Introduction: Basal cell carcinoma (BCC) is the most common malignancy in humans and represents a growing public health care problem. BCC is primarily caused by dysregulation of sonic Hedgehog signaling pathway in basal cells of the skin. BCC can be classified into low risk non-aggressive and high-risk aggressive subtypes. Distinction of BCC subtypes is essential for prognosis and for better disease management and treatment strategies.
Objective: The aim of this study was to assess the correlation between angiogenic agents, e.g. IL-6, VEGF-A, mast cell, and the aggressiveness of BCC in histopathology.
Methods: Nineteen patients achieved paraffin embedded blocks containing BCC were examined using immunohistochemical techniques for the antigen specific to Interleukin (IL)-6, vascular endothelium growth factor (VEGF), and Toluidine Blue staining to assess mast cell density.
Results: A strong VEGF-A expression was found significant more frequently in high risk aggressive BCC than in non-aggressive BCC (p <0.05). The highest number of mast cell was found in the micronodular BCC subtype, and there was a significant difference in mast cell number between high risk aggressive BCC and in non- aggressive BCC. IL-6 expressions were found in 79% of the BCC samples. A significant relationship between IL-6 expression and the BCC subtype (p<0.001) has been proven .
Conclusion: An increase in the angiogenic agents VEGF-A, mast cells, and IL-6 provides a hint for BCC’s nature of aggressiveness. Hence, in certain cases where the distinction between low risk non-aggressive and high-risk aggressive BCC is conventionally hard to determine, assessing to the angiogenic agents will be helpful.
angiogenic agent, basal cell carcinoma, aggressiveness
Basal cell carcinoma (BCC) is a non-melanoma skin cancer originating from the basal layer of epidermis and non-keratinized cells [1]. BCC is locally invasive, aggressive and destructive, but it’s metastatic tendency is very low [2]. Until the present time the etiology of BCC is still unknown [3]. In Regional General Hospital Dr. Moewardi, Surakarta, Indonesia, the occurrence of BCC is in the first rank of all skin cancers. There were 56 cases of BCC during 2010-2011. The prevalence reached 74.13% and was more common in women than men, 58.13% and 41.87%, respectively [4].
The most common genetic deviation in human skin cancer is related to the gene p53. This gene encodes phosphoprotein which involved in the cell cycle and in the maintaining of chromosomal stability [5]. It can be found that histologically aggressive BBC is significantly associated with increased expression of p53, which may be a sign of a form of mutation, though it still cannot be certainly determined [6]. Ultraviolet (UV) radiation considered as an etiological factor for the pathogenesis of BCC. UVB is an absorbed carcinogen and directly damages DNA [7]. Total UV radiation can also trigger the release of pro-inflammatory cytokines such as Interleukin (IL)-6 and Tumor Necrosis Factor (TNF)- α in human epidermal keratinocytes [7]. IL-6 dysregulation has been reported to be associated with various types of tumors, and IL-6 has an important role in regulating apoptosis from various cell types. Previously, the occurrence of myeloid cell leukemia 1 (MCL-1) in BCC with overexpression of IL-6 was significantly increase underlining the anti-apoptotic activity [8].
Mast cells are unique immune cells that are in the tissue with the ability to release various biologic active compounds that can trigger, regulate and suppress immune responses. The presence of mast cells in human tumors was first reported by Paul Ehrlich towards the 19th century. Since then various studies have found the presence of mast cell infiltration in various solid and hematological tumors [9]. On the other hand, an increase of growth factors such as basal fibroblast growth factor (FGF), including vascular endothelial growth factor-A (VEGF-A) and mast cells, will enhances the activity of angiogenesis, while angiogenesis itself is a major factor of tumor growth [10]. The aim of the study is to prove that high expression of angiogenic agent related to its aggressiveness.
This research was an analytic observational study with a cross sectional approach, namely collecting data that was assessed simultaneously in one period and then carried out statistical tests to assess the data obtained. This study has obtained ethical approval from the Health Research Ethics Committee of the University of Sebelas Maret, Surakarta-Indonesia (803/V/HREC/2018).
The samples of this study were specimens of skin lesions from patients with BCC on face and head who were biopsied either excision or punch biopsy and were diagnosed with histopathological examination of BCC. The technique of sampling was consecutive, which is all BCC patients who came in our clinic, who have fulfilled the inclusion criteria including the age of patients more than or equal to 18 years, patients with primary BCC, predilection in the face/head, and willing to take part in the study by signing an informed consent. The exclusion criteria, namely the age of patients less than 18 years old, recurrent BCC (had received prior treatment), BCC with predilection other than on the face and head, not accompanied by other malignant abnormalities, and not willing to follow/reject the study.
Nineteen patients achieved formalin-fixed, paraffin embedded tissue samples of BCC using the standard Hematoxylin and Eosin stain. The BCC specimens were then examined by immune-histochemical techniques for the antigen specific to Interleukin (IL)-6 by using anti IL-6 antibody (Ab9324 AbcamÒ), vascular endothelium growth factor (VEGF-A) by using VEGF-A antibody: sc-7269, Santa Cruz BiotechnologyÒ, and Toluidine Blue staining for asses mast cell density.
Histopathological types of BCC were determined and classification of the lesions was performed according to criteria proposed by Dixon and Jacobs et al. [11,12] IL-6 measurements are based on reactive cytoplasm, based on the IHJ Profiler plugin J Image. Positive high: 3+, positive: 2+; weak positive: 1+; negative: 0. Meanwhile VEGF-A measurements were carried out manually observing immune-histochemical reactivity in reactive tissue, analyzed independently by two expert pathologists and scored as strong positive (3+), moderate positive (2+), weak positive (1+) and negative (0) if<10% [13]. Kruskal-Wallis or Mann-Whitney tests and Spearman’s rho statistical analysis were performed pertaining to expression of IL-6, VEGF-A and mast cell among the subtypes of the BCC (Figures 1-3).
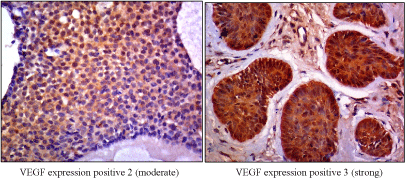
Figure 1. VEGF expression
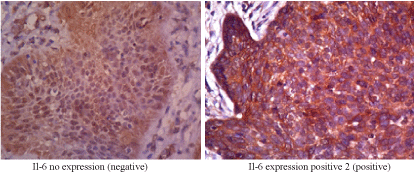
Figure 2. Il-6 expression
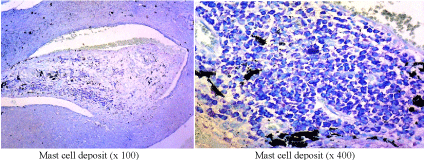
Figure 3. Mast cell deposit
The demographic data are divided according to gender, age, and occupation (Table 1). In this study female patients outnumber male patients with a ratio of 13:6 (68.4%: 31.6%). The ratio in the aggressive BCC group is 11: 5 (57.9%: 26.3%), while in the non- aggressive BCC group, 2:1 (10.5%: 5.3%). The age of the subjects ranges from 40-75 years old (average 57.5 ± 17.5) both in aggressive and in non-aggressive BCC groups, but mostly aged >50 years old (84.2%). Farmer is the highest proportion in occupation (26.2%). It has been found that the aggressive BCC is 1.5 times the non-aggressive BCC in women, but statistically not significant (p >0.05).
Table 1. Demographic data
|
Number |
Percentage (%) |
Aggressive BCC* |
Non-Aggressive BCC |
p-Value |
Gender
Men
Woman |
6
13 |
31.6
68.4 |
5 (26.3%)
11 (57.9%) |
1 (5.3%)
2 (10.5%) |
p=0.943 |
Age
40-50 years old
51-60 years old
61-70 years old
> 70 years old |
3
8
4
4 |
15.8
42.0
21.1
21.1 |
3 (15.8%)
6 (31.6%)
4 (21.1%)
3 (15.8%) |
0
2 (10.5%)
0
1 (5.3%) |
p=0.556 |
Occupation
Housewife
Government Employees
Labor
Entrepreneur
Farmer |
3
3
4
4
5 |
15.8
15.8
21.1
21.1
26.2 |
3 (15.8%)
1 (5.3%)
3 (15.8%)
4 (21.1%)
5 (26.3%) |
0
2 (10.5%)
1 (10.5%)
0
0 |
p=0.080 |
*BCC: basal cell carcinoma
The clinicopathological data (Table 2) are described according to the characteristics of tumor predilection, tumor diameter, and BCC subtype. Tumor location is based on Baker classification [11] which divides face predilection into three groups, i.e. upper face, mid face (middle face and ears), and lower face. Based on Walling criteria [14], the tumor size with diameter >2.5-3.0 cm contributes to aggressive type of BCC. Subjects are grouped into subtypes of histopathological features of BCC as follows: (i) nodular, (ii) micronodular (iii) superficial, (iv) morpheaform, and (v) mixed.
Table 2. Clinicopathologic data
|
Number |
Percentage (%) |
Aggressive BCC |
Non-aggressive BCC |
p-Value |
Tumor location
Upper face
Mid face
Lower face |
5
13
1 |
26.2
68.4
5,3 |
4 (21.1%)
11 (57.9%)
1 (5.3%) |
1 (5.3%)
2 (10.5%)
0 |
p=0.880 |
Tumor Diameter
< 2 cm
> 2 cm |
9
10 |
47.4
52,6 |
7 (36.8%)
9 (47.4%) |
2 (10.5%)
1 (5.3%) |
p=0.466 |
BCC subtype
Nodular
Micronodular
Superficial
Morpheaform
Mix type |
4
2
2
4
7 |
15.8
10,5
10,5
21.1
36.8 |
1 (5.3%)
2 (10.5%)
2 (10.5%)
4 (21.1%)
7 (36.8%) |
3 (15.8%)
0
0
0
0 |
p < 0.001 |
Comparison of VEGF-A expression interpretations by the two pathologists is analyzed by kappa test. Based on kappa test, the coefficient of Cohen's kappa is κ=0.683 with a significant value of 0.003. In the aggressive BCC group, 14 samples (73.7%) show an aggressivety score of +3 and two samples (10.5%) with a score of +2; while in the non- aggressive BCC group there are only one sample with a score of +3 (5.3%) and two samples with a score of +2 (10.5%).
Two of each aggressive BCC samples and non-aggressive BCC samples do not show IL-6 expression. However, IL-6 expression is found positive in the remaining 15 samples (78.9%). Based on Spearman’s rho statistical analysis it can be determined that there is a significant relationship between IL-6 expression and BCC subtypes (p<0.001) (Table 3).
Table 3. VEGF-A and IL-6 expression in BCC
Expression |
Aggressive
BCC |
Non-aggressive BCC |
p value |
VEGF-A |
16 |
3 |
p < 0.003 |
IL-6 |
13 |
2 |
p < 0.001 |
The increase of mast cell deposit is higher in aggressive BCC compared to non-aggressive BCC, while the mast cell deposits among the various subtypes of the BCC are significantly different for each other (p <0.05).
Basal cell carcinoma (BCC) is the most common skin cancer with a prevalence of 70% of keratinocyte tumors [10]. Some studies report BCC is more prevalent in men than in women. This is caused by occupation where men experience repeated UV exposure. This study finds that BCC is more common in women than in men, with a ratio of 68.4%:31.6%. This is conform with study results by Marcelina et al., which reported more women affected by BCC than men [12]. Based on age group, the biggest proportion of patients with BCC aged >50 years old (84.2%), with an average of 57.5 ± 17.5 years. The incidence of BCC increases at ages >50 years [12], which generally occurs at the age of 40-80 years [14]. However, the incidence of BCC increases relatively high in women aged <40 years [15,16]. The peak incidence occurs between the ages of 60 and 80 years with >95% of patients are aged >65 years old. One of the risk factors for the occurrence of BCC is the ability of the skin to be tanned, thereby increasing its incidence in young people aged <40 years old especially women because of the use of tanning beds indoors. Skin which tans easily has a higher tendency to develop BCC after chronic and intermittent UV exposure compared to skin which tans with difficulty [11].
In cancer where the epidermal cell layer is differentiated, the VEGF-A level is higher than when the epidermal cell layer is undifferentiated. Several studies employing immunohistochemical techniques and hybridization in situ have shown the increase of VEGF- A level in tumor cells compared to normal epidermal cells. In this study, VEGF-A expression is found in 100% of samples with moderate to strong scoring variations. It statistically shows a significant difference between VEGF-A expression in aggressive BCC compared to non-aggressive BCC (p <0.05), hence VEGF-A can be used as a prognostic for assessing the risk of tumors to become invasive. This is in line with the previous study results by Oh et al. [17], who reported that high levels of VEGF-A affect to the aggressiveness and metastasis of BCC. Most human tumors express VEGF and VEGFR- 2 which play an important role in tumor angiogenesis [18,19].
In our study, mast cell deposit has been significantly increased in aggressive BCC compared to non-aggressive BCC, moreover mast cell deposit in various subtypes also found significantly different for each other (p <0.05). Recent data supports the additional role of mast cells in the development and progression of skin cancer suggesting mast cells may play contradictory roles in tumor biology, and that local conditions may determine whether mast cells will have a promoting or inhibitory effect on tumors. Thus, certain studies lead to the evidence that mast cells might play a dual role in the pathogenesis of skin tumors, including BCC. Mast cells have a very extensive mediator warehouse, with promoting and inhibitory effects on malignancy [20]. The increase in mast cell deposit is shown significantly different upon BCC subtype, it can therefore be taken as a prognostic factor in the management of BCC. Mast cell deposits were found in aggressive BCC, especially in morpheaform, micronodular and mixed subtypes. Published studies have also shown increased mast cell deposits in morpheaform subtype of BCC [21-23].
IL-6 is a pro-inflammatory cytokine that mediates chronic inflammation and may play an important role in tumorigenesis triggered by inflammation. IL-6 activates transcription factors in cells through phosphorylation process, both through STAT-1 and STAT-3 or JAK-2 [21]. High levels of STAT-3 have been observed in various tumors, associated with tumorigenesis induced by IL-6. IL-6 is also capable to regulate cells for survival, growth, and differentiation into cancer cells [22]. In this study, Il-6 expression is found in 78,9 % of samples; and there is a significant relationship between IL-6 expression and BCC subtypes (p<0.001) (Table 3).
Because of the small sample size the study results should be further proven by another large scale investigation, and differences in some risk factors of demographic data compared to previous studies elsewhere cannot be ruled out.
Distinction of the aggressive nature of BCC becomes a consideration in managing BCC properly with the aim to reduce risk of recurrence, to find an appropriate treatment, and not at least to save a good quality of life. An enhance in activity of the angiogenic agents VEGF-A, mast cells, and IL-6 can be hints for aggressiveness of the examined cutaneous tumor. Hence, if the distinction between low risk non-aggressive and high-risk aggressive BCC is histopathologically hard to determine, assessing to these angiogenic agents will be helpful.
- Basal cell carcinoma (BCC) is the most frequently diagnosed skin cancer worldwide. The dysregulation of sonic Hedgehog signaling pathway in basal cells thought to be the initial reason for the emergence of BCC.
- BCC can histopathologically be classified into low risk non-aggressive and high-risk aggressive subtypes. The identification of the subtype is relevant for prognosis and particularly for proper choice of treatment.
- A strong VEGF-A expression was found significant more frequently in high-risk aggressive BCC than in non-aggressive BCC (p <0.05). Likewise, the number of mast cell was significantly increased in aggressive BCC. A significant relationship between IL-6 expression and the BCC subtype (p <0.001) has also been proven.
- In certain cases where the distinction between low risk non-aggressive and high-risk aggressive BCC is conventionally, histopathologically hard to determine, assessing to specific angiogenic agents, e.g. VEGF-A expression, mast cells number, and IL-6 of the examined cutaneous tumor can provide a helpful tool.
Acknowledgement: The authors would like to thank all study coworkers and patients who participated in this study.
Funding. No funding or sponsorship was received for this study and no Rapid Service Fee was received by the journal for the publication of this article.
Disclosures. All authors have nothing to disclose.
Authorship. All named authors meet the International Committee of Medical Journal Editors (ICMJE) criteria for authorship for this article, take responsibility for the integrity of the work as a whole, and have given their approval for this version to be published.
Author contribution statement: All authors contribute to the study conception and design. Material preparation, data collection and analysis were performed by PM, IJ, ND, SST and EPW. Critical review, commentary, and revisions by PM and IE.
Compliance with ethics guidelines. Ethical approval of this research was obtained from the Health Research Ethics Committee of the University Sebelas Maret, Surakarta-Indonesia (803/V/HREC/ 2018). Informed consent was gained from all participants
Data availability. Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
- Carucci JA, Leffel D (2008) Basal cell carninoma. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS et al. (editor). Fitzpatrick’s dermatology in general medicine. 7th Ed. New York: Mc Graw Hill, pp: 1036-1041.
- Zwann E, Haas NK (2010) Genetic of basal cell carcinoma. Aus J Dermatol 51: 81-94.
- Tilli CML, Steensel MAM, Krekels GAM, Neumann HAM, Ramaekers FC (2005) Molecular etiology and pathogenesis of basal cell carcinoma. Br J Dermatol 152: 1108- 1124. [Crossref]
- Mawardi P, Kalim H, Kusworini, Fitri LE, Mintaroem K, et al. (2016) Mid-face location of primary basal cell carcinoma related to cancer aggressivity. Asian Pac J Trop Dis 6: 650-653.
- Katayama H, Sasai K, Kawai H, Yuan ZM, Bondaruk J, et al. (2004) Phosphorylation by aurora kinase A induce Mdm2-mediated destabilization and inhibition of p53. Nat Genet 36: 55-62.
- Rossenstein P, Phelps RG, Weinstocks MA, Bernstain JL, Gordon ML, et al. (1999) P53 mutation in basal cell carcinoma arising in routine users of sunscreen. Photochem Photobiol 70: 798-806. [Crossref]
- Brash DE, Heffeman T (2008) Carcinogenesis: Ultraviolet radiation. In: Goldsmith LA, Katz SI, Gilchrest BA, Paller AS et al. (editor). Fitzpatrick’s dermatology in general medicine. 7th Ed. New York: Mc Graw Hill, pp: 999-1004.
- Shimizu T (2010) The role of macrophage migration inhibitory factor (MIF) in ultraviolet radiation-induced carcinogenesis. Cancers 2: 1555-1564.
- Aponte-López A, Fuentes-Pananá EM, Cortes-Muñoz D, Muñoz-Cruz S (2018) Mast Cell, the neglected member of the tumor microenvironment: role in breast cancer. J Immunol Res 2018: 1-11.
- Winter J, Kneitz H, Bröcker EB (2011) Blood vessel density in basal cell carcinoma and benign trichogenic tumors as a marker for differential diagnosis in dermatopathology. J Skin Cancer 10: 1-5.
- Dixon AY, Lee SH, McGregor DH (1989) Factors predictive of recurrence of basal cell carcinoma. Am J Dermatopathol 11: 222-23.
- Jacobs G, Rippey J, Altini M (1982) Prediction of aggressive behavior in basal cell carcinoma. Cancer 49: 533-537.
- Bowden J, Brennan PA, Umar T, Cronin A (2002) Expression of vascular endothelial growth factor in basal cell carcinoma and cutaneous squamous cell carcinoma of the head and neck. J Cutan Pathol 29: 585-589.
- Baker SR (2007) Reconstruction of the Nose. In: Baker’s local flap in facial reconstruction. Philadelphia: Mosby, pp: 415-74.6
- Gauci J, Muscat G, Aquilina S (2017) A local perspective on basal cell carcinoma: frequency of subquent skin tumors. Malta Medical School Gazette 1: 46-55.
- Marcelina P, Mappiasse A, Anwar AI, Ganda IJ, Hatta M, et al. (2016) Expression of patched-1 protein in aggressive and nonaggressive basal cell carcinoma. Int J Clin Exp Med 4: 122-128.
- Dourmishev L, Rusinova D, Botev I (2013) Clinical variants, stages, and management of basal cell carcinoma. Indian Dermatol Online J 4: 12-17.
- Walling HW, Fosko SW, Geraminejad PA, Whitaker DC, Arpey CJ (2004) Aggressive basal cell carcinoma: presentation, pathogenesis, and management. Cancer Metastasis Rev 23: 389-402. [Crossref]
- Hoorens I, Vossaert K, Ongenae K, Brochez L (2016) Is early detection of basal cell carcinoma worthwhile? Systematic review based on the WHO criteria for screening. Br J Dermatol 174: 1258-1265.
- Dębski T, Lembas L, Jethon J (2012) Basal cell carcinoma. In: Agullo FJ (editor). Current concepts in plastic surgery. Croatia: In Tech, pp: 15-48.
- Apalla Z, Lallas A, Sotiriou E, Lazaridou E, Vakirlis E, et al. (2016) Farmers develop more aggressive histologic subtypes of basal cell carcinoma. Experience from a Tertiary Hospital in Northern Greece. J Eur Acad Dermatol Venereol 30: 17-20. [Crossref]
- Oh CK, Kwon YW, Kim YS, Jang HS, Kwon KS (2003) Expression of basic fibroblast growth factor and thrombospondin-1 related to microvessel density in nonaggressive and aggressive basal cell carcinoma. J Dermatol 30: 306-313. [Crossref]
- Salem Yehya AH, Asif M, Petersen SH, Subramani AV, Kono K, et al. (2018) Angiogenesis: Managing the culprits behind tumorigenesis and metastasis. Medicina 54: 1-20. [Crossref]
- Macedo F, Ladeira K, Longatto-Filho A, Martins SF (2017) Gastric cancer and angiogenesis: Is VEGF a useful biomarker to assess progression and remission? J Gastric Cancer 17: 1-10.
- Heidarpour M, Rajabi P, Heidarpour M, Khalife A (2010) Mast cell in basal cell carcinoma. Pak J Med Sci 26: 398-440.
- Erkiliç S, Erbağci Z (2001) The significance of mast cell associated with basal cell carcinoma. J Dermatol 28: 312-315.
- Theoharides TC, Conti P (2004) Mast Cells: the JEKYLL and HYDE of tumor growth. Trends Immunol 25: 235-241.
- Chang Q, Bournazou E, Sansone P, Berishaj M, Gao SP, et al. (2013) The IL- 6/JAK/Stat3 feed-forward loop drive tumorigenesis and metastases. Neoplasia 15: 848-862. [Crossref]
- Gasche JA, Hoffmann J, Boland CR, Goel A (2011) Interleukin-6 promotes tumorigenesis by altering DNA methylation in oral cancers cells. Int J Cancer 129: 1053-1063.
Editorial Information
Editor-in-Chief
Dr. Dennis Mans
Article Type
Research Article
Publication history
Received date: February 03, 2021
Accepted date: March 05, 2021
Published date: March 10, 2021
Copyright
©2021 Mawardi P. This is an open-access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Citation
Mawardi P, Julianto I, Dharmawan N, Toh SS, Putra E, et al. (2021) The difference of angiogenic agent expression in basal cell carcinoma related to its aggressiveness. Glob Dermatol 8: DOI: 10.15761/GOD.1000230
Corresponding author
Prasetyadi Mawardi
MD, Department of Dermatology and Venereology, Faculty of Medicine, Sebelas Maret University, Dr. Moewardi Regional General Hospital, Jalan Ir. Sutami, Surakarta, Jawa Tengah, Indonesia 57126
E-mail : bhuvaneswari.bibleraaj@uhsm.nhs.uk



