Introduction: Cluster randomized crossover (CRXO) trials involve randomly allocating clusters to a sequence of interventions. In CRXO trials, researchers must determine if clusters will crossover between the two treatment groups once or multiple times. There is concern that multiple crossovers may increase the risk of treatment contamination. We sought to determine the incidence of treatment contamination within CRXO trials that use alternating two-month recruitment periods.
Methods: The PREP-IT Trials is a master protocol for CRXO trials that evaluate different surgical antiseptic skin solutions for fracture fixation. Three separate cohorts are enrolling patients across 43 clusters, with each cluster randomized to a starting solution and alternating its treatment intervention every two months. We used descriptive statistics and regression analysis to report the incidence of treatment contamination.
Results: The majority of clusters had no contamination during the run-in. The incidence of contamination during enrollment was 4.0% (242/6096), with statistically significant difference between two of the trials (p<0.001). Variation was observed across clusters (range, 0% to 19.0%), with 13.9% (6/43) of clusters having contamination rates greater than 10%. There was no clustering of contamination immediately following the treatment crossover (p=0.701).
Discussion: The PREP-IT Trials demonstrates low treatment contamination using the two-month multiple CRXO design. The run-in phase allowed for the confirmation of acceptable treatment compliance and was critical to cluster success. There are minimal changes in the incidence of contamination over the treatment periods and over time. There is variation in contamination by cluster, suggesting that more oversight and training may be needed.
clinical trial design, cluster randomized crossover, contamination, surgical site infection
Cluster randomized crossover trials (CRXO) involve randomly allocating naturally occurring groups or clusters to enroll participants under one treatment group for a specified period of time and then crossing over to the other treatment group. When designing these trials, researchers must determine if clusters will crossover between treatment groups just once or multiple times throughout the course of the trial. A previously conducted simulation study suggested that increasing the number of crossovers in a CRXO trial from one to three (resulting in two or four treatment periods respectively) yields substantial increases in statistical efficiency, but that increasing the number of crossovers beyond this yields diminishing returns [1]. However, when determining the optimal number of crossovers, researchers may also need to consider other practical issues.
CRXO designs can be used to assess different infection prevention interventions. In infection prevention studies, it is necessary to account for seasonal variability in surgical site infections (SSI) and their associated infectious organisms [2]. For example, clusters can enroll for a 12-month period under the first treatment intervention and then crossover to the second treatment intervention and enroll for an additional 12 months. While this approach also matches for seasonal variability and is a very simple design, there are important limitations. The primary limitation is the risk of confounding if new SSI preventative measures are introduced during the trial. Secondly, there is also a possible risk of treatment imbalance if clusters are unable to enroll for 24 months or if enrollment needs to be extended beyond 24 months. An alternative approach is to have clusters crossover more frequently, such as every two months. This approach accounts for seasonal variability in SSI incidence and their associated infectious organisms, as each crossover period covers a season.
Although increasing the number of crossovers in a cluster randomized trial may improve statistical efficiency, one potential disadvantage is increased use of the incorrect treatment intervention (contamination). A recently published infection prevention trial compared preoperative skin disinfection with 0.5 percent chlorhexidine–alcohol and one percent iodine–alcohol in patients undergoing breast, colorectal, vascular, orthopaedic, and gallbladder surgery using a CRXO design in which treatment periods crossed over every three months for two years (seven crossover events and eight treatment periods) [3]. The authors did not report the rate of treatment contamination, which is likely due to the pragmatic nature of their trial and limited data collection (e.g. not collecting contamination at the surgery level). The PREP-IT Investigators are conducting two similar infection prevention CRXO trials, in three cohorts of fracture patients, in which the incidence of contamination are collected. The purpose of this analysis is determine the incidence of treatment contamination within the PREP-IT CRXO trials that use alternating two-month recruitment periods.
PREP-IT Overview
The PREP-IT (Program of Randomized trials to Evaluate Pre-operative antiseptic skin solutions In orthopaedic Trauma) consists of two ongoing pragmatic CRXO trials [4]. Aqueous-PREP: A Pragmatic Randomized trial Evaluating Pre-operative aqueous antiseptic skin solutions in open fractures and 2) PREPARE: A Pragmatic Randomized trial Evaluating Pre-operative Alcohol skin solutions in FRactured Extremities) (Figure 1). The Aqueous-PREP trial will enroll at least 1,540 patients with open fractures to determine the effectiveness of aqueous pre-operative antiseptic skin preparation with 10% povidone-iodine versus 4% chlorhexidine gluconate. The PREPARE trial will enroll at least 1,540 patients with open fractures (PREPARE-Open) and 6,280 patients with closed lower extremity and pelvic fractures (PREPARE-Closed) and compares alcohol-based pre-operative antiseptic skin preparation with iodine povacrylex (0.7% free iodine) (DuraPrepTM) versus 2% chlorhexidine gluconate (ChloraPrepTM). PREPARE-Open and PREPARE-Closed will be analyzed separately as they are two distinct patient populations. The primary outcome of both trials is post-fracture SSI within 90 days of the fracture as defined by the Centers for Disease Control and Prevention (CDC) [5] and unplanned fracture-related reoperations within 12 months to manage infection, wound healing problems, and fracture healing problems. Both trials are registered on clinicaltrials.gov (NCT03385304 and NCT03523962) and the master protocol has been published [4].
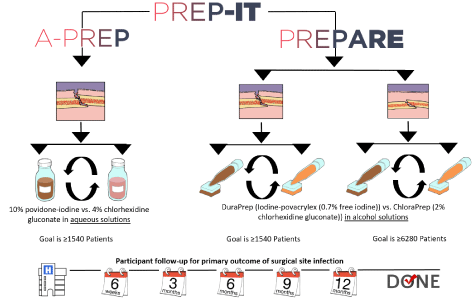
Figure 1. PREP-IT Trial. Reproduced from “Cluster identification, selection, and description in cluster randomized crossover trials: the PREP-IT trials” by Sprague, et al. [6]
Cluster Definition
Clusters are defined as orthopaedic practices within a hospital and are described previously [6]. Twenty-six different clusters are participating in PREP-IT. Twelve clusters have enrolled patients into Aqueous-PREP trial, 14 clusters have enrolled patients into the PREPARE-closed cohort, and 17 clusters have enrolled patients into the PREPARE-Open cohort. Of note, clusters often participate in more than one cohort (e.g. PREPARE-Open and PREPARE-Closed). As each cohort will be analyzed and results presented separately in most of our analyses, there are 43 clusters across all three cohorts.
Number and Duration of Treatment Crossovers
In PREP-IT, the unit of randomization is the orthopaedic practices within clinical sites (clusters). Recruitment for each treatment group will be performed in multiple iterations of approximately two-month periods. Each orthopaedic practice was initially randomized to use one of two pre-operative surgical skin preparation solutions for fracture surgeries (Figure 2). Upon completion of the two-month period, each orthopaedic practice crosses over to the alternative treatment allocation and completes another two-month recruitment period. Each cluster is anticipated to enroll for 24-months; however, some clusters may have a shorter total recruitment duration (e.g., a participating site who joins the trial later, high volume clinical sites, etc.). The two-month treatment periods will help account for seasonal variability in SSI incidence and their associated infectious organisms [2], as each crossover period will cover a season. In addition, for those clusters enrolling beyond 12 months, the distribution of recruitment periods for each solution may be seasonally matched by reversing the order of the alternating allocation after 12 months of recruitment. Prior to commencing enrollment, each cluster completed a 15-patient or one-month run-in phase, with the possibility to extend it to three months.
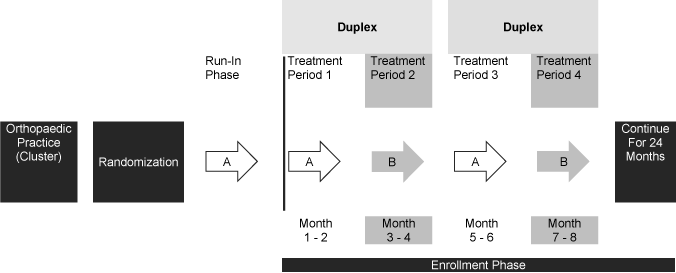
Figure 2. Cluster Crossover Schedule
**Figure 2 shows an example of a cluster that is randomized to begin with solution A.
Contamination Definitions
Depending on the severity of the injury, fracture patients may require multiple surgeries over multiple days and sometimes weeks to manage their injury and, therefore, their limb is prepped for surgery on multiple occasions. The PREP-IT protocols require that the limb be prepped with the same antiseptic solution at each planned surgery related to their fracture, as per the treatment period that the participant was in during their first fracture related surgery. If the incorrect solution is used, this is considered a contamination. For example, if a participant in the PREPARE trial had their first surgery during a ChloraPrepTM treatment period but was prepped with DuraPrepTM during their initial surgery, or any subsequent planned surgery, this would be considered a contamination. Solution compliance for the run-in phase and for the trial is documented in the electronic data capture (EDC) system. Contamination may be reported by participant or by surgery, depending on the research question.
Research Objectives and Hypotheses
The specific research objectives of this analysis are to determine: 1) the incidence of contamination by trial and by cluster during the run-in phase and the enrollment phase; 2) if the incidence of contamination differs between the three fracture patient cohorts; 3) if the incidence of contamination differs between the clusters; 4) if the incidence of contamination increases at beginning of a treatment period (e.g. when the clusters change from one treatment to the next); and 5) determine if the incidence of contamination changes as the trials progress.
We hypothesize that the incidence of contamination will be different across the three fracture patient cohorts due to differences in the fracture populations (open fractures vs. closed fractures) and difference in the application and texture of the preparation solutions. Therefore, we believe that the Aqueous-PREP trial is likely to have a higher incidence of contamination as the population includes only open fractures and the surgical preparation solutions are different in texture, which may influence surgeon preference, and are applied without an applicator.
We hypothesize that the incidence of contamination differs between the clusters due to differences in cluster characteristics including the size of the trauma centre, patient volume, number of surgeons, number of operating rooms, and existing research infrastructure. Larger clusters with a higher patient volume, more surgeons and operating rooms, and limited research resources are likely to have a higher incidence of contamination.
We hypothesize that the incidence of contamination will increase at beginning of a treatment period (e.g. when the clusters change from one treatment to the next). Our rationale is that errors may be made at this time as clinical personnel may not be aware of the treatment crossover or they may have forgotten about the treatment crossover.
We hypothesize that the incidence of contamination may decrease as the trial progresses due to clinical personnel and research personnel becoming more aware of the trial procedures.
Incidence of contamination by trial and by cluster during the run-in phase and the enrollment phase
Descriptive statistics (frequencies and percentages) were used to report the percentage of participants with at least one treatment contamination during the run-in phase, each treatment period, and the entire recruitment duration stratified by cluster and by trial.
Incidence of contamination between the three fracture patient cohorts
Logistic regression was used to determine if the incidence of contamination differs between Aqueous-PREP, PREPARE-Open, and PREPARE-Closed. The unit of analysis was the patient and the dependent variable was patient contamination of final prep solution in any planned surgeries. The independent variable was trial cohort (Aqueous-Prep, PREPARE-Open, PREPARE-Closed) and cluster was entered as a random effect. Odds ratios (OR), corresponding 95% confidence intervals, and associated p values were reported.
Incidence of contamination between the clusters
Logistic regression was used to determine if the incidence of contamination differs between clusters. The unit of analysis was the patient and the dependent variable was patient contamination of final prep solution in any planned surgeries. The independent variables were cluster and trial cohort.
Incidence of contamination at the beginning of a treatment period
Logistic regression was used to determine if the incidence of contamination increases at the initiation of a treatment period. The unit of analysis was planned surgery. The dependent variable was treatment contamination (yes vs. no) with the final prep solution during the planned surgery. The main independent variable was the time in days from the start of the treatment period to the planned fracture surgery. The number of planned surgeries per participant, number of PREP-IT trials that the cluster was enrolling for at the time, and treatment solution were also included as independent variables in the logistic regression model. Trial cohort (Aqueous-Prep, PREPARE-Open, vs. PREPARE-Closed), cluster and participant were included as random effects. Odds ratios, corresponding 95% confidence intervals, and associated p values were reported.
We observed the relationship of treatment contamination and time in a scatter plot, and were prepared to transfer the independent variable if necessary during the data analysis.
Incidence of contamination as the trials progress
A logistic regression model was used to determine if the incidence of contamination decreases as the trial progresses. The unit of analysis was the participant. The dependent variable was participant contamination. Participant contamination was defined as participants who had one or more contamination of final preparation solution during a planned fracture surgery. The independent variables were the duplex number (duplex is defined as two months using one solution followed by two months using the other solution). Treatment solution was also included as an independent variable. Trial cohort and cluster were included as random effects. Odds ratios, corresponding 95% confidence intervals, and associated p values were reported.
All tests were two-tailed with an alpha of 0.05. All analyses were conducted using R version 3.6.2.
Incidence of contamination by trial and by cluster during the run-in phase and the enrollment phase
During the run-in phase, the overall incidence of contamination by fracture surgery was 4.1% (45/1,096) and is shown for each cluster in Aqueous-PREP, PREPARE-Open and PREPARE-Closed. Aqueous-PREP had the highest incidence of contamination (13.4%) during the run-in phase, followed by PREPARE-Open (3.6%), and PREPARE-Closed (1.3%) (Table 1). Most clusters (66.8%; 30/43) had no contamination during the run-in phase. Contamination ranged from 0% to 37.5% in Aqueous-PREP, 0% to 18.7% in PREPARE-Open and 0% to 9.1% in PREPARE-Closed. One cluster in Aqueous-PREP had a 37.5% (21/56) incidence of contamination and was unable to enroll in the trial. One cluster completed a successful run-in phase for PREPARE-Open and PREPARE-Closed but decided to only participate in PREPARE-Closed to manage workflow. During the enrollment phase, the overall contamination rate by fracture surgery was 4.0% (242/6,096) and the overall contamination rate by participant was 4.4% (204/4,668) (Table 1).
Table 1. Treatment Contamination by Trial and by Clinical Site (Cluster)
| Trial and Cluster
By Site Number*
|
Contamination During the Run-In Phase
N (%)
|
Contamination by Participant During the Enrollment Phase
N (%)
|
Contamination by Surgery During the Enrollment Phase
N (%)
|
Aqueous-PREP |
Site 1 |
0 (0) |
6 (7.2) |
8 (5.4) |
Site 2 |
0 (0) |
3 (1.8) |
6 (2.9) |
Site 3 |
0 (0) |
2 (3.8) |
10 (11.9) |
Site 4 |
2 (10.5) |
14 (9.3) |
10 (3.8) |
Site 5 |
0 (0) |
27 (14.0) |
36 (8.9) |
Site 6 |
21 (37.5) |
- |
- |
Site 7 |
3 (10.3) |
15 (14.9) |
13 (8.7) |
Site 8 |
2 (10.5) |
9 (14.8) |
2 (2.4) |
Site 9 |
0 (0) |
13 (8.7) |
18 (6.4) |
Site 10 |
0 (0) |
12 (13.2) |
30 (20.1) |
Site 11 |
0 (0) |
1 (4.8) |
0 (0.0) |
Site 12 |
0 (0) |
8 (22.9) |
7 (14.9) |
Site 13 |
1 (9.1) |
3 (7.9) |
2 (3.1) |
Total |
28 (13.4) |
113 (9.9) |
142 (7.4) |
PREPARE-Open |
Site 1 |
1 (2.5) |
7 (2.9) |
8 (2.4) |
Site 31 |
0 (0) |
11 (21.2) |
12 (17.9) |
Site 32 |
0 (0) |
2 (3.7) |
4 (4.0) |
Site 33 |
0 (0) |
- |
- |
Site 34 |
0 (0) |
1 (4.3) |
1 (2.4) |
Site 35 |
3 (18.7) |
0 (0.0) |
0 (0.0) |
Site 36 |
0 (0) |
1 (4.3) |
1 (2.9) |
Site 37 |
0 (0) |
2 (15.4) |
2 (11.1) |
Site 38 |
1 (11.1) |
1 (5.6) |
2 (8.3) |
Site 39 |
0 (0) |
0 (0.0) |
0 (0.0) |
Site 40 |
2 (10) |
5 (4.9) |
6 (4.6) |
Site 41 |
0 (0) |
0 (0.0) |
0 (0.0) |
Site 42 |
0 (0) |
1 (4.5) |
1 (3.6) |
Site 43 |
0 (0) |
4 (23.5) |
4 (14.8) |
Site 44 |
0 (0) |
0 (0.0) |
0 (0.0) |
Total |
7 (3.6) |
35 (5.0) |
41 (4.1) |
PREPARE-Closed |
Site 1 |
1 (2.3) |
4 (1.1) |
4 (1.0) |
Site 2** |
- |
9 (6.0) |
9 (5.8) |
Site 4** |
- |
1 (1.6) |
1 (1.4) |
Site 31 |
2 (3.5) |
15 (5.0) |
18 (5.0) |
Site 32 |
0 (0) |
3 (1.2) |
3 (1.1) |
Site 33 |
2 (9.1) |
1 (0.7) |
1 (0.6) |
Site 34 |
0 (0) |
2 (1.4) |
2 (1.2) |
Site 35 |
1 (1.7) |
1 (0.8) |
1 (0.7) |
Site 36 |
0 (0) |
1 (0.7) |
1 (0.6) |
Site 37 |
0 (0) |
3 (3.1) |
3 (3.0) |
Site 38 |
0 (0) |
3 (2.6) |
3 (2.6) |
Site 39 |
3 (6.2) |
1 (1.4) |
1 (1.2) |
Site 40 |
0 (0) |
4 (1.9) |
4 (1.7) |
Site 41 |
0 (0) |
1 (0.3) |
1 (0.3) |
Site 42 |
0 (0) |
5 (2.7) |
5 (2.5) |
Site 43 |
0 (0) |
2 (3.1) |
2 (2.5) |
Site 44 |
0 (0) |
0 (0.0) |
0 (0.0) |
Total |
9 (1.3) |
56 (2.0) |
59 (1.9) |
Total for All Cohorts |
44 (4.1) |
204 (4.4) |
- (4.0)
|
*Site number is unique for each cluster and assigned at the beginning of the trial by the Methods Centre
**Site 2 and 4 did not complete a run-in period for PREPARE-Closed as they are participating in the Aqueous-PREP trial.
Incidence of contamination between the three fracture patient cohorts
The Aqueous-PREP trial had the highest contamination rate (7.4% by fracture surgery and 9.9% by participant), followed by PREPARE-Open (4.1% by fracture surgery and 5.0% by participant), and PREPARE-Closed (1.9% by fracture surgery and 2.0% by participant). No statistically significant difference in incidence of contamination was found in PREPARE-Open compared with Aqueous-PREP [odds ratio (OR) 0.65 (95% CI 0.36 to 1.22, p=0.148]; however, there was a significant difference between PREPARE-Closed compared with Aqueous-PREP [OR 0.22 (95% CI 0.13 to 0.39, p<0.001] in our multivariable logistic regression model which adjusted for cluster.
Incidence of contamination between the clusters
Contamination rates between clusters was significantly different (range, 0% to 19%) (p<0.001) (Table 1).
Incidence of contamination at the beginning of a treatment period
The incidence of contamination did not increase at the beginning of a new treatment period [OR 1.003 for every day further out from the crossover (95% CI 0.987 to 1.02 p=0.701)] (Figure 3). In other words, no clustering of contamination was observed in the days immediately following a treatment crossover.
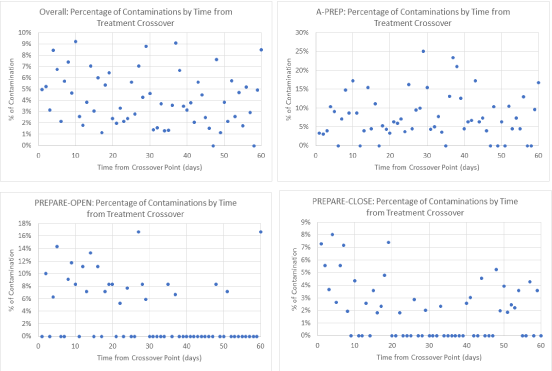
Figure 3. Contamination by Time from Treatment Crossovers
Incidence of contamination as the trials progress
The incidence of contamination by treatment period are reported in Table 2. The logistic regression did not show a change in contamination as the trials progressed (Table 3).
Table 2. Contamination by Treatment Period
Trial |
First Treatment Period* |
Second Treatment Period* |
Third Treatment Period* |
Fourth Treatment Period* |
Treatment Periods 5-10* |
Total |
Aqueous-PREP |
18/357 (5.0%) |
21/348 (6.0%) |
22/265 (8.3%) |
21/190 (11.1%) |
60/756 (7.9%) |
142/1916 (7.4%) |
PREPARE – Open |
13/218 (6.0%) |
6/185 (3.2%) |
4/159 (2.5%) |
13/174 (7.5%) |
5/265 (1.9%) |
41/1001 (4.1%) |
PREPARE – Closed |
14/735 (1.9%) |
13/700 (1.9%) |
14/593 (2.4%) |
8/545 (1.5%) |
10/606 (1.7%) |
59/3179 (1.9%) |
Overall |
45/1310 (3.4%) |
40/1233 (3.2%) |
40/1017 (3.9%) |
42/909 (4.6%) |
75/1627 (4.6%) |
242/6096 (4.0%) |
*A treatment period is defined as approximately 2 months using one solution
** Unit of analysis was planned fracture surgery
Table 3. Incidence of Contamination in Each Treatment Duplex
Duplex |
Contamination, n (%) |
Logistic Regression |
OR (95% CI) |
P-Value |
Duplex 1 |
85 (3.34%) |
Ref |
Ref |
Duplex 2 |
82 (4.26%) |
1.09 (0.78-1.53) |
0.60 |
Duplex 3 |
43 (3.92%) |
0.74 (0.49-1.13) |
0.17 |
Duplex 4 |
31 (6.74%) |
0.66 (0.36-1.18) |
0.16 |
Duplex 5 |
1 (1.85%) |
0.656 (0.14-3.02) |
0.59 |
*Treatment Duplex is defined as two months using one solution followed by two months using the other solution.
Comparative effectiveness research compares interventions with proven effectiveness in real-world settings and are often implemented as pragmatic trials. One potential criticism of pragmatic trials is that intervention fidelity, that is the adherence to the trial intervention as outlined in the protocol, may not be well captured. While the current study provides some insight on the rate of contamination for the PREP-IT trials, it is also likely a good estimate of contamination in similar surgical preparation CRXO trials with unreported contamination such as the one published by Charehbili, et al. in 2019. Additionally, this study provides insight into where and when the contaminations are occurring.
One of the concerns at the onset of this trial was that introducing multiple crossovers may increase treatment contamination beyond the thresholds outlined in the trial protocol. This analysis of the PREP-IT trials demonstrates that it is possible to maintain an acceptable level (e.g. less than 10%)4 of contamination using the two-month multiple crossover trial design. The run-in phase allowed for the confirmation of acceptable treatment compliance prior to cluster initiation and was critical to setting clusters up for success by allowing them to work out issues causing treatment contamination before initiating enrollment. Most clusters had little contamination during the run-in phase, and many have successfully used the run-in phase to resolve local logistical challenges.
During enrollment, the incidence of contamination in A-PREP and PREPARE were below the thresholds outlined a priori in the trial protocol. Variations in contamination between trials were observed and may be explained by multiple factors differentiating the trials. Aqueous-PREP and PREPARE-Open include open fractures which are more frequently treated urgently and outside of standard operating hours in comparison to PREPARE-Closed which involves closed fractures that can often be scheduled for treatment during standard operating hours. Increased surgeries outside of regular operating times likely contributed to the increased contamination in open fracture patients since less oversight by the research team was available to minimize contamination. Open fractures are also more likely to receive multiple surgeries which increases the probability of a patient level contamination. Additionally, other specialists who may not be aware of the study, such as vascular and plastic surgeons, are more frequently involved in the management of open fractures, which likely contributes to the increased contamination seen in open fractures.
We also found that the incidence of contamination varies across the clusters. The characteristics of the clusters also vary, which may help to explain differences. For example, clusters have differing number of orthopaedic surgeons (two to 12), number of operating rooms for fracture surgeons (one to 32), and fracture volume. Additionally, research infrastructure and organization also vary across the clusters, which may also contribute to differences in contamination rates across clusters. This variation in contamination by cluster also suggests that more focused oversight and training may be needed to prevent inadvertent contamination at clusters with higher contamination rates.
We anticipated that increased contamination would be observed immediately following the treatment crossover date. There was no clustering of contamination immediately following the treatment crossover date in Aqueous-PREP and minimal clustering of contamination immediately following the treatment crossover date in PREPARE-Open and PREPARE-Closed. Contamination occurred across the entire treatment period for all three cohorts, which suggests that they are occurring due to reasons other than the treatment crossover.
Surprisingly, overall, there were minimal changes in the incidence of contamination as the trials progressed, with no trends towards increases or decreases in contamination. It may be a result of establishing a low initial rate of contamination using a run-in phase which allowed clinical sites to establish procedures for preventing contamination. This may also be indicative of a high level of vigilance of study personnel as the trial continues.
The study team collaboratively established and implemented several strategies to increase adherence to the study solutions and minimize the number of contaminations. This included site initiation visits and training, use of a crossover checklist which summarizes the steps to take prior to changing treatments (e.g. change posters, email clinical team, re-arrange solutions in the operating room), and flexibility in the date of the treatment crossover. While clusters were encouraged to adhere to their crossover schedule as closely as possible, they were permitted to adjust this date if it fell on a date that increased the risk of contamination (e.g. weekend, holiday, etc.). The Methods Centre evaluated performance monthly and concerns were escalated to the local site investigator. These approaches have likely contributed to the low number of contaminations over time.
There are some limitations with the current analysis, including that new clusters are being initiated as the trials are ongoing. This resulted in a small number of treatment periods at clusters that initiated enrollment later. This analysis is strengthened by including multiple clusters in different settings, including two trials with three different fracture cohorts, including both open fractures and closed fracture patient populations, and including four different surgical preparation solutions.
This study describes the success of a novel approach to account for seasonal variation in CRXO trials. The PREP-IT trials demonstrate that it is possible to maintain an acceptable incidence of treatment contamination using the two-month multiple crossover trial design. The run-in phase provided a useful opportunity for clusters to work through challenges in administering the correct solution. Surprisingly, there are minimal changes in the incidence of contamination over the treatment periods and over time from treatment crossover. There is variation in contamination by cluster, suggesting that more focused oversight and training may be needed to prevent inadvertent contamination at clusters with higher contamination rates.
Ethics approval and consent to participate
Ethics approval for PREPARE and A-PREP was obtained through the Hamilton Integrated Research Ethics Board (Project #4913 and #4336) and Advarra (Pro00028360 and Pro00023709). Informed consent was obtained for all study participants enrolled in the PREP-IT trials. All methods were carried out in accordance with relevant guidelines and regulations.
Consent for publication
Not applicable.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
SS, MB, and GS conceived the study. SS, CSL, DHA, DP, and LT analyzed and interpreted the data. SS, CSL, DHA, DP, SD, JL drafted the manuscript. All authors (SS, CSL, DHA, DP, SD, JL, MB, MJP, SM, MJG, MJW, MH, TJ, LT and GS) critically revised the manuscript and gave final approval of the version to be published.
The PREPARE trial is funded by the Patient-Centered Outcomes Research Institute (PCORI) (PCS-1609-36512) and the Canadian Institutes of Health Research (CIHR) (Foundation Grant); the Aqueous-PREP trial is funded by the US Department of Defense (W81XWH-17-1-070) and the CIHR (Foundation Grant). McMaster University Surgical Associates funded start-up activities at the Methods Centre and The Physician Services Incorporated provided funding to the Methods Centre and Hamilton Health Sciences for the Aqueous-PREP trial. The views in this publication are solely the responsibility of the authors and do not necessarily represent the views of the PCORI, its board of governors or methodology committee.
We acknowledge and appreciate the many patients, nurses, national organizations, and others who have contributed to the success of this trial. The names, affiliations, and roles of the PREP-IT team are listed in detail below; The Executive Committee is responsible for the overall conduct of the trial, and is comprised of the Principal Investigators and a patient partner. The Executive Committee are advised by a Steering Committee, multiple clinical, research, and stakeholder specialty cores, and experts in patient engagement (University of Maryland PATIENTS Program). An Adjudication Committee reviews participant eligibility and reported study events. The Methods Centre is responsible for the day-to-day management of the PREP-IT trials, which includes clinical site management, data management, and data analysis. The Administrative Centre is responsible for piloting each trial protocol, contracting with each clinical site, and overseeing the Central Institutional Review Board activities.
Executive Committee
Gerard P. Slobogean (Principal Investigator, University of Maryland School of Medicine, Baltimore, MD); Sheila Sprague (Principal Investigator, McMaster University, Hamilton, ON); Jeffrey Wells (Patient Representative, Trauma Survivors Network, Falls Church, VA); Mohit Bhandari (Principal Investigator, McMaster University, Hamilton, ON).
Steering Committee
Gerard P. Slobogean (Co-Chair, University of Maryland School of Medicine, Baltimore, MD); Mohit Bhandari (Co-Chair, McMaster University, Hamilton, ON); Sheila Sprague (Principal Investigator, McMaster University, Hamilton, ON); Jean-Claude D’Alleyrand (Walter Reed National Military Medical Center, Bethesda, MD); Anthony D. Harris (University of Maryland School of Medicine, Baltimore, MD); Daniel C. Mullins (University of Maryland, Baltimore, MD); Lehana Thabane (McMaster University, Hamilton, ON); Jeffrey Wells (Trauma Survivors Network, Falls Church, VA); Amber Wood (Association of periOperative Registered Nurses, Denver, CO).
Adjudication Committee
Gregory J. Della Rocca (Chair, University of Missouri, Columbia, MO); Anthony D. Harris, (University of Maryland School of Medicine, Baltimore, MD); Joan Hebden (University of Maryland, Baltimore, MD); Kyle J. Jeray (Greenville Health System, Greenville, SC); Lucas Marchand (University of Maryland, Baltimore, MD); Lyndsay M. O’Hara (University of Maryland School of Medicine, Baltimore, MD); Robert Zura (LSU Health, New Orleans, LA).
Data and Safety Monitoring Committee
Michael J. Gardner (Chair, Stanford University School of Medicine, Palo Alto, CA); Jenna Blasman (Patient Representative, Kitchener, ON); Jonah Davies (University of Washington, Seattle, WA); Stephen Liang (Washington University, St. Louis, MO); Monica Taljaard (Ottawa Hospital Research Institute, Ottawa, ON).
Research Methodology Core
PJ Devereaux (McMaster University, Hamilton, ON); Gordon H. Guyatt (McMaster University, Hamilton, ON); Lehana Thabane (McMaster University, Hamilton, ON); Diane Heels-Ansdell (McMaster University, Hamilton, ON).
Patient Centred Outcomes Core
Debra Marvel (Patient Representative, Baltimore, MD); Jana Palmer (Patient Representative, Baltimore, MD); Jeffrey Wells (Patient, Trauma Survivors Network, Falls Church, VA); Jeff Friedrich (Editor, Slate Magazine, Washington DC); Daniel C. Mullins (University of Maryland, Baltimore, MD); Nathan N. O’Hara (University of Maryland School of Medicine, Baltimore, MD); Ms. Frances Grissom (Trauma Survivor Network, Baltimore, MD).
Orthopaedic Surgery Core
Gregory J. Della Rocca (University of Missouri, Columbia, MO); I. Leah Gitajn (Dartmouth University, Hanover, NH); Kyle J. Jeray (Greenville Health System, Greenville, SC); Saam Morshed (San Francisco General Hospital, San Francisco, CA); Robert V. O’Toole (University of Maryland School of Medicine, Baltimore, MD); Bradley A. Petrisor (Hamilton Health Science, Hamilton, ON).
Operating Room Core
Megan Camara (R Adams Cowley Shock Trauma Center, Baltimore, MD); Franca Mossuto (Hamilton Health Science, Hamilton, ON).
Infectious Disease Core
Anthony D. Harris (University of Maryland School of Medicine, Baltimore, MD); Manjari G. Joshi (University of Maryland School of Medicine, Baltimore, MD).
Military Core
Jean-Claude D’Alleyrand (Walter Reed National Military Medical Center, Bethesda, MD); Justin Fowler (United States Army, USA); Jessica Rivera (San Antonio Military Medical Center, San Antonio, TX); Max Talbot (Canadian Armed Forces, Montreal, QC).
McMaster University Methods Center
(Hamilton, ON): Sheila Sprague (Principal Investigator); Mohit Bhandari (Principal Investigator); Shannon Dodds (Research Coordinator); Alisha Garibaldi (Research Coordinator); Silvia Li (Research Coordinator); Uyen Nguyen (Research Coordinator); David Pogorzelski (Research Coordinator); Alejandra Rojas (Research Coordinator); Taryn Scott (Research Coordinator); Gina Del Fabbro (Research Assistant); Olivia Paige Szasz (Research Assistant); Diane Heels-Ansdell (Statistician); Paula McKay (Manager).
University of Maryland School of Medicine Administrative Center
(Baltimore, MD): Gerard P. Slobogean (Principal Investigator); Nathan N. O’Hara (Manager); Andrea Howe (Project Manager); Joshua Rudnicki (Project Manager); Haley Demyanovich (Project Manager); Kelly Little (Financial Manager).
University of Maryland School of Pharmacy, The PATIENTS Program
(Baltimore, MD): C. Daniel Mullins (Executive Director); Michelle Medeiros (Director of Research); Eric Kettering (Senior Instructional Technology and Dissemination Specialist); Diamond Hale (Project Manager).
Executive Committee: Gerard P. Slobogean (Principal Investigator, University of Maryland School of Medicine, Baltimore, MD); Sheila Sprague (Principal Investigator, McMaster University, Hamilton, ON); Jeffrey Wells (Patient Representative, Trauma Survivors Network, Falls Church, VA); Mohit Bhandari (Principal Investigator, McMaster University, Hamilton, ON)
Steering Committee: Gerard P. Slobogean (Co-Chair, University of Maryland School of Medicine, Baltimore, MD); Mohit Bhandari (Co-Chair, McMaster University, Hamilton, ON); Sheila Sprague (Principal Investigator, McMaster University, Hamilton, ON); Jean-Claude D’Alleyrand (Walter Reed National Military Medical Center, Bethesda, MD); Anthony D. Harris (University of Maryland School of Medicine, Baltimore, MD); Daniel C. Mullins (University of Maryland, Baltimore, MD); Lehana Thabane (McMaster University, Hamilton, ON); Jeffrey Wells (Trauma Survivors Network, Falls Church, VA); Amber Wood (Association of periOperative Registered Nurses, Denver, CO)
Adjudication Committee: Gregory J. Della Rocca (Chair, University of Missouri, Columbia, MO); Anthony D. Harris, (University of Maryland School of Medicine, Baltimore, MD); Joan Hebden (University of Maryland, Baltimore, MD); Kyle J. Jeray (Greenville Health System, Greenville, SC); Lucas Marchand (University of Maryland, Baltimore, MD); Lyndsay M. O’Hara (University of Maryland School of Medicine, Baltimore, MD); Robert Zura (LSU Health, New Orleans, LA)
Data and Safety Monitoring Committee: Michael J. Gardner (Chair, Stanford University School of Medicine, Palo Alto, CA); Jenna Blasman (Patient Representative, Kitchener, ON); Jonah Davies (University of Washington, Seattle, WA); Stephen Liang (Washington University, St. Louis, MO); Monica Taljaard (Ottawa Hospital Research Institute, Ottawa, ON)
Research Methodology Core: PJ Devereaux (McMaster University, Hamilton, ON); Gordon H. Guyatt (McMaster University, Hamilton, ON); Lehana Thabane (McMaster University, Hamilton, ON); Diane Heels-Ansdell (McMaster University, Hamilton, ON)
Patient Centred Outcomes Core: Debra Marvel (Patient Representative, Baltimore, MD); Jana Palmer (Patient Representative, Baltimore, MD); Jeffrey Wells (Patient, Trauma Survivors Network, Falls Church, VA); Jeff Friedrich (Editor, Slate Magazine, Washington DC); Daniel C. Mullins (University of Maryland, Baltimore, MD); Nathan N. O’Hara (University of Maryland School of Medicine, Baltimore, MD); Ms. Frances Grissom (Trauma Survivor Network, Baltimore, MD)
Orthopaedic Surgery Core: Gregory J. Della Rocca (University of Missouri, Columbia, MO); I. Leah Gitajn (Dartmouth University, Hanover, NH); Kyle J. Jeray (Greenville Health System, Greenville, SC); Saam Morshed (San Francisco General Hospital, San Francisco, CA); Robert V. O’Toole (University of Maryland School of Medicine, Baltimore, MD); Bradley A. Petrisor (Hamilton Health Science, Hamilton, ON)
Operating Room Core: Megan Camara (R Adams Cowley Shock Trauma Center, Baltimore, MD); Franca Mossuto (Hamilton Health Science, Hamilton, ON)
Infectious Disease Core: Anthony D. Harris (University of Maryland School of Medicine, Baltimore, MD); Manjari G. Joshi (University of Maryland School of Medicine, Baltimore, MD)
Military Core: Jean-Claude D’Alleyrand (Walter Reed National Military Medical Center, Bethesda, MD); Justin Fowler (United States Army, USA); Jessica Rivera (San Antonio Military Medical Center, San Antonio, TX); Max Talbot (Canadian Armed Forces, Montreal, QC)
McMaster University Methods Center (Hamilton, ON): Sheila Sprague (Principal Investigator); Mohit Bhandari (Principal Investigator); Shannon Dodds (Research Coordinator); Alisha Garibaldi (Research Coordinator); Silvia Li (Research Coordinator); Uyen Nguyen (Research Coordinator); David Pogorzelski (Research Coordinator); Alejandra Rojas (Research Coordinator); Taryn Scott (Research Coordinator); Gina Del Fabbro (Research Assistant); Olivia Paige Szasz (Research Assistant); Diane Heels-Ansdell (Statistician); Paula McKay (Manager)
University of Maryland School of Medicine Administrative Center (Baltimore, MD): Gerard P. Slobogean (Principal Investigator); Nathan N. O’Hara (Manager); Andrea Howe (Project Manager); Joshua Rudnicki (Project Manager); Haley Demyanovich (Project Manager); Kelly Little (Financial Manager)
University of Maryland School of Pharmacy, The PATIENTS Program (Baltimore, MD): C. Daniel Mullins (Executive Director); Michelle Medeiros (Director of Research); Eric Kettering (Senior Instructional Technology and Dissemination Specialist); Diamond Hale (Project Manager)
Lead Clinical Site (Aqueous-PREP and PREPARE)
University of Maryland School of Medicine, R Adams Cowley Shock Trauma Center, Baltimore, MD: Robert V. O'Toole, Jean-Claude D'Alleyrand, Andrew Eglseder, Aaron Johnson, Christopher Langhammer, Christopher Lebrun, Theodore Manson, Jason Nascone, Ebrahim Paryavi, Raymond Pensy, Andrew Pollak, Marcus Sciadini, Gerard P. Slobogean, Yasmin Degani, Haley K. Demyanovich, Andrea Howe, Nathan N. O’Hara, Katherine Joseph, Joshua Rudnicki, Megan Camara
Aqueous-PREP and PREPARE
Hamilton Health Sciences – General Site, Hamilton, ON: Brad A. Petrisor, Herman Johal, Bill Ristevski, Dale Williams, Matthew Denkers, Krishan Rajaratnam, Jamal Al-Asiri, Jordan Leonard, Francesc A. Marcano-Fernández***, Jodi Gallant, Federico Persico, Marko Gjorgjievski, Annie George
IU Health Methodist Hospital, Indianapolis, IN: Roman M. Natoli, Greg E. Gaski, Todd O. McKinley, Walter W. Virkus, Anthony T. Sorkin, Jan P. Szatkowski, Joseph R. Baele, Brian H. Mullis, Lauren C. Hill, Andrea Hudgins, Methodist OR Core II Staff
San Antonio Military Medical Center, San Antonio, TX: Patrick Osborn, Justin Fowler, Sarah Pierrie, Eric Martinez, Joseph Kimmel
Prisma Health - Upstate, Greenville, SC: Kyle J. Jeray, John D. Adams, Michael L. Beckish, Christopher C. Bray, Timothy R. Brown, Andrew W. Cross, Timothy Dew, Gregory K. Faucher, Richard W. Gurich Jr, David E. Lazarus, S. John Millon, M. Jason Palmer, Scott E. Porter, Thomas M. Schaller, Michael S. Sridhar, John L. Sanders, L. Edwin Rudisill, Jr, Michael J. Garitty, Andrew S. Poole, Michael L. Sims, Clark M. Walker, Robert M. Carlisle II, Erin Adams Hofer, Brandon S. Huggins, Michael D. Hunter, William A. Marshall, Shea Bielby Ray, Cory D. Smith, Kyle M. Altman, Julia C. Bedard, Markus F. Loeffler, Erin R. Pichiotino, Austin A. Cole, Ethan J Maltz, Wesley Parker, T. Bennett Ramsey, Alex Burnikel, Michael Colello, Russell Stewart, Jeremy Wise, M. Christian Moody, Stephanie L. Tanner, Rebecca G. Snider, Christine E. Townsend, Kayla H. Pham, Abigail Martin, Emily Robertson
University of California, San Francisco, San Francisco, CA: Saam Morshed, Theodore Miclau, Utku Kandemir, Meir Marmor, Amir Matityahu, R. Trigg McClellan, Eric Meinberg, David Shearer, Paul Toogood, Anthony Ding, Erin Donohoe, Jothi Murali, Tigist Belaye, Eleni Berhaneselase, Alexandra Paul***, Kartik Garg
Aqueous-PREP
McGovern Medical School at UTHealth Houston, Houston, TX: Joshua L. Gary, Stephen J Warner, John W. Munz, Andrew M. Choo, Timothy S. Achor, Milton L. “Chip” Routt, Mayank Rao, Guillermo Pechero, Adam Miller***
University of Florida, Gainesville, FL: Jennifer E. Hagen, Matthew Patrick, Richard Vlasak, Thomas Krupko, Kalia Sadasivan***, Chris Koenig, Daniel Bailey***, Daniel Wentworth***, Chi Van, Justin Schwartz
The CORE Institute, Phoenix, AZ: Niloofar Dehghan, Clifford B Jones***, J Tracy Watson, Michael McKee, Ammar Karim***, Michael Talerico, Debra L Sietsema, Alyse Williams, Tayler Dykes
Vanderbilt Medical Center, Nashville, TN: William T Obremskey, Amir Alex Jahangir, Manish Sethi, Robert Boyce, Daniel J. Stinner, Phillip Mitchell, Karen Trochez, Andres Rodriguez***, Vamshi Gajari, Elsa Rodriguez, Charles Pritchett
Banner University Medical Center – Tucson, Tucson, AZ: Christina Boulton, Jason Lowe, Jason Wild***, John T. Ruth, Michel Taylor, Andrea Seach, Sabina Saeed, Hunter Culbert, Alejandro Cruz, Thomas Knapp***, Colin Hurkett***, Maya Lowney
Wright State University, Dayton, OH: Michael Prayson, Indresh Venkatarayappa, Brandon Horne, Jennifer Jerele, Linda Clark
Hospital Universitari Parc Tauli, Barcelona, Spain: Francesc Marcano-Fernández, Montsant Jornet-Gibert, Laia Martínez-Carreres, David Martí-Garín, Jorge Serrano-Sanz,
Joel Sánchez-Fernández, Matsuyama Sanz-Molero, Alejandro Carballo, Xavier Pelfort, Francesc Acerboni-Flores, Anna Alavedra-Massana, Neus Anglada-Torres, Alexandre Berenguer, Jaume Cámara-Cabrera, Ariadna Caparros-García, Ferran Fillat-Gomà, Ruben Fuentes-López, Ramona Garcia-Rodriguez, Nuria Gimeno-Calavia, Guillem Graells-Alonso, Marta Martínez-Álvarez, Patricia Martínez-Grau, Raúl Pellejero-García, Ona Ràfols-Perramon, Juan Manuel Peñalver, Mònica Salomó Domènech, Albert Soler-Cano, Aldo Velasco-Barrera, Christian Yela-Verdú, Mercedes Bueno-Ruiz, Estrella Sánchez-Palomino
Vall d’Hebron Hospital, Barcelona, Spain: Ernesto Guerra-Farfan, Jordi Tomas-Hernandez, Jordi Teixidor-Serra, Vicente Molero-Garcia, Jordi Selga-Marsa, Juan Antonio Porcel-Vasquez, Jose Vicente Andres-Peiro, Joan Minguell-Monyart, Yaiza Garcia-Sanchez, Jorge Nuñez-Camarena, Eladia Tauste-Rubio, Marta Gonzalez-Amigo
MetroHealth Medical Center, Cleveland, OH: Nicholas M. Romeo, Heather A Vallier, Mary A Breslin***, Joanne Fraifogl, Eleanor S Wilson***, Leanne K Wadenpfuhl***, Paul G. Halliday
FRASER HEALTH AUTHORITY/Royal Columbian Hospital, New Westminster, BC: Darius G. Viskontas, Kelly L. Apostle, Dory S. Boyer, Farhad O. Moola, Bertrand H. Perey, Trevor B. Stone, H. Michael Lemke, Mauri Zomar, Ella Spicer, Chen “Brenda” Fan, Kyrsten Payne
Carolinas Medical Center, Atrium Health Musculoskeletal Institute, Charlotte, NC: Kevin Phelps, Michael Bosse, Madhav Karunakar, Laurence Kempton, Stephen Sims, Joseph Hsu, Rachel Seymour, Christine Churchill, Claire Bartel, Robert Miles Mayberry, Maggie Brownrigg, Cara Girardi, Ada Mayfield
Inova Fairfax Medical Campus, Falls Church, VA: Robert A. Hymes, Cary C. Schwartzbach, Jeff E. Schulman, A. Stephen Malekzadeh, Michael A. Holzman, Lolita Ramsey, James S. Ahn, Farhanaz Panjshiri***, Sharmistha Das, Antoinisha D. English, Sharon M. Haaser, Jaslynn A. N. Cuff
Wake Forest Baptist Health, Winston-Salem, NC: Holly Pilson, Eben A. Carroll, Jason J. Halvorson, Sharon Babcock, J. Brett Goodman, Martha B. Holden, Debra Bullard, Wendy Williams
University of Utah, Salt Lake City, Utah: Thomas F. Higgins, Justin M. Haller, David L. Rothberg, Lucas S. Marchand, Ashley Neese, Mark Russell, Zachary M. Olsen.
Dartmouth-Hitchcock Medical Center, Lebanon, NH: I. Leah Gitajn, Marcus Coe, Kevin Dwyer, Devin S. Mullin, Clifford A. Reilly, Peter DePalo, Amy E. Hall
Massachusetts General Hospital, Boston, MA: Marilyn Heng, Mitchel B. Harris, R. Malcolm Smith, David W. Lhowe, John G. Esposito, Mira Bansal
University of Mississippi Medical Center, Jackson, MS: Patrick F. Bergin, George V. Russell, Matthew L. Graves, John Morellato, Heather K. Champion, Leslie N. Johnson, Sheketha L. McGee, Eldrin L. Bhanat
University of Pennsylvania, Philadelphia, PA: Samir Mehta, Derek Donegan, Jaimo Ahn, Annamarie Horan, Mary Dooley, , Ashley Kuczinski, Ashley Iwu
Sanford Health, Sioux Falls, SD: David Potter, Robert VanDemark III, Branden Pfaff, Troy Hollinsworth
Brigham Women's Hospital, Boston, MA: Michael J. Weaver, Arvind G. von Keudell, Michael F. McTague, Elizabeth M. Allen
University of Maryland Prince George’s Capital Region Health: Cheverly MD: Todd Jaeblon, Robert Beer, Haley K. Demyanovich
Duke University Hospital, Durham, NC: Mark J. Gage, Rachel M. Reilly, Cindy Sparrow
*** Individual is no longer actively working on the Aqueous-PREP and / or PREPARE trial
- Grantham KL, Kasza J, Heritier S, Hemming K, Litton E, et al. (2019) How many times should a cluster randomized crossover trial cross over? Stat Med 38: 5021-5033. [Crossref]
- Gustilo RB, Anderson JT (1976) Prevention of infection in the treatment of one thousand and twenty-five open fractures of long bones: retrospective and prospective analyses. J Bone Joint Surg Am 58: 453-458. [Crossref]
- Charehbili A, Koek MBG, A de Mol van Otterloo JC, Bronkhorst MWGA, van der Zwaal P, et al. (2019) Cluster-randomized crossover trial of chlorhexidine-alcohol versus iodine-alcohol for prevention of surgical-site infection (SKINFECT trial). BJS open 3: 617-622. [Crossref]
- Slobogean GP, Sprague S, Wells J, Bhandari M, Rojas A, et al. (2020) Effectiveness of Iodophor vs Chlorhexidine Solutions for Surgical Site Infections and Unplanned Reoperations for Patients Who Underwent Fracture Repair: The PREP-IT Master Protocol. JAMA Netw Open 3: e202215. [Crossref]
- CDC (2021) Centers for Disease Control and Prevention, Surgical Site Infection (SSI) Event.
- Sprague S, Hebden J, O’Hara LM, Slobogean GP (2020) Cluster Identification, Selection, and Description in Cluster randomized Crossover Trials: The PREP-IT Trials. Trials 1: 712. [Crossref]



