Abstract
Introduction: The management of unilateral, uncomplicated Wilms Tumour (WT) is relatively straightforward, and most patients are expected to survive following chemotherapy and radical nephrectomy. While there is still some debate on upfront biopsy and the timing of surgery the underlying principle remains the same; to remove all tumour, without rupture, or vascular compromise and to provide accurate surgical (histological) staging. However, there remains a unique subset of patients that continue to present a significant challenge to the surgeon. These include patients with intravascular extension, Bilateral disease, Wilms tumour in a Horseshoe kidney, tumour in a solitary kidney, Wilms tumour infiltrating the neighbouring organs and the acute presentation with rupture or massive haemorrhage. Cases presentation: One case each of bilateral WT and unilateral Wilms with contralateral nephroblastomatosis were managed in infants with good outcome. WT with IVC extension, in a three-year-old was managed successfully with cardiovascular surgical team. WT in a horseshoe kidney in a six-year-old was diagnosed pre-operatively and thus we were ready for the complex anatomy. WT with persisting liver invasion despite neoadjuvant chemotherapy could be successfully dealt with due to the surgeon’s experience in liver resections. A case which could be diagnosed only post-operatively and had a fatal outcome was of a pre-operative rupture of suspected WT in a 21-month-old child. Conclusions: This paper seeks to describe the surgical difficulties faced in managing a complex Wilms Tumour. Controversies over the approach to a patient with bilateral WT and IVC tumour thrombus are discussed as is the surgical and Interventional approach to horseshoe WT and the ruptured tumour.
Key words
bilateral wilms; horseshoe kidney; nephron sparing surgery; tumour thrombus; case series
Introduction
Wilms Tumour (WT), or nephroblastoma, is the most common genitourinary malignant Tumour in children with a peak incidence between 2 and 3 years of age [1]. Long- term disease free survival rates in unilateral Wilms without metastasis is currently greater than 90% [2]. This demonstrates significant progress compared to an overall survival of less than 30% in the past and is due to the evolution of more effective chemotherapy protocols, safer surgical techniques and team-based management [2].
Nevertheless, a small group of patients still present surgical challenges, these include bilateral Wilms tumour, Wilms tumour with intravascular thrombus, tumour in a horseshoe kidney and the acute presentation with rupture and or haemorrhage. Complete surgical clearance and meticulous technique is of paramount importance in staging and long-term management. With optimal pre-operative work up and careful surgical timing and planning, excellent results can also be achieved in this, more complex, group of patients. This review is based on our experience at King Hamad University Hospital, Kingdom of Bahrain and Nhi Dong Bien Hi- HCMC, Vietnam from 2013 to 2019. In this period, we had 1 patient with bilateral Wilms tumour, 1 child with Wilms tumour and contralateral nephroblastomatosis, 1 child with Wilms tumour with IVC thrombus, 2 children with Wilms tumour in a horseshoe kidney, 1 child with Tumour invading segments of the liver, and 1 patient with suspected rupture at presentation.
Case series
Bilateral Wilms
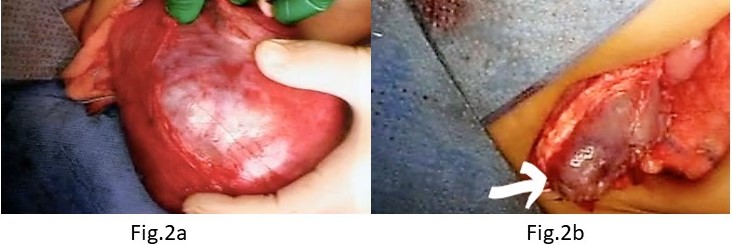
An 8 month old girl with hypoplastic corpus callosum, epilepsy and bilateral hydronephrosis presented with abdominal distension due to an abdominal mass occupying almost all quadrants. Ultrasonography could not define the organ of origin and CECT (contrast enhanced CT) abdomen showed a right-side renal mass (11 × 10.5 cm) occupying the mid and lower pole with hydronephrosis, and three well defined masses in the upper, mid, and lower pole of the left kidney, measuring 7 × 5cm, 4.3 × 4cm and 7 × 6.7cm respectively (Figure 1). A bilateral trucut biopsy revealed nephroblastoma on either side. Metastatic work up was negative, and she underwent neoadjuvant chemotherapy with Vincristine and Actinomycin. Reassessment with CT abdomen after 6 weeks showed good response in the left kidney, however only slight reduction in size of the right-side mass. The child underwent an initial, Nephron-Sparing Surgery (NSS) on the right side (Figures 2a,2b) with negative margins and negative sampled lymph nodes (LN). Hence post-operative chemotherapy was administered as local Stage II. After a month, DMSA scintigraphy (Dimercaptosuccinic Acid) showed good function in the residual right kidney and a left radical nephrectomy was done (nodes negative). Histopathology on both sides revealed a favourable type of WT. The child is now 5 year old, and at latest follow has no recurrence and excellent renal function.
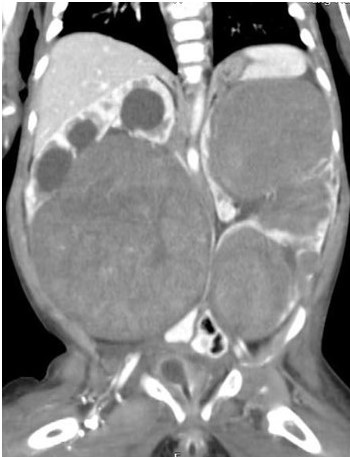
Figure 1. CECT showing bilateral Wilms tumor
Unilateral Wilms and contralateral nephroblastomatosis
A 1-year-old girl child presented with a symptomatic abdominal mass. An MRI suggested bilateral renal mass (suspected nephroblastomatosis), a 16 × 10 right renal mass with centrally located compressed, functional renal parenchyma and multiple 2-3 cm subcapsular masses in the upper and lower pole of the left kidney (Figure 3). Needle biopsy from either side revealed nephroblastomatosis. An MRI after 2 agent chemotherapy showed total regression of the left-sided lesions but little response on the right side. A needle biopsy of the right-side lesion revealed nephroblastoma. The child underwent right side nephron sparing surgery (heminephrectomy) and biopsy of the left kidney which showed nephrogenic rests. The histology was unfavourable. Margins and lymph node samples were negative (local stage II). Post-operative chemotherapy was completed, and child is on close follow–up for the last 4 years. No recurrence on the right side or any new lesions on the left side have been noted.
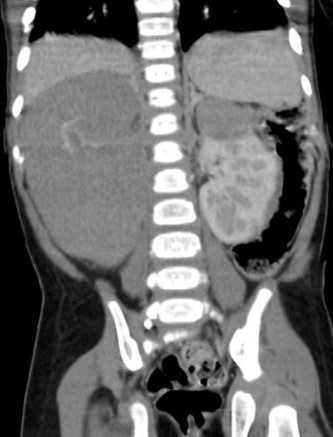
Figure 3. MRI showing bilateral renal mass
Wilms Tumour with IVC extension
A three-year-old boy presented with a large left renal mass which was evaluated with imaging and Tru-cut biopsy and found to be WT with an asymptomatic Inferior vena cava (IVC) thrombus extending from the left renal vein and kidney up to the right atrium (Figures 4a, 4b). He received neoadjuvant chemotherapy. However, after 6 weeks of chemotherapy, no regression in the size of the thrombus was noted; although, there was significant reduction of the left sided primary. Planned surgery included a left radical nephrectomy with IVC thrombectomy in a cardiac OT and with the cardiac surgical team in attendance. A radical left nephrectomy with a cuff of Renal Vein/IVC insertion was performed and the IVC opened under distal and sub-diaphragmatic control. Massive bleeding from the IVC (presumed lumbar/azygous vein collaterals) made this impossible and following a limited distal thrombectomy and IVC closure, the residual proximal IVC and atrial Tumour thrombus was removed under cardiac bypass. An acute SVC thrombus in the immediate post op period warranted urgent re- thoracotomy and additional anti-coagulation but was without further sequelae. Adjuvant chemotherapy and radiotherapy was completed post-operatively. Histology of the left kidney showed favourable histology WT and was node negative. The tumour thrombus itself was necrotic with no evidence of viable WT. He is well at 5 years follow up with no evidence of recurrence.
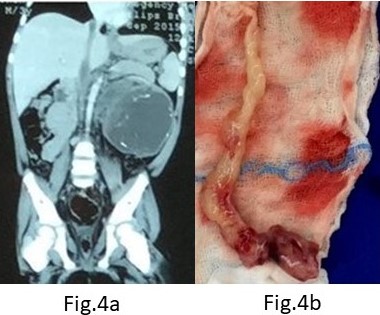
Figure 4. (A) CECT showing IVC thrombus; (B) Retrieved tumor thrombus
Wilms in horseshoe Kidney
A. A six-year-old was admitted with a history of blunt trauma to the abdomen during play. Abdominal palpation revealed a large mass in the left flank. An Ultrasound showed a large Wilms tumour in an apparent horseshoe kidney. A CT abdomen (Figure 5a) confirmed a horseshoe kidney with a large central mass consistent with WT – Stage V and no metastases. A Tru-cut biopsy confirmed favourable WT and he was commenced on 12 weeks of neo-adjuvant chemotherapy. At surgery, a 12 × 10 cm mass was noted to be arising from the isthmus of the horseshoe with both kidneys torted anteriorly and medially. The tumour was closely related to the lower pole of the right kidney but clear of the lower left pole. The ureters were identified from each kidney and carefully preserved while the tumour with a portion of the lower pole of the right kidney excised. Tumour margins were negative as were sampled nodes from both sides. He is well with no evidence of recurrence 4 years after surgery and after two agent chemotherapy.
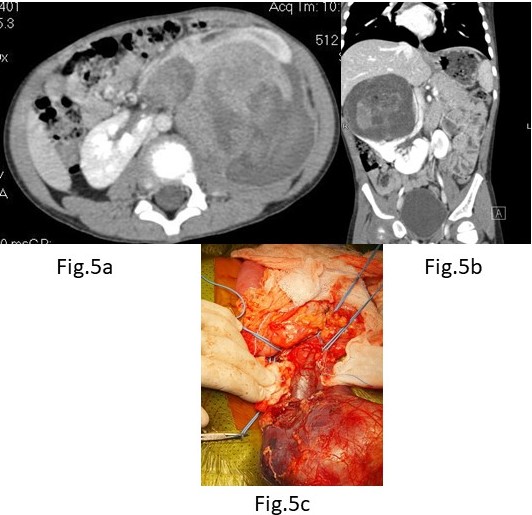
Figure 5. (A) CECT(axial view) WT in Horseshoe Kidney; (B) CECT(coronal view) WT in Horseshoe Kidney;
B. A four-year-old boy presented with hematuria and abdominal mass of a week’s duration. Evaluation with CT showed right side WT sparing the lower pole and isthmus in a horseshoe kidney (Figure 5b). He underwent an upfront nephrectomy of the right side. Tumour was occupying the renal pelvis (Figure 5c). Histopathology showed a favourable histology with hilar lymph nodes being positive. He is undergoing radiotherapy and chemotherapy.
WT invading the liver
A 3-year boy had right sided WT invading the segment V and VI of Liver even after pre-operative chemotherapy (Figure 6). He underwent a right-side radical nephrectomy with segmental resection of the liver en-bloc for complete tumour free margins with a successful outcome. At 5 years of follow up, he has no evidence of disease.

Figure 6. WT invading liver segment
Acute abdomen with suspected pre-operative tumour rupture
A 21-month-old boy presented with abdominal pain of 2 weeks’ duration which had become more severe over 4-5 hours. An Ultrasound showed a large (9 × 11 cm) mass in the upper pole of the right-side kidney.
An urgent CECT abdomen showed a large 11 × 12 cm Tumour in the upper pole of right kidney (Figure 7). The mass was heterogeneous, contrast enhancing and with areas of necrosis and haemorrhage. The hilar nodes were enlarged and there was a moderate amount of free fluid with several suspicious metastatic lesions in the liver. The impression was of a suspected ruptured Wilms Tumour
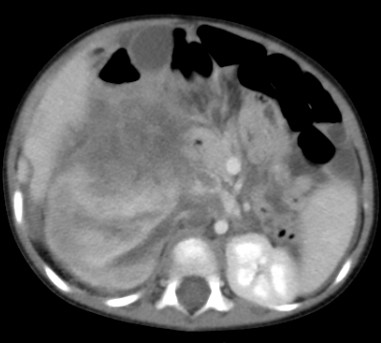
Figure 7. CECT suspicious of tumor rupture
Following the CT, his condition deteriorated acutely with signs of hypovolemic shock. Aggressive resuscitation with blood, inotropes, and ventilation partially controlled his hypovolemic state but ongoing bleeding prompted an urgent laparotomy with a provisional diagnosis of rupture and haemorrhage from a right sided WT.
At surgery, he was noted to have a massive tumour of the right kidney involving the right lobe of liver with rupture of the renal component and massive bleeding. It was unclear if this was a primary renal tumour invading the liver or a primary hepatoblastoma invading the kidney. An en-masse liver and kidney resection were performed with difficulty but the child succumbed on the tenth post-operative day. Histology confirmed an hepatoblastoma with an elevated, preoperative AFP.
Discussion
Wilms tumour is the most common renal malignancy in childhood and is responsible for nearly 6% of all childhood cancers [3]. Surgery is one of the cornerstones in management of Wilms tumour. Other aspects of management include chemotherapy and radiation therapy. Dramatic advances in the management of Wilms tumour have resulted in more than 90% of patients with localized disease being completely cured and 70% surviving metastatic disease [4].
A small fraction of children with Wilms tumour which include the patients managed by our team still pose a significant challenge to the surgical team.
Bilateral disease represents 5-10% of all Wilms patients (BWT), which may present as synchronous or metachronous bilateral Tumours [5]. At least 10% of synchronous BWTs have unfavourable histology, and up to 22% are associated with genitourinary abnormalities, aniridia, WAGR (WT, aniridia, genitourinary anomalies, and retardation) syndrome, Denys–Drash syndrome, hemihypertrophy or one of the other overgrowth syndromes. The management of a child with bilateral Wilms Tumour (BWT) is indeed challenging in achieving disease clearance while preserving adequate renal function. The right balance must be achieved between preservation of the optimal amount of renal parenchyma to prevent renal insufficiency, and simultaneously, complete resection to optimize the chances to cure malignancy. The presence of nephroblastomatosis complicates the diagnosis of WT and mandates frozen section biopsy to ensure clear surgical margins.
Because other paediatric renal tumours are almost never bilateral, neoadjuvant chemotherapy for presumed BWT can be initiated without first performing a biopsy of any of the Tumours [6]. The feasibility of bilateral nephron-sparing surgery should be assessed after six weeks of therapy by multiphased contrast-enhanced CT scan of the abdomen and pelvis (Figure 8). To preserve maximal residual renal parenchyma, a marginal resection or enucleation technique is often used for bilateral nephron-sparing surgery [7]. A positive margin following NSS requires radiation but has not found to increase the incidence of local recurrence except in cases with diffuse anaplasia [8,9]. Hence, nephron preservation should be the primary aim despite assuming the risk of positive surgical margins. A margin which contains nephrogenic rests has no bearing on patient prognosis or local disease relapse [9]. Growth of a tumour on chemotherapy should cause concern as this may indicate unfavourable histology.
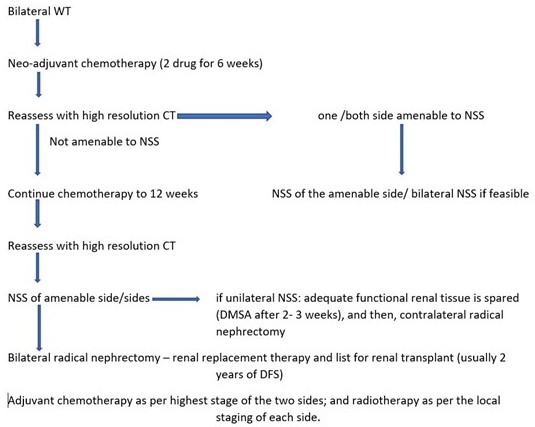
Figure 8. Algorithm for bilateral WT
Intravascular extension (Renal Vein, IVC, Right Atrial) of tumour thrombus is a well-recognized, but thankfully rare, condition in Wilms, and is usually asymptomatic [10]. It can extend into the renal vein, inferior vena cava (IVC) and atrium with a prevalence of 11.3%, 4.1% to 8.1%, and 1%, respectively [11-13]. It should be considered at the diagnostic stage as it is usually found concurrently. Ultrasonography with Doppler has become the first choice for preliminary diagnosis of Wilms tumours and intravenous tumour thrombus with an accuracy of 60-100% [14]. Its advantages are avoidance of radiation, efficiency and its non-invasive nature. CT, which is routinely used for staging the disease was found to be less accurate in identifying the tumour thrombus and defining its extent [15]. MRI is more sensitive however and can accurately diagnose the intravenous and intra-atrial tumour thrombus displaying accurate location, size of the thrombus and its relationship to the vessel wall [14]. Its main disadvantage however is the length of time required to capture images, which may require sedation or anaesthesia especially in younger children.
Daum described a staging system for intravascular tumour thrombus, which is largely followed by many [16]. Stage I as a small <5 cm thrombus extension in the IVC below the level of the hepatic vessels; Stage II as a large thrombus >5 cm, yet, still below the hepatic vessels; Stage III as extending to the level of the hepatic vessels and above providing more operative difficulty obtaining proximal control; and Stage IV as tumour thrombus extending to the atrium. Abullah et al. modified this (Figure 9) and presented a more comprehensive staging system to assess the degree of thrombus extension [17]. The difficulties and operative techniques differ according to the stage of thrombus.
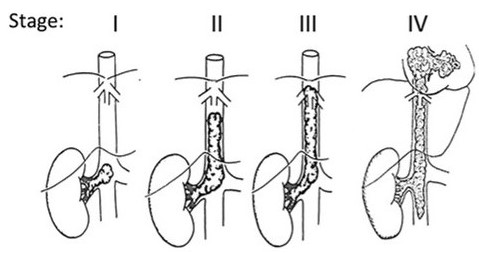
Figure 9. IVC thrombus Staging system [17]
Despite the different management protocols between the three main collaboratives (NWTS, SIOP and COG) after the NWTS 5 study, all bodies agreed that identification of intravascular tumour thrombus into the IVC at or above the level of the hepatic veins should ideally be treated with preoperative chemotherapy. A decrease in the size of the intravascular extension has been reported by many authors as a result of preoperative therapy regardless of the extent of thrombus [18,19]. It has also been suggested that preoperative therapy may decrease the risk of surgical complications [11].
Removal of the residual thrombus after neoadjuvant chemotherapy would seem to be important. However, not all the thrombus remains viable after preoperative chemotherapy. Boam et al., in a meta-analysis confirmed that neoadjuvant chemotherapy was effective in achieving thrombus non-viability in around 50 per cent of patients with tumour extension into the vena cava [20]. Shamberger et al. noted that up to 40 % had necrotic or fibrotic tissue at resection among the children who underwent initial chemotherapy. Also interestingly, there was no increased incidence of relapse in the patients in whom Tumour thrombus was not resected, which happened in 18 out of 164 operated children in their study and 9 of them had not received pre-operative chemotherapy [12]. It has been reported in various studies, that intravascular extension of Wilms Tumour is associated with an increased frequency of surgical complications [21].
One of the post-operative complications after inferior vena cavotomy to retrieve the tumour thrombus, is occlusion of the IVC [11]. It is notable however, that in a large study by Shamberger et al., although postoperative occlusion of the IVC was documented in 14 children, only 1 was symptomatic, with lower extremity edema, which resolved over time [12]. They concluded that adequate retroperitoneal collaterals develop to overcome the obstruction, and this prevents significant long term complications.
Hence, we would like to suggest making a case for avoiding a major procedure causing significant morbidity, to retrieve the tumour thrombus. Even Boam et al., in the systemic review concluded that although it is impossible with current imaging technology to determine whether a thrombus is biologically viable or not before surgery, if this could be determined more accurately with innovative tools then the extent of surgery might well be modified in future [20]. In our case, we faced significant bleeding, which, in the absence of the cardiac bypass, would have resulted in a catastrophe. The risks of the retrieval of tumour thrombus must be weighed in thoroughly, before undertaking one. At any rate, all such tumours should be treated with the cardiac surgical team on standby.
Although rare, an increased incidence of WT has been noted in children with a horseshoe kidney (HK) [22]. It has been documented to represent approximately 0.4% to 0.9% of all WT. And these present not only a therapeutic challenge but a diagnostic challenge too. In the report from NWTSG on WT in HK, 13 out of the total of 41 (32%) patients had not been diagnosed pre-operatively as WT in a Horseshoe Kidney [23]. There are anatomical variations in the relation of the great vessels and the ureter in a horseshoe kidney. These have to be considered in the pre-operative evaluation, if needed with a CT angiography.
The surgical treatment involves removal of the involved kidney along with the isthmus in the majority of cases. However, if the isthmus is the site of the tumour, then bilateral lower pole heminephrectomy with isthmusectomy is the surgery of choice as in our case.
When the WT is found to be invading the adjacent structures on primary imaging, en-bloc resection of adjacent solid organs (liver, spleen, or pancreas) except part of the diaphragm is not recommended. Pre-operative chemotherapy causes regression of the invasion and makes it safer for surgery. However, in our case the tumour was found invading into the segments of the liver intra-operatively despite good response of the tumour to pre-operative chemotherapy. Ideally, the surgeon should be prepared to deal with such situations, but every surgeon may not be comfortable doing a liver resection. If considered possible, then a hepatobiliary surgeon should be available.
The child with suspected rupture of Wilms Tumour on presentation is a rare situation but most of the times can be managed conservatively at presentation with adequate resuscitation. A focussed analysis of the CT imaging may distinguish between the intra-peritoneal rupture from retro-peritoneal ones, which may significantly guide the treatment. The emergency surgery rate for tumour rupture is very low and has been estimated at between 0.8% and 1.8% [24,25].
Our case was treated as a rupture and bleed from a Wilms tumour although later on it was shown not to be a Wilms tumour. Very few patients will require immediate embolization by the interventional radiology team and/or surgery to control abdominal bleeding. Hemodynamically stable patients may be treated by neoadjuvant chemotherapy and secondary surgery. These patients should be included as stage III regardless of other pathologic criteria and should be treated by chemotherapy and RT despite toxicity.
Conclusion
We have highlighted the difficulties a surgeon encounters when planning an appropriate surgical approach for the unique subset of complicated WT. With meticulous evaluation and preparedness these difficult situations can be managed to give a good outcome.
Declaration of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Authors Contribution
RS: Collected the data, Contributed to the data, Wrote the Paper; HZ: Collected the data, Wrote the paper ( first 2 cases); PRP: Collected the data( concised the details of the case presentation); TTN: Contributed data, Other contribution( Operating surgeon in 2 cases); MC: Conceived and designed , Other contribution (operating surgeon, reviewed and edited)
References
- Dénes FT, Duarte RJ, Cristófani LM, Lopes RI (2013) Pediatric genitourinary oncology. Front Pediatr 1: 48. [Crossref]
- Davidoff AM (2012) Wilms tumor. Adv Pediatr 59: 247-267. [Crossref]
- Erginel B (2016) Wilms Tumor and Its Management in a Surgical Aspect. In: van den Heuvel-Eibrink MM, editor. Wilms Tumor [Internet]. Brisbane (AU): Codon Publications. Ch 4. [Crossref]
- Bhatnagar S (2009) Management of Wilms' tumor: NWTS vs SIOP. J Indian Assoc Pediatr Surg 14: 6-14. [Crossref]
- Charlton J, Irtan S, Bergeron C, Pritchard-Jones K (2017) Bilateral Wilms tumour: a review of clinical and molecular features. Expert Rev Mol Med 19: e8. [Crossref]
- Ehrlich P, Chi YY, Chintagumpala MM, Hoffer FA, Perlman EJ, et al. (2017) Results of the First Prospective Multi-institutional Treatment Study in Children With Bilateral Wilms Tumor (AREN0534): A Report From the Children's Oncology Group. Ann Surg 266: 470-478. [Crossref]
- Kieran K, Davidoff AM (2015) Nephron-sparing surgery for bilateral Wilms tumor. Pediatr Surg Int 31: 229-236. [Crossref]
- Kieran K, Williams MA, Dome JS, McGregor LM, Krasin MJ, et al. (2013) Margin status and tumor recurrence after nephron-sparing surgery for bilateral Wilms tumor. J Pediatr Surg 48: 1481-1485. [Crossref]
- Murphy AJ, Davidoff AM (2018) Bilateral Wilms Tumor: A Surgical Perspective. Children (Basel) 5: 134. [Crossref]
- McMahon S, Carachi R (2014) Wilms' tumor with intravascular extension: A review article. J Indian Assoc Pediatr Surg 19: 195-200. [Crossref]
- Ritchey ML, Kelalis PP, Breslow N, Offord KP, Shochat SJ, et al. (1988) Intracaval and atrial involvement with nephroblastoma: review of National Wilms Tumor Study-3. J Urol 140: 1113-1118. [Crossref]
- Shamberger RC, Ritchey ML, Haase GM, Bergemann TL, Loechelt-Yoshioka T, et al. (2001) Intravascular extension of Wilms tumor. Ann Surg 234: 116-121. [Crossref]
- Lall A, Pritchard-Jones K, Walker J, Hutton C, Stevens S, et al. (2006) Wilms' tumor with intracaval thrombus in the UK Children's Cancer Study Group UKW3 trial. J Pediatr Surg 41: 382-387. [Crossref]
- Xu S, Sun N, Zhang WP, Song HC, Huang CR (2019) Management of Wilms tumor with intravenous thrombus in children: a single center experience. World J Pediatr 15: 476-482. [Crossref]
- Szavay P, Luithle T, Semler O, Graf N, Fuchs J (2004) Surgery of cavoatrial tumor thrombus in nephroblastoma: a report of the SIOP/GPOH study. Pediatr Blood Cancer 43: 40-45. [Crossref]
- Daum R, Roth H, Zachariou Z (1994) Tumor infiltration of the vena cava in nephroblastoma. Eur J Pediatr Surg 4: 16-20. [Crossrefv]
- Abdullah Y, Karpelowsky J, Davidson A, Thomas J, Brooks A, et al. (2013) Management of nine cases of Wilms' tumour with intracardiac extension - a single centre experience. J Pediatr Surg 48: 394-399. [Crossref]
- Habib F, McLorie GA, McKenna PH, Khoury AE, Churchill BM (1993) Effectiveness of preoperative chemotherapy in the treatment of Wilms tumor with vena caval and intracardiac extension. J Urol 150: 933-935. [Crossref]
- Berberoğlu S, Akyüz C, Büyükpamukçu M (1996) Successful treatment of intracaval and atrial extension of Wilms' tumour by chemotherapy. Postgrad Med J 72: 749-750. [Crossref]
- Boam TD, Gabriel M, Shukla R, Losty PD (2021) Impact of neoadjuvant chemotherapy on thrombus viability in patients with Wilms tumour and caval extension: systematic review with meta-analysis. BJS Open 5: zrab020. [Crossref]
- Ritchey ML, Kelalis PP, Breslow N, Etzioni R, Evans I, et al. (1992) Surgical complications after nephrectomy for Wilms' tumor. Surg Gynecol Obstet 175: 507-514. [Crossref]
- Neville H, Ritchey ML, Shamberger RC, Haase G, Perlman S, et al. (2002) The occurrence of Wilms tumor in horseshoe kidneys: a report from the National Wilms Tumor Study Group (NWTSG). J Pediatr Surg 37: 1134-1137. [Crossref]
- Neville H, Ritchey ML, Shamberger RC, Haase G, Perlman S, et al. (2002) The occurrence of Wilms tumor in horseshoe kidneys: a report from the National Wilms Tumor Study Group (NWTSG). J Pediatr Surg 37: 1134-1137. [Crossref]
- Brisse HJ, Schleiermacher G, Sarnacki S, Helfre S, Philippe-Chomette P, et al. (2008) Preoperative Wilms tumor rupture: a retrospective study of 57 patients. Cancer 113: 202-213. [Crossref]
- Godziński J, Weirich A, Tournade MF, Gauthier F, Buerger D, et al. (2001) Primary nephrectomy for emergency: a rare event in the International Society of Paediatric Oncology Nephroblastoma Trial and Study no. 9. Eur J Pediatr Surg 11: 36-39. [Crossref]