silver alchemists’ producing material from silver without any proper identity of what it really is. A new and more precise direction must be sought in order to legitimise ‘water suspended quantum silver’.
After much study, research and experimentation starting in early 2008, it became obvious that those involved in the ‘Wet Chemistry’ of colloidal silver were operating in complete darkness: Chemists working with Silver Nitrate, High voltage ablation and various forms of electrolysis. Also manufacturing concerns and private individuals experimenting with electro-chemistry using simple AC/DC power supplies feeding into an electrode pair made of unpure silver and distilled water without knowing what they were doing and not aware of the difference between neutral and ionic silver. The chances, especially with the use of extremely high elevated voltages, production of silver isotopes or transmutation may be real. Many thought and still do so today, only produce ionic silver without even realising its toxicity. As one scientist wrote to me: he had one customer who claimed that they were manufacturing colloidal silver guaranteed to be 100% pure ionic. Those researchers and scientists conducting clinical trials using silver proved equally ignorant and complacent and I am yet to see a paper where so-called colloidal silver is properly described and identified with a proper specification as to type of manufacture, cluster size and concentration. Some even choose the easy-way out by purchasing finely pulverised metallic silver that only required to be dumped in water and acquire colloidal silver that way. Research from Denmark has shown that this type of metallic silver is toxic for human cells and should be avoided [3-5].
The introduction of a new technology
It was noticed at the start, that low voltage direct current as well as alternating current took many hours to build up to a reasonable level. For some that meant adding salt to increase the conductivity. Salt however is made of Sodium and chlorine (NaCl) and any ionic silver produced would quickly attach to chlorine to form Silver Chloride having a surplus electron. Silver Chloride is a rather insoluble product and not conducive to ever becoming neutral silver. The silver intended to become colloidal silver is thus lost permanently. To even get involved in this seemed a waste of silver, energy and time. So, something more efficient needed to be found.
As a lifelong electronic hobbyist and owning a vast array of electronic test equipment, a series of experiment were conducted in deliberate areas, explained as follows:
- How to obtain a specific level of current to flow in either distilled or deionised water and at what voltage potential.
- To step away from a low DC voltage and a high current such as 12 volt and 100mA respectively and determine the viability of using alternating current.
- The conditions under which a pure silver product could be produced consistently and suitable for medical applications.
- To determine the actual science involved and how to avoid inconsistencies and artefacts.
- A choice of necessary instrumentation for determining concentration and Quality Control during and after production.
In less than a year and after extensive research covering a variety of scientific disciplines ranging from Quantum Physics (photo-electric effect), ultra-pure water science and cluster formation, Astronomy (amateur telescope making) and electro-chemistry. The result produced the following:
- Use of alternating current was rejected for the simple reason that the current was alternated between the cathode and anode. A Direct current of 500 micro ampere (0.5 mA) at a voltage potential of 300 vdc across two pure silver electrodes would run from switch-on to switch-off for whatever time was needed. A special electronic circuit could be adjusted to control and simultaneously limit the current applied.
- The natural absorption of visible wavelength by water needed to be overcome or a choice made of a spectral colour that would not be absorbed. This information was learned from Astronomy stating that it was difficult to photograph blue stars such as Rigel, the brightest star in the constellation of Orion and water science, about absorption charts. It was determined that silver absorbs violet light between 417-420 nm, parts of the 3% that silver does not reflect and the unique super transparency of water at these exact wavelengths, all depending on the purity of both the silver and the water. The scene was set to try out what was learned.
- A power supply was designed and built using a voltage double circuit that produced both 336 vdc and 672 vdc respectively. This secondary voltage was later to prove capable of increasing the current level to 1,500 micro amperes (1.5 mA), a three-fold increase in production. At such a low current of 500 micro ampere the process of producing nano silver in an acceptable concentration took 48 hours and provided neutral atomic silver clusters ranging in sizes from 3.6 to 10.1 nm at a concentration of 10ppm consistently and without any ionic silver present.
- The actual science involved is the conversion of bulk metallic silver into ionic silver, using electrochemistry and by simultaneous irradiation of 417 to 420 nm intense light, neutralise and reconstituting the silver atoms by covalent bonding, i.e. the removal of an electron by an electric current and returning the electron by photonic energy. Since this happening occurs within the confines of the atom, it is considered quantum mechanical. The name Electro-Photochemistry is an accurate description of this process.
- Immediately seeing the need for verification of the product, a number of prototype instruments were designed and constructed that include: 10,000 Million Ohm input resistance meter for checking out the purity of the water with great precision; a Linear polarising light scattering spectrophotometer functioning like a turbidity meter and nepholometer with the ability to separately quantify the light scattering of silver and contamination level and a separate electronic instrument for concentration testing using a capacitance reactance principle.
Silver an effective biocide
Many clinical trials using samples provided to Griffith University as well as Queensland University (School of Dentistry and the Institute of Molecular biology) done in vitro as well in topical applications have proven beyond a doubt its ability to eradicate a variety of pathogens and some infectious Fungi and recently also a biofilm species of entero-coccus faecalis in the mouth, at very low concentrations in the single digits ppm. The size of the suspended atomic clusters was between 3.6 and 10.1 nm with the highest concentration between 5 and 7nm in size. So far it has been agreed by all concerned to only use the substance in a topical way, i.e. on the skin and in the mouth since there appear to have been no clinical trials ‘in vivo’ so far. The production process here discussed is the relatively new electro-photochemical way referred to as ‘Photo Electron Transfer”. The obtained very narrow band of cluster sizes and high surface area to volume ratio is more effective than other production methods that may be made up of a high assortment of sizes as large as ½ micron, i.e. 500 nm [5-10].
The electro-photochemical production explained
The electro-photochemical method of producing nano metre sized neutral atomic silver clusters suspended in water is a relatively new procedure. It is based on the physics of using a controlled low current at an elevated low DC voltage of 300 vdc or 600 vdc for converting neutral bulk metallic silver into ionic silver (Ag+) and using photonic collisions with hydrated electrons to reconstitute the silver ions into neutral silver atomic clusters, albeit in a semi-permanent repelling electrical suspension in water.
Conditions for this process are as follows:
- Application of temperature control: between 4 and 10 degrees such as provided by a domestic refrigerator.
- No other light during the production process other than irradiation by an intense light source of around 420 nm.
- The use of a direct voltage and current of 300 volts and 500 micro amperes across the cathode and anode silver electrodes for the duration of the production process, i.e. until such time as the previously determined concentration has been reached.
- The use of a high grade of deionised water no higher in conductivity than 0.22 micro Siemens or its equivalent in micro ampere.
- A tank made of a stable clear acrylic or borosilicate glass and to avoid soda glass at all costs due to leaching of Sodium (Na for Natrium).
- Storage to be in containers as above and well-sealed and topped to avoid the formation of carbon dioxide by exposure to air.
Note: Experimentations have shown that using multiple channels and electrodes as well as a doubling of the voltage to 600 vdc, commercial production figures can be obtained at least 24 times faster and 72 times faster respectively.
The actual process is activated by the voltage potential and current flow, creating ionic silver (cations) by removal of the outer electron of a silver atom coming away from the anode. The cations so formed are attracted to the cathode and must not get there. To prevent this, the electrodes must be separated by at least 200mm and simultaneous when the cations are formed to irradiate them with a high dose of violet light at around 417 to 420 nm. This will neutralise the ionic silver and reconstitute it to neutral locally produced atomic silver clusters suspended in water on a semi-permanent basis.
Some of the more peculiar properties of reconstituted suspended silver
Any activity with silver at a molecular level is referred to as “Classical Physics”. However what changes are affected inside a silver atom occurs in the realm of Quantum Physics. Not subject to gravity, Quantum confinement of electrons and Local plasmon resonances are the consequences when atomic silver clusters are of a size at 10nm or less. At such small dimensions also, the ratios of surface area to volume become very large indeed and much more able to get very close to pathogens.
Quantum confined electrons and plasmon resonances
Electron flow in bulk metallic silver is virtually unimpeded due an unpaired valence electron (number 47) in the outer shell. This makes silver such a good electrical conductor. However, when silver has been electrically reconstituted and suspended in water as a cluster of atoms, stability has been created by each atom donating this outer electron. The action results in a covalent bonding of the local concentration of atoms into a cluster. As a direct consequence, electrons are now quantum confined due to a lack of space. This containment requires energy
Incoming long wavelength photons impacting and colliding with such a quantum confined electron will cause an oscillatory vibration called a plasmon resonance and creation of a Polariton (a virtual particle) making the electron shake. When the disturbance dies out, the photon moves away again but instead of having lost energy (red shifting) it has now gained energy and is said to be blue shifted. Red shifting due to long wave resonances normally causes thermal agitation such as heat, blue shifting resonances however at 420 nm (violet) and shorter wavelengths such as Ultraviolet possess a higher electron level starting at 2.95 eV which is an ionizing energy. Such conditions may be even more disastrous for pathogens.
The water in which quantum pico and nano silver is produced and contained
Water for use to produce pico and nano metre sized silver should be as pure and stable as possible. There is little point in producing a very low concentration of silver clusters such as at 3 or 4ppm, if the combined contamination of both organic and inorganic material is in excess of that concentration. The silver is likely to become unsuitable for its intended purpose and actually cause infections rather than eliminate the problem. To produce an effective biocide, even if used topically, requires great care. It may really be a case of ’only the best will do’! [11-14].
Note! Nothing in the Universe is actually stable and certainly not matter at our scale and size where changes occur relatively fast. Water is certainly capable of change, hydrogen bonding in water never ceases and it is claimed to do so at a time frame of 10-18 seconds. In comparison with other fluids, many changes in water can be observed in seconds and longer.
It is obvious that the metallic silver used must be as pure as commercially available and the target so far has been a purity of 99.998%. At produced concentrations of around 10 ppm and a purity of the stated 99.998%, Arsenic levels have been tested at 10 ppb and below. Needless to say, the water used must be equally pure and in here lies a problem. It must be noted here also that unless nano silver is used in clinical trials, its production method and all other properties must be known. Unfortunately, that is almost never the case and it is anyone’s guess what quality water is actually used. Water is subject to the same uncertainties and more often than not far more contaminated with uncharged organic and inorganic junk that is only made visible by light scattering and microscopy. For our purpose commercial quality deionised water of around 0.22 micro Siemens conductivity works well but 0.1 micro Siemens (equivalent to 10 million Ohm) is preferred. Tests have borne out that commercial distilled water is generally unsuitable due to excessive amounts of carbon dioxide and other organic contamination, such as Ammonia. In addition to organic and inorganic materials (in the form of non- ionic states) can also affect the quality of the product. In some instances, current flow during production can be opposed. It is a certainty that there exists no real ultra-pure water and if it ever did, the environment and the Universe itself would make it change within seconds. It has been determined that Cosmic Rays rain down on Earth at the rate of 200 per square meter every second. Having stated this, the need for testing the purity of water is paramount. With the present situation where no affordable adequate instrumentation is commercially available, alternative measures must be created.
Existing practices for testing quantum nano silver and water and the many shortcomings and anomalies that stand in its way
Some facts and the associated anomalies about so-called colloidal silver and the water it is contained in that are not openly shared. This has severely halted progress into a proper research to establish if this material that eradicates pathogens by electrical charges is a friend or foe. Only the anomalies and shortcomings related to pico and nano silver suspended in water and the water used for this process are listed here.
Anomaly 1. What constitutes Silver nano particles?
So-called “colloidal silver particles” are in reality not a colloid and do not exist as particles either. Neither are the claimed particles distributed as a dispersion. Instead the best and most accurate way to describe this substance is by stating the following;
“Pico and nano metre sized co-valent bonded reconstituted atomic silver clusters in a static electrical suspension in an aqueous medium”.
This can further be qualified by identifying this substance as:
- A mutually repelling material by way of a hydrophobic relationship with the water it is contained in;
- subject to quantum physics when of an individual size of an atomic cluster is 10nm or smaller and
- A source of ‘free radicals’ and the ability to eradicate pathogens indiscriminately by an action known as the Oligo-Dynamic Effect.
The fact that so-called colloidal silver has never been scientifically described and sufficiently identified as to what really constitutes this so-called colloidal silver and a standard never having been introduced. From this would it would follow the conclusion that it really does not even exist as an identifiable substance. This is evidenced by many papers written and published and providing no technical nor scientific details about the material, the subject of the research but for a vague description such as AGNPs. This absence of a clear description of pico and nano metre sized silver and what it is able to do and used for, has halted progress for decades. Most likely ignorance and complacency in its production and use was made worse by a void created by the availability of appropriate instrumentation that could determine the properties of the material.
Anomaly 2. The problem with silver as an effective biocide
It is stated that silver has the highest level of reflectivity of all metals, in fact 97% of all visible light. From that statement can be concluded that the remaining 3% of all visible light must be absorbed. Some of this actually occurs at around 420nm (violet light), where such light is actually absorbed although this information is not generally written about. Also, not mentioned is that at 420nm, photons possess a small amount of ionizing energy at 2.95eV (a frequency of 714 Tera Hertz), or generally reserved for Ultraviolet wavelengths shorter than 400nm. Curious is also a misplaced silence and avoidance of the words violet and the slightly longer wavelength of indigo. At best both indigo and violet are referred to as BLUE. Most red shifted spectral colours from green to red have a refractive index. That means that whilst part of the light is reflected, part of the light is more or less absorbed depending on the refractive index of the material. It is an interesting question if indeed part of 420nm both is absorbed and also reflected. Perhaps not, as the ionizing energy would not create thermal agitation that longer and red-shifted wavelengths would. Some other questionable anomalies are discussed below.
Anomaly 3. Identifying the properties of water used for water-suspended silver
It is absolutely remarkable that the present testing of water’s conductivity is using methods and standards originally designed for solid metallic conductors yet adopted as if water can be measured that way as well. Both Conductivity and its alleged reciprocal Resistivity (Resistivity Specific) are concepts measured in Siemens per cm, when in fact it has nothing whatsoever to do with water. The concept of the word Siemens was coined by a German Industrialist by that name when a standard of resistance for copper telegraph cable formulated in the late 1800s. It consisted of a column of Mercury 1 metre high that measured 0.95 Ohm. Resistivity Specific related to the measure of current flow along the skin of a (three dimensional) solid metallic cube such as 1cm3. It expressed the current between two opposing surfaces and would flow on the outside from one surface to the other along the side. It should be noted that electrical current generally flows on the outside of a metallic conductor and it is for that reason that multi-strand cable allows more current to flow than a single solid strand of the same thickness (Figure 1).
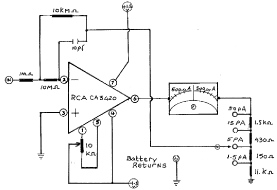
Figure 1. This is a particulary interesting circuit of one of a number of operational amplifiers designed and produced in the mid 1900 th are very low in price. Nevertheless, they boast input resistances of 1 million 1 million Ohm or abreviated to 1 Tera Ohm. Both current and resistance can be measured at 10-12 (pico) ampere and 1012 (tera) Ohms respectively with great accuracy. This particular operational amp was designed by Radio Corporation of America.
According the present standard of testing the conductivity of water the following conditions apply:
- The actual probe must contain two opposing metallic foils measuring 10x10 mm in area and separated by 10mm between the two foils. The conductivity of water is measured between the two foil electrodes as if the water is a solid conductor and expected to be in a straight line, i.e. Siemens/cm.
- At a temperature of 25º Centigrade and at a pH of 7, the maximum expected equivalent value of resistivity, i.e. 18.24 million Ohm, the conductivity will be 0.0548 micro Siemens/cm. It is also claimed that 1 micro-Siemens equals 1 million OHM resistivity. In a recent report from NIST in the USA, its scientists specifically quote cm2 in their calculations. What is missing in all this are the accepted norms for current (amperage per hour), resistance in Ohms and voltage potential as if these values are irrelevant. What is even more surprising is the concept of Parallel Resistance, Impedance and Input resistance not taken into consideration [15-24].
It is true, that it is debatable if in fact water has a resistance factor as a dielectric and insulator and it is really the ionic content of the water that is measured (Figure 2).
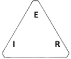
Figure 2. This is the conventional diagram used to illustrate the relationship between Voltage Potential (E), Current (I) and Resistance (R) for wich the folowing rule applies under Ohm’s Law, i.e. at a potential of 1 volt across a resistance of 1 OHM, 1 ampere of current flows. It should be noted at this point, that Ohms, Resistivity, Conductivity, Siemens and even Mho (reverse spelling of Ohm) all relate to current flow through solid metal conductors. It should also be noted that the value of 0.0548 micro Siemens is made up of two hypothetical figures of conductance, i.e. 0.0349 micro Siemens for H+ and 0.01983 micro Siemens for OH-. I have searched for the origin of these two figures but have so far been unsuccessful in finding any recorded evidence of these claims. How these figures were arrived at is another mystery!.
It is accepted by many researchers that for every anion there MUST be a cation and ‘vice versa’ and indeed that appears to be the case generally. However, there is definite hiatus when the conductance meter only ever shows a deviation ‘One Way’. In the case of an analogue panel meter from left to right, i.e. from no deflection to right swing deflection and by digital read-out from a low numerical figure to high one. The question now is: What is being measured? The cations or the anions or simply the difference between the two? Research so far has shown that measuring conductivity of anion does not get much of a mention, yet if cations are flowing toward the cathode (by attraction), anions attracted to the anode must flow in the opposite direction. This should give either a negative deflection on a center Zero meter or a minus figure read out, i.e. a below zero reading (Figures 3 and 4).

Figure 3. Illustration of two mid 1900 conductivity meters including a reference to the use of MHO.
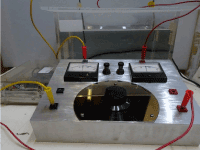
Figure 4. A modern example of a very simple instrument (bottom right) capable of determining the parallel resistance value with just a small analogue electronic power supply (bottom left) two panel meters and a variable resistor, measuring the resistance of water without actually being in the water compared to two submersed silver electrodes in a 9 litre of deionised water in tank (top right). The two panel meters both rated at 50 micro ampere FSD show a reading of 25 micro ampere. The actual resistance measured across the variable resistance proved to be 173,000 Ohm at the time of the test.
Anomaly 4. The problem with the absorption of water
Water is known for absorbing heat. It is very efficient in doing so. It is equally efficient in releasing the stored heat. Water also blocks shorter wavelengths, i.e. ultraviolet light. At around 200 nm even water droplets would impede these photons and only allow radiation in a vacuum. For some yet to be explained scientific phenomenon, water at 420 nm is said to become super transparent, allowing photons at around 417 to 420 nm to travel virtually unimpeded. It is claimed that pure water has a bluish tinge.
Note! This is a fortunate anomaly and well suited to the conversion (reduction) of ionic silver into neutral silver by irradiation at 420 nm.
Anomaly 5. The problem with ions in water
It is stated that for every ion there is a counter ion, indicating that the number of cations (positive charges) and anions (negative charges) MUST BE the same. At the same time another core assumption (dogma) explains ions as incomplete atoms. Whilst that may be true in some instances such as cations, made up of atoms that have lost one or more electrons. An unusual example would be a hydrogen ion known as a proton. It only needs to lose its one electron to do so. One could ask the question, “is a proton really an ion or should it be categorised by another definition?”. It is not an incomplete atom in the sense of being a fundamental particle but for the three quarks it is made up from. The matter of identifying ions is with negative ions. It cannot be said that an anion is an incomplete atom, rather it is over-complete by having an excess of an electron or more. There may be a case for thinking of negative ions as negative charges as they cannot be explained as an incomplete atom. Another example of confusion over negative ions is the so-called hydrated or solvated electron. In the process of oxidising silver by an applied DC current and voltage potential, metallic bulk silver loses its outer unpaired electron that winds up in the water. Its negative charge attracts positive charges such as the hydrogen atoms of a water molecule. It is quickly surrounded by the water. An individual hydrated electron cannot possible be called an incomplete atom, so it to does not qualify to be called an ion either. Nevertheless, it is the opposite charge to the positive silver ion, also in the water.
It has come to my attention that NO attention is paid to negative ions. Conductance/conductivity meters measure from zero to a high value, i.e. on an analogue panel meter from left (no deflection) to the right (maximum deflection or better known as full scale deflection). This relates to the measurement of positive ions only and begging the question “What about the negative ions or for lack of a better description “negative charges?” It may seem pedantic, but, positive current flow to the cathode and negative current flow to the anode are in opposite direction to each other. If, however a need exists for a current or charge carrier to flow in a specific direction, i.e. toward the cathode, a stronger current in the opposite direction may interfere with this. Such is often the case with distilled water, having elements of opposite charges such as ammonia and carbon dioxide.
Comment! I am in the process of designing an instrument able to quantify negative ions or charges in water regardless if they exist or not as well as completely separately also quantify positive ions or charges. It has been interesting but also frustrating research due to not able to locate reference material or research on negative ions. It is almost as if these do not exist or no one wishes to discuss it. Nevertheless, the technology for this is available but care needs to be taken to avoid artefacts. What is required is to detect negative ions or negative charges in the water, electronically process these as negative charges and present them unaltered at the output and accurately quantified as negative charges. Seeking such differences in negative and positive ions may be difficult in that their individual polarities may not necessarily equate to positive and negative voltages or current levels.
Anomaly 6. Conductivity and resistivity or more accurately named “Resistance Specific”
This distorted corner of ‘wet chemistry’ is the most questionable anomaly of all. It claims that conductivity (flow of current or charge carriers) has its reciprocal in resistivity (resistance specific) in Ohms. Resistivity Specific means at some agreed and standardised specification. In the case of Resistance Specific, it means current flow through a solid (metallic) conductor between opposing surfaces such as a solid metallic cube that can be 10×10×10 mm or 1meterx1metrex1 metre (1m3 or cubic) or any size in between. However, in conductivity measurements distortion is created by using cm2 or meter square as if the third dimension is irrelevant. The conductivity concept also insists that both conductivity and its alleged reciprocal resistivity must be:
- Measured in Siemens or parts thereof over distance, i.e. S/cm instead of current in Ampere over time.
- Only measured at a pH of 7 (where water is considered neutral) and (c) At a temperature of 25 degrees Centigrade and not be any higher in water’s resistance than 18.24 million Ohm. All this without any regard to the actual quantity of water: in other words: “it would make no difference with Siemens to measure the conductivity of an ocean or just a small amount of the same water in a 1 litre beaker. It further makes the claim that at a maximum resistance of 18.24 million Ohm the conductivity of water conforms to its reciprocal in conductivity of 0.0548 micro Siemens (Figures 5-7) [16-20,25].

Figure 5. A self-constructed 10,000 million Ohm input resistance analogue based meter shown here measuring a sample of deionised water with virtually no loading of the water at a reading of just 2 micro ampere in the cell on the far left.

Figure 6. The Same instrument showing the large increase of conductivity of ordinary tap water at a deflection of the panel meter of 43 micro ampere.

Figure 7. Proof that this instrument is also able to measure conductivity of Urine at around 50 micro amperes.
Anomaly 7. The need to optically observe and quantify contaminated water
In the mid-1900s optical instrumentation that could make contamination visible, operated on two distinctly different methodologies, i.e. nepholometry (light scattering) and obscurity (densitometry and turbidity) respectively, by optical means as follows:
These instruments each had a light source focused on a square or rectangular glass cell containing a liquid (usually water) to observe light scattering off particles in the water. This meant using a nephelometer (seeing in the dark) for or determining the purity or impurity of the water. The turbidity meter or densitometer was a way to determine to what extent the light was being obscured by foreign matter in the water. It was at a more popular method other than the measurement of pH and conductivity. The glass cells at the time were generally square in shape and transparent through all four surfaces. Some of these instruments were fitted with two of these cells and a choice to irradiate the light through the cell of choice and compare any difference between the two. Much could be learned about the extent of the contamination and measured exactly by a sensitive photo cell. Suddenly, these instruments disappeared from the market including the square glass cells and cuvettes used in them. Nowadays, we rely solely on pH and conductivity meters and any glass cells and cuvettes still around have a mottled impression or indenture on two opposing surfaces to make right angle light scattering observation impossible. The strategy for that change was claimed to have enough grip and not drop these cells and break them (Figure 8).
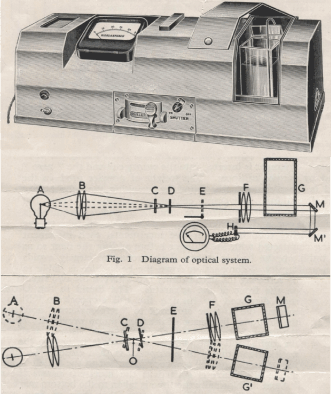
Figure 8. Details of what a turbidity or densitometer looked like. At the top an external view of such an instrument, including the square glass cells mentioned and below a schematic representation of its operation. Illustrations courtesy of Catalogue 56 S Chemical Laboratory Apparatus published by Griffin & George Limited (UK) undated and unable to trace. I personally think it is a great idea that should not have vanished.
Seeing a definite need for such instruments and in order to establish a means to observe contamination levels, a combined nephelometer/densitometer has been designed to also include linear cross polarization and sensitive photo-diodes to quantify changes to the light after it leaves the sample in the cell. It will have a window for visible observation and a second window for photographic or video recording and a third window linea recta for electronic photo sensing. With this optical/electronic instrument the following properties can be seen and recorded:
- Light scattering from all matter in the water, i.e. nano metre sized atomic silver clusters as well as organic and non-organic materials.
- By means of the cross-polarizing light scattering cause light scattering from silver to remain visible but any other material in the water to become invisible. This is due to most light in the visible wavelength having a refractive index that reflects and refracts. Metals do not have a refractive index at visible wavelength and as such remain visible. Silver and gold do have a refractive index, with silver absorbing light at between 417 and 420 nm (violet light, often referred to erroneously as blue light) [26].
- The electronic photo-sensing is capable of not only quantifying the blocking of light through the liquid in the cell, but also incorporating a violet pass filter at 417 to 420 nm to prove that there is light scatter from silver, except at this frequency due to its absorption. If a diffraction grating were to be also incorporated other metals which absorb wavelengths in the ultraviolet regions could also be identified. An illustration of such a representative prototype electronic/optical instrument is shown hereunder (Figure 9).
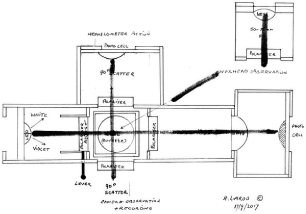
Figure 8. Rough sketch and modern concept of a combined light scattering nephelometer and densitometer enhanced with linear cross polarization to enable metals such as silver to be isolated for observation and quantifying for concentration. Light scattering combined with cross polarisation techniques works best at right angles to the light path, although backward scatter or scatter from different angles can also be an advantage in determining shape and size of particles or small collections of atomic clusters, i.e. in particular an elongated shape would show light scatter out of phase from one end to the other and so provide another bit of information of the material so detected.
When scientific and technical matters appear difficult, common sense is always available for the truth of the matter. Generally, it is a case of ‘an easy way out and hope for the best’ attitude. This reeks of a lack of precise thinking and an unwillingness to rationalise the problem at hand. So, it is with the inconsistencies mentioned in this draft. When measuring the conductivity of water, directions were taken that did not make any sense such as using alternating current instead of DC and grabbing words and description already owned by the world of solid metallic conductors and forgetting that water is a dielectric and thus an insulator. On its own water does not allow current to flow, it is the substances in water that do. If there is a resistance possessed by water, it would be infinitely high and beyond measurement by conventional instrumentation. That technology is available to become more precise in what we measure was demonstrated yesterday when I acquired a digital multimeter with an ability to measure resistance of 2,000 million Ohm. However, to measure water’s resistance we may need to go a factor of a million times higher.
- Ernest Dorsey N (1873) Properties of Ordinary Water-Substance, in all its phases: Water-vapor, Water, and all its Ices.
- Powell JL (1965) Quantum Mechanics. Wesley Publishing Company.
- Laroo H (2016) When a Particle is really a Cluster, a Dispersion a Suspension and there is no colloid in sight, you have the recipe for Colloidal Silver that is not a colloid either. Int J Nano Med & Eng 1: 50-56.
- Laroo H (2013) Colloidal Nano Silver- Its Production Method, Properties, Standards and its Bio-efficacy as an Inorganic Antibiotic. J Phys Chem Biophys 3: 130.
- Verano-Braga T, Miethling-Graff R, Wojdyla K, Rogowska-Wrzesinska A, Brewer JR, et al. (2014) Insights into the cellular response triggered by silver nanoparticles using quantitative proteomics. ACS Nano 8: 2161-2175.
- Cock I, Mohanty S, White A, Whitehouse M (2012) Colloidal Silver (CS) as an Antiseptic: Two opposing viewpoints. Pharmacognosy Communications 2: 47-56.
- Rai M, Yadav A, Gade A (2009) Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv 27: 76-83
- Lkhagvajav N, Yasa I, Celik E, Sari O (2011) Antimicrobial activity of colloidal silver nanoparticles prepared by sol-gel method. Dig J Nanomater Biostruct 6: 149-154.
- DMBT Disaanayake, Faoagali J, Laroo H, Hancock G, Whitehouse MW (2014) Preventing/Treating Rheumatoid Arthritis? Efficacy of some Colloidal Silver and Silver Salts against Proteus Species.
- Daming Fu (2013) Evaluation of the Antibacterial Efficacy of Silver Nanoparticles against Enterococcus faecalis biofilm. 2013 American Association of Endodontists. ACS Nano.
- Laroo H (2017) The Fragile and Flawed existence of Questionable Water Purity as used in Wet Chemistry and in particular where such water is destined for Medical Applications such as Vaccines. The International Journal of Vaccines and Vaccinations (MedCrave) 4: 00087.
- Nilsson A, Pettersson LGM (2015) The structural origin of anomalous properties of liquid water. Nature Communication.
- Laroo H (2016) Testing of metal derived nanometre sized particles using analogue methodologies. Mol Biophys Biochem.
- Malmberg CG, Maryott AA (1956) Dielectric Constant of Water from 0º to 100ºC. Journal of Research of the National Bureau of Standards 56.
- Shreiner RH, Pratt KW (2004) Standard reference materials: Primary standards and standard reference materials for electrolytic conductivity. NIST 142-260.
- Thornton Associates, Inc, Waltham, Massachusetts. Paper presented at the 1998 Semiconductor Pure Water and Chemicals Conference.
- Morash KR, Thornton RD, Saunders CH, Bevilacqua AC (1994) Measurement of the Resistivity of Ultrapure Water at elevated temperatures. Ultrapure Water Journal.
- Sokhan VP, Jones AP, Cipcigan FS, Crain J, Martyna GJ (2015) Signature properties of water: Their Molecular Electronic Origins. Proc Natl Acad Sci U S A 12: 6341-6346. [Crossref]
- Malmberg CG, Maryott AA (1956) Dielectric Constant of Water from 0º to 100ºC. Journal of Research of the National Bureau of Standards 56: 2641.
- Bevilacqua AC (1998) Ultrapure Water-The Standard for Resistivity Measurements of Ultrapure Water, presented in March 1998.
- Morash KR. Thornton Associates, Inc Waltham, MA 02154. Measurement of the Resistivity of Ultrapure Water at elevated temperatures. Published in the Ultrapure Water Journal in December of 1994 and presented at the Water EXPO. It was pre-printed with permission of Tall Oaks Publishing.
- Chaplin M (2017) Anomalous properties of water; Water structure and Science; Water molecule structure; Water clusters: overview; Water absorption spectrum and Water’s Two-State cluster history.
- Shields RM, Temelso B, Archer KA, Morrell TE, Shields GC (2010) Accurate Predictions of Water Cluster Formation, (H2O)n=2-10. J Phys Chem 114: 11725-11737.
- Pope RM, Fry ES (1997) Absorption Spectrum (380-700nm) of pure water. II. Integrating cavity measurements. Applied Optics 36.
- Bilenko Y, Shturm I, Bilenko O, Shatalov M, Gaska R (2010) New UV Technology for Point-of-Use Water Disinfection. Sensor Electronic Technology.
- Cryczynski Z, Lukomska J, Lakowicz JR, Matveeva EG, Gryczynski I (2006) Depolarised light scattering from silver nanoparticles. Chemical Physics Letters 421: 189-192









