Abstract
Introduction: Tobacco smoking represents the leading preventable cause of morbidity and mortality in adults. Nicotine is responsible for tobacco addiction and is known to elevate brain dopamine, leading to feelings of pleasure and reward. The MAOA enzyme is responsible for degrading dopamine. It is known that a polymorphism in the promoter region of the MAOA gene has been implicated in smoking behavior. In this study, we investigated the association between the MAOA VNTR polymorphism and smoking behavior in Brazilian males.
Methods: A cross-sectional study was conducted with 121 Brazilian males over 18 years of age. The polymorphism was genotyped using PCR. Multiple logistic regression were used to verify the association of MAOA gene polymorphisms with smoking status.
Results: We not found significant association between the MAOA polymorphism and smoking status. Likewise, no association of the VNTR polymorphism with elements of smoking behavior, such as smoking duration, smoking initiation, relapses, smoking cessation, and degree of nicotine dependence, was observed.
Conclusions: In conclusion, no association was observed between the MAOA polymorphism, smoking status and smoking behavior in population of Brazilian males studied. Further studies with larger sample sizes will be needed to elucidate these issues.
Key word
MAOA gene, smoking behavior, polymorphism, nicotine dependence, tobacco
Introduction
Tobacco smoking is a worldwide public health problem, representing the leading preventable cause of morbidity and mortality in adults [1-4]. Moreover, smoking increases the risk for a number of pathologies, including several types of cancer [5,6]. Over 5300 compounds have been identified in tobacco smoke, and over 70 carcinogens with sufficient evidence for carcinogenicity [7,8].
Nicotine is the major psychoactive substance in tobacco [1,9]. The addictive nature of nicotine is multifactorial, involving both environmental and genetic factors [1,10,11]. Studies have shown that most smokers wish to quit smoking, but definitive cessation is a rare outcome, with frequent relapses [12-14]. Birth decade, low socio-economic status, low education level, alcohol consumption, and beginning smoking during adolescence have been found to be environmental risk factors for tobacco smoking [15-17].
Genetic factors also affect smoking behavior [4,18,19]. In fact, genetic variation may influence specific elements of smoking status [20]. Estimates indicate that genetic variation may account for 44-56% of smoking initiation; 46-59% of persistent smoking; and 67-75% of nicotine addiction [21,22]. The interaction between nicotine and dopamine should be considered when exploring these genetic factors, since nicotine activates dopaminergic neurons and enhances dopamine release, leading to feelings of pleasure and reward [23-24]. Several dopamine-related genes have been investigated regarding a possible association with smoking behavior [1,19].
The Monoamine oxidase A gene (MAOA) encoding the enzyme that catalyzes the oxidative deamination of biogenic amines, such as dopamine, noradrenaline, serotonin and histamine, is an important candidate gene to investigate a possible association with smoking behavior [4,13,18,25,26]. This gene is located on the X chromosome and contains a common functional variable number tandem repeat (VNTR) polymorphism that has been studied in relation to smoking behavior [1,2,19,25,27,28]. This polymorphism consists of 2, 3, 3.5, 4, and 5 repeats of a 30 bp sequence in the promoter region. Transcription efficiency was shown to be two- to three-fold higher for longer alleles (3.5, 4, and 5 repeats) than for shorter alleles (3 and a rare 2 repeat allele) [18,25,28-30]. Differences in MAOA enzyme activity among individuals are partly determined by this MAOA gene polymorphism [2,31].
In the present study, we evaluated the association between smoking behavior and functional VNTR MAOA polymorphism in a Brazilian population. We also assessed smoking behavior elements, such as smoking initiation, cigarette consumption, relapses and nicotine dependence among current smokers.
Methods
Study population
A total of 121 subjects were enrolled in a cross-sectional study. The study was open to participation for Brazilian males over 18 years of age. Only males were evaluated because the genotyping of the MAOA gene in males is simpler than in females, since it is located on the X chromosome [1,2,32]. The study was approved by the ethical committee of the Sergio Arouca National School of Public Health (ENSP), Oswaldo Cruz Foundation, number CAAE 38593714.1.0000.5240, and all participants signed an informed consent form.
Individuals were interviewed by trained investigators, using a detailed questionnaire including sociodemographic characteristics, current and previous smoking behaviors, medical history, and occupational data. In addition, the standardized Fagerstrom Test for Nicotine Dependence (FTND) was used to measure the degree of nicotine dependence. A blood sample of about 5 mL was collected from each participant and stored under refrigeration until analysis at the CESTEH Toxicology Laboratory, FIOCRUZ.
Definition of smoking status
All participants were divided into three groups according to their smoking status: Never smokers, Former smokers, and Current smoker. Never smokers were defined as persons who never smoked or smoked less than 100 cigarettes in their lifetime; Former smokers were defined as those who had previously smoked more than 100 cigarettes but had quit smoking for more than 1 year; and Current smokers were defined as those who smoked more than 100 cigarettes in their life and currently smoked or quit smoking for less than 1 year [1,25]. FTND scores are considered as the best measurement tools in the assessment of nicotine dependence, and reflect the level of severity [33]. The evaluation of the degree of nicotine dependence for Current smokers was determined as a FTND score of four or more, which is a sensitive and specific cut-off for smoking [34].
Genotyping
Genomic DNA was extracted from 500 µL of whole blood samples by the salting-out method, and DNA integrity was verified by agarose gel electrophoresis. The MAOA VNTR polymorphism was analyzed according to Ito et al. (2003) [25]. Briefly, PCR was performed on a final volume of 50 μl containing 250 ng of DNA, 0.0002 mmol L-1 of each primer, 0,05 mmol L-1 KCl, 0,01 mmol L-1 Tris-HCl (pH 8.3), 0,2 mmol L-1 of dNTPs, 0,0015 mmol L-1 of MgCl2 and 1-2.5 units of Taq DNA polymerase (Invitrogen, Life Technologies, ThermoFisher). The following primers were used for amplification: 5’-ACAGCCTGACCGTGGAGAAG-3’ (forward) and 5’-GAACGGACGCTCCATTCGGA-3’ (reverse). Initial denaturation was at 95°C for 5 min, followed by 30 cycles at 94°C for 40 s, 58°C for 1 min and 72°C for 1 min. PCR products were resolved by 2.5% agarose gel electrophoresis and stained with GelRedTM (Biotium) Nucleic Acid Gel staining. All genotypes were evaluated and independently confirmed by at least two people. A total of 10% of DNA samples were selected randomly for repeat analyses in order to verify the accuracy of the method, and the concordance rate was 100%. The 2, 3, 3.5, 4 and 5 repeat alleles produced fragments of 291 bp, 321 bp, 336 bp, 351 bp and 381 bp, respectively.
Statistical analyses
Statistical analyses were carried out using the SPSS 20.0 statistical software package (Chicago, IL, USA). The normality of the data distribution was assessed with the Kolmogorov-Smirnov test. The χ2-test and ANOVA test were used to analyze differences between the groups. The effect of the variables age, education, and alcohol consumption on smoking status were tested using logistic regressions, that were also used to examine the relationship between the MAOA VNTR polymorphism and smoking behavior, using comparisons between Low efficiency alleles (2R e 3R) and Long efficiency alleles (3.5R, 4R e 5R). The elements of smoking behavior, such as smoking duration, smoking initiation, relapses, smoking cessation, and degree of nicotine dependence were tested using the t-test, χ2-test and Mann-Whitney test. The significance level for all tests was set at p ≤ 0.05.
Results
Subjects were classified into three groups according to smoking status: Never smokers (61.1%, n=74), Current smokers (17.4%, n=21) and Former smokers (21.5%, n=26). The demographic data for the groups are displayed in Table 1. Differences regarding age, education, and alcohol consumption were statistically significant across the three groups (P < 0.05). The age of smoking initiation was around 16 years old, while the mean quitting age was 33. A mean smoking duration of 22 years was observed. The relapse mean among smoking participants (Current and Former smokers) was of 2 times (twice throughout the smokers life). Forty two percent of the smoking participants smoked more than 10 cigarettes per day. Industrial cigarettes represented 100% of the kind of the tobacco used among the smoking participants. Most of this group presented a degree of nicotine dependence (FNTD) less than 4 (71.4%).
Table 1. Demographic characteristics of the study population per smoking status categories (121 subjects).
Characteristicsa |
Total
N (%) |
Never smokers
N (%) |
Current smokers
N (%) |
Former smokers
N (%) |
p-valueb |
N |
121 (100) |
74 (61.2) |
21 (17.4) |
26 (21.5) |
|
Age (mean) |
42.26 ± 11.685 |
39.19 ± 10.675 |
43.95 ± 11.267 |
49.62 ± 11.597 |
<0.001 |
Education level (years) |
11.02 ± 2.924 |
11.61 ± 2.460 |
10.76 ± 3.548 |
9.54 ± 3.153 |
0.006 |
Ethnic background/
Skin color |
|
|
|
|
0.423 |
Brown skin |
62 (51.2) |
38 (51.4) |
7 (33.3) |
17 (65.4) |
|
White |
33 (27.3) |
19 (25.7) |
9 (42.9) |
5 (19.2) |
|
Black |
25 (20.7) |
16 (21.6) |
5 (23.8) |
4 (15.4) |
|
Asian |
1 (0.8) |
1 (1.4) |
0 (0.0) |
0 (0.0) |
|
Alcohol consumption |
|
|
|
|
0.004 |
No |
44 (36.4) |
32 (43.2) |
1 (4.8) |
11 (42.3) |
|
Yes |
77 (63.6) |
42 (56.8) |
20 (95.2) |
15 (57.7) |
|
N = number of individuals
aContinuous variables are expressed as mean ± standard deviation, and the categorical variables are expressed as n (%).
bp value by χ2 test and ANOVA.
Table 2. Adjusted association of smoking status and MAOA polymorphism, specifically for transcription efficiency.
|
Ever smoker vs. Never smoker |
Current smoker vs. Former smoker |
OR (95% CI) |
p |
OR (95% CI) |
p |
Low efficiency |
reference |
reference |
High efficiency |
1.59 (0.68 – 3.74) |
0.287 |
0,497 (0.12 – 2.04) |
0.332 |
| |
|
|
|
|
|
The MAOA VNTR polymorphism in the promoter region in the study population was determined by PCR. Figure 1 displays a representative MAOA genotyping result on agarose gel electrophoresis with the 5 different MAOA genotypes.
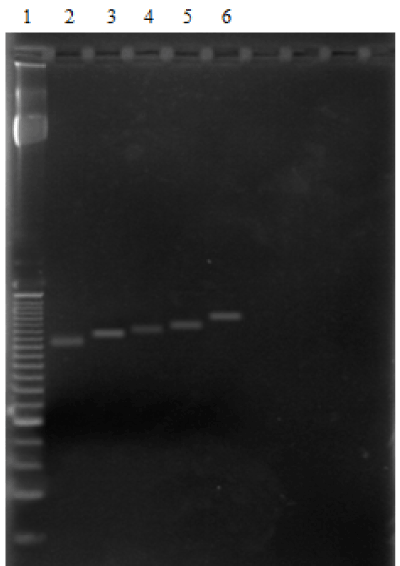
Figure 1. Genotyping of MAOA-u VNTR by PCR. The electrophoresis run on agarose gel stained with GelRed displays the 5 different genotypes observed in the study population. Lane 2-2R allele (291 bp); lane 3-3R allele (321 pb); lane 4-3.5R allele (336 pb); lane 5-4R allele (351 pb); lane 6-5R allele (381 pb).; and lane 1- 25bp DNA ladder.
The following genotype frequencies of the MAOA VNTR polymorphism in the population were found: 2R-5%, 3R-43.8%, 3.5R-14%, 4R-35.5%, and 5R-1.7%. The genotype frequencies in each smoking status category are displayed in Figure 2. The graph indicates a similar distribution among the groups. Regarding the frequency of high efficiency alleles, no statistical difference was observed.
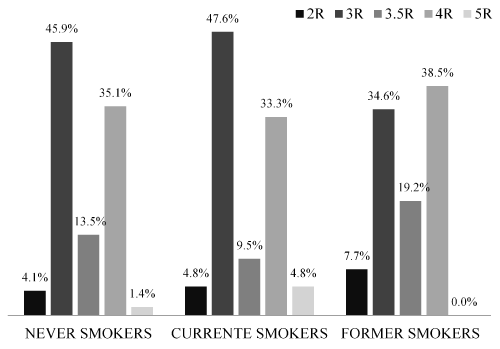
Figure 2. Genotype frequencies of the MAOA VNTR polymorphism per smoking status categories.
The analysis of the association between smoking status and the MAOA polymorphism, specifically transcription efficiency, was adjusted by age, education level, and alcohol consumption. The transcription efficiency of the genotype showed no significant associations with the smoking status. Logistic regressions demonstrated that other variables, such as age and education level, had an impact on the smoking behavior This study also found no significant association between smoking behavior elements (smoking initiation, cigarrete consumption, relapses and nicotine dependence) and MAOA polymorphism (results not shown).
Discussion
We evaluated the relationship between smoking status and the MAOA VNTR polymorphism in Brazilian males. Most of the study population comprised participants who has never smoked (61.1%), followed by former smokers (21.5%), and current smokers (17.4%). These findings were similar to the surveillance of risk factors and protection for chronic diseases by telephone inquiry data (VIGITEL) [35], which is indicated owed a decreased prevalence of smoking in the Brazilian population. There was a decrease in the prevalence of cigarette smoking from 16.2% (data from 2006) to 10.8% (data from 2014) in 26 Brazilian states and in the Federal District [35,36]. These data may reflect the public effort to control smoking in Brazil, such as imposing advertising restrictions, raising fees on tobacco products and banning smoking indoors [37,38].
The mean of smoking initiation age corresponded to adolescence, which is indicated by the literature as the phase of greatest risk [39]. Similarly, to other studies [40,41], we found that tobacco consumption was in the form of industrial cigarettes. In relation to allele frequencies, our findings showed higher frequencies of 3R and 4R compared to other genotypes. This is consistent with earlier studies that present these as the most frequent alleles [2,19,25,29,30,42-44].
Studies have reported that current smokers with an expected high MAOA activity have a significantly higher FTND score compared to those with low enzyme activity [25,45]. Authors suggest that potential MAO activity may be related to the level of smoking required for satisfaction [12,25]. Thus, genotypes responsible for higher enzymatic activity may be related to an increased risk of smoking. However, there is no consensus on which genotypes present the greatest risk [25,30]. Some studies have pointed to high efficiency alleles, while others have considered low efficiency alleles as risk alleles [12,25,46]. Herein, low efficiency alleles showed slightly higher values for most of the smoking behavior characteristics, such as smoking duration (years), relapses, and amount of cigarettes, in comparison to high efficiency alleles.
The relationship between the MAOA VNTR polymorphism and tobacco smoking is still unclear. Previous studies have reported conflicting results [1,4,19,25,30,47,48]. In agreement with other authors [2,19,25], the present study also found no significant associations between MAOA VNTR polymorphism with smoking habits in the study population. Several possible reasons for these contradictions exist, such as differences in the study designs, differences between studied populations, and the effect of gene-gene interactions on smoking behavior [19]. Moreover, it may also be important to consider the effect of environmental factors, such as occupation, educational status, age, medical history, and daily life stress on smoking behavior [19]. In this study, age, educational level and alcohol consumption showed differences regarding smoking status, similarly to other studies [2,35,49].
We took into account certain concerns when carrying out the study, such as adjustment for possible confounding factors by a logistic model. A reduced sample size might be a limitation of our study. To our knowledge, this is the first study to investigate the association between MAOA gene polymorphism and smoking status in a Brazilian population.
In conclusion, this study found no evidence for an association between the MAOA VNTR polymorphism and smoking behavior. Further studies with larger sample sizes will be needed to assess the relation of MAOA genotypes and smoking.
Caroline de Lima Mota: Preparation of the text, bibliographic research, revision of the formatting and laboratorial analyses.
Simone Mitri Nogueira: Preparation of the text, bibliographic research, revision of the formatting and supervision of laboratorial analyses.
Cristiane Barata-Silva: Preparation of the text and bibliographic research.
Thelma Pavesi: Preparation of the text and revision of the formatting
Josino Costa Moreira: Preparation of the text, bibliographic research and revision of the formatting.
Acknowledgments
The authors thank the Center for Tobacco and Health Studies (Cetab/Fiocruz), the Campus Administration Directorship (Dirac/Fiocruz), the Germano Sinval Faria Scholl Health Center, and the Newton Alves Cardoso Polyclinic. Coordination of Superior Level Staff Improvement (CAPES), and National Council for Scientific and Technological Development (CNPQ).
This study was partially supported by the following agencies: Cristiane Barata-Silva and Caroline Lima by FIOCRUZ and CAPES; Thelma Pavesi, Simone Mitri and Josino Costa Moreira by CNPQ.
There are no known conflicts of interest associated with this publication and there has been no significant financial support for this work that could have influenced its outcome.
References
- Jin Y, Chen D, Hu Y, Guo S, Sun H, et al. (2006) Association between monoamine oxidase gene polymorphisms and smoking behaviour in Chinese males. Int J Neuropsychopharmacol 9: 557-564. [Crossref]
- Tang X, Guo S, Sun 2021 Copyright OAT. All rights reserve-gene interactions of CYP2A6 and MAOA polymorphisms on smoking behavior in Chinese male population. Pharmacogenet Genomics 19: 345-352. [Crossref]
- World Health Organization (2011) WHO Report on the Global Tobacco Epidemic. 2011: Warning about the Dangers of Tobacco. Geneva.
- Yang X, Chen H, Li S, Wang Q, Pan L, et al. (2015) Association between monoamine oxidase gene polymorphisms and smoking behavior: A meta-analysis. Drug Alcohol Depend 153: 350-354. [Crossref]
- Instituto Nacional de Câncer (2007) Tabagismo Um Grave Problema de Saúde Pública.
- World Health Organization (2013) WHO Report on the Global Tobacco Epidemic, 2013: Enforcing Bans on Tobacco Advertising, Promotion and Sponsorship. Geneva.
- Hecht SS (2012) Research opportunities related to establishing standards for tobacco products under the Family Smoking Prevention and Tobacco Control Act. Nicotine Tob Res 14: 18-28. [Crossref]
- International Agency for Research on Cancer (2004) IARC. Lyon.
- Wonnacott S, Sidhpura N, Balfour DJ (2005) Nicotine: from molecular mechanisms to behaviour. Curr Opin Pharmacol 5: 53-59. [Crossref]
- Agrawal A, Verweij KJ, Gillespie NA, Heath AC, Lessov-Schlaggar CN, et al. (2012) The genetics of addiction-a translational perspective. Transl Psychiatry 2: e140. [Crossref]
- Caron L, Karkazis K, Raffin TA, Swan G, Koenig BA (2005) Nicotine addiction through a neurogenomic prism: ethics, public health, and smoking. Nicotine Tob Res 7: 181-197. [Crossref]
- Berlin I, Anthenelli RM (2001) Monoamine oxidases and tobacco smoking. Int J Neuropsychopharmacol 4: 33-42. [Crossref]
- Lewis A, Miller JH, Lea RA (2007) Monoamine oxidase and tobacco dependence. Neurotoxicology 28: 182-195. [Crossref]
- Batra V, Patkar AA, Berrettini WH, Weinstein SP, Leone FT (2003) The genetic determinants of smoking. Chest 123: 1730-1739. [Crossref]
- Paavola M, Vartiainen E, Haukkala A (2004) Smoking, alcohol use, and physical activity: a 13-year longitudinal study ranging from adolescence into adulthood. J Adolesc Health 35: 238-244. [Crossref]
- Romberger DJ, Grant K (2004) Alcohol consumption and smoking status: the role of smoking cessation. Biomedecine Pharmacother 58: 77-83.
- Wetter DW, Cofta-Gunn L, Fouladi RT, Irvin JE, Daza P, et al. (2005) Understanding the associations among education, employment characteristics, and smoking. Addict Behav 30: 905-914. [Crossref]
- Ducci F, Newman TK, Funt S, Brown GL, Virkkunen M, et al. (2006) A functional polymorphism in the MAOA gene promoter (MAOA-LPR) predicts central dopamine function and body mass index. Mol Psychiatry 35: 495-519. [Crossref]
- Tochigi M, Suzuki K, Kato C, Otowa T, Hibino H, et al. (2007) Association study of monoamine oxidase and catechol-O-methyltransferase genes with smoking behavior. Pharmacogenet Genomics 17: 867-872. [Crossref]
- Shiels MS, Huang HY, Hoffma SC, Shugart YY, Bolton JH, et al. (2008) A community-based study of cigarette smoking behavior in relation to variation in three genes involved in dopamine metabolism: Catechol-O-methyltransferase (COMT), dopamine beta-hydroxylase (DBH) and monoamine oxidase-A (MAO-A). Prev Med 47: 116-122. [Crossref]
- Li MD, Cheng R, Ma JZ, Swan GE (2003) A meta-analysis of estimated genetic and environmental effects on smoking behavior in male and female adult twins. Addiction 98: 23-31. [Crossref]
- Vink JM, Willemsen G, Boomsma DI (2005) Heritability of smoking initiation and nicotine dependence. Behav Genet 35: 397-406. [Crossref]
- Benowitz NL (2010) Nicotine addiction. N Engl J Med 362: 2295-2303. [Crossref]
- Fowler CD, Kenny PJ (2014) Nicotine aversion: Neurobiological mechanisms and relevance to tobacco dependence vulnerability. Neuropharmacol 76: 533-544. [Crossref]
- Ito H, Hamajima N, Matsuo K, Okuma K, Sato S, et al. (2003) Monoamine oxidase polymorphisms and smoking behaviour in Japanese. Pharmacogenetics 13: 73-79. [Crossref]
- Rendu F, Peoc'h K, Berlin I, Thomas D, Launay JM (2011) Smoking related diseases: the central role of monoamine oxidase. Int J Environ Res Public Health 8: 136-147. [Crossref]
- McGrath LM, Mustanski B, Metzger A, Pine DS, Kistner-Griffin E, et al. (2012) A latent modeling approach to genotype–phenotype relationships: maternal problem behavior clusters, prenatal smoking, and MAOA genotype. Arch Womens Ment Health 15: 269-282. [Crossref]
- Huang CL, Ou WC, Chen PL, Liu CN, Chen MC, et al. (2015) Effects of Interaction Between Dopamine D2 Receptor and Monoamine Oxidase A Genes on Smoking Status in Young Men. Biol Res Nurs 17: 422-428. [Crossref]
- Deckert J, Catalano M, Syagail YV, Bosi M, Okladnova O, et al. (1999) Excess of high activity monoamine oxidase A gene promoter alleles in female patients with panic disorder. Hum Mol Genet 8: 621-624. [Crossref]
- Sabol SZ, Hu S, Hamer D (1998) A functional polymorphism in the monoamine oxidase A gene promoter. Hum Genet 103: 273-279. [Crossref]
- Wiesbeck GA, Wodarz N, Weijers HG, Dursteler-MacFarland KM, Wurst FM, et al. (2006) A functional polymorphism in the promoter region of the monoamine oxidase A gene is associated with the cigarette smoking quantity in alcohol-dependent heavy smokers. Neuropsychobiol 53: 181-185. [Crossref]
- Lu RB, Lee JF, Ko HC, Lin WW, Chen K, et al. (2002) No association of the MAOA gene with alcoholism among Han Chinese males in Taiwan. Prog Neuropsychopharmacol Biol Psychiatry 26: 457-461. [Crossref]
- Carmo JT do, Pueyo AA (2002) A adaptaçäo ao português do Fagerström Test for nicotine dependence (FTND) para avaliar a dependência e tolerância à nicotina em fumantes brasileiros. Rev Bras Med 58: 73-80.
- Olfson E, Bloom J, Bertelsen S, Budde JP, et al. (2016) CYP2A6 metabolism in the development of smoking behaviors in young adults. Addict Biol. [Crossref]
- Brasil, Ministério da Saúde. Secretaria de Vigilância em Saúde (2015) Secretaria de Gestão Estratégica e Participativa, Monteiro CA, et al. Vigitel Brasil 2014: vigilância de fatores e risco e proteção para doenças crônicas por inquérito telefônico. Brasília: Ministério da Saúde.
- Brasil, Ministério da Saúde. Secretaria de Vigilância em Saúde (2007) Secretaria de Gestão Estratégica e Participativa, Monteiro CA, et al. Vigitel Brasil 2006: vigilância de fatores e risco e proteção para doenças crônicas por inquérito telefônico. Brasília: Ministério da Saúde;.
- Instituto Nacional de Câncer (2012) INCA - Agência de notícias -Políticas antitabagismo no Brasil já salvaram mais de 400 mil vidas.
- Szklo AS, de Souza MC, Szklo M, de Almeida LM (2016) Smokers in Brazil: who are they? Tob Control 25: 564-570. [Crossref]
- Pinto D da S, Ribeiro SA (2007) Variáveis relacionadas à iniciação do tabagismo entre estudantes do ensino médio de escola pública e particular na cidade de Belém - PA. J Bras Pneumol 33: 558-564.
- Mitrouska I, Bouloukaki I, Siafakas NM (2007) Pharmacological approaches to smoking cessation. Pulm Pharmacol Ther 20: 220-232. [Crossref]
- NIH-U.S. National Cancer Institute and World Health Organization (2016) The Economics of Tobacco and Tobacco Control. National Cancer Institute Tobacco Control Monograph 21. NIH Publication No. 16-CA-8029A. Bethesda, MD: U.S. Department of Health and Human Services, National Institutes of Health, National Cancer Institute; and Geneva.
- Contini V, Marques FZC, Garcia CED, Hutz MH, Bau CHD (2006) MAOA-uVNTR polymorphism in a Brazilian sample: further support for the association with impulsive behaviors and alcohol dependence. Am J Med Genet Part B Neuropsychiatr Genet 141B: 305-308. [Crossref]
- Courts C, Grabmüller M, Madea B (2013) Monoamine oxidase A gene polymorphism and the pathogenesis of sudden infant death syndrome. J Pediatr 163: 89-93. [Crossref]
- Nishioka SA, Perin EA, Sampaio AS, Cordeiro Q, Cappi C, et al. (2011) O papel do polimorfismo funcional VNTR da região promotora do gene MAOA nos transtornos psiquiátricos. Rev Psiquiatr Clínica 38: 34-42.
- Fowler JS, Volkow ND, Wang GJ, Pappas N, Logan J, et al. (1996) Brain monoamine oxidase A inhibition in cigarette smokers. Proc Natl Acad Sci U S A 93: 14065-14069. [Crossref]
- Balciuniene J, Emilsson L, Oreland L, Pettersson U, Jazin E (2002) Investigation of the functional effect of monoamine oxidase polymorphisms in human brain. Hum Genet 110: 1-7. [Crossref]
- Huang S, Cook DG, Hinks LJ, Chen XH, Ye S, et al. (2005) CYP2A6, MAOA, DBH, DRD4, and 5HT2A genotypes, smoking behaviour and cotinine levels in 1518 UK adolescents. Pharmacogenet Genomics 15: 839-850. [Crossref]
- McKinney EF, Walton RT, Yudkin P, Fuller A, Haldar NA, et al. (2000) Association between polymorphisms in dopamine metabolic enzymes and tobacco consumption in smokers. Pharmacogenetics 10: 483-491. [Crossref]
- Piasecki TM, McCarthy DE, Fiore MC, Baker TB (2008) Alcohol consumption, smoking urge, and the reinforcing effects of cigarettes: An ecological study. Psychol Addict Behav 22: 230-239. [Crossref]


