Key words
Lower leg; Soft tissue defect; Coverage; Hemisoleus muscle flap
Abbreviations
VAC: Vacuum Assisted Closure; NFCSAF: neurofasciocutaneous sural artery flap; HSMF: hemisoleus muscle flap; ORIF: open reduction and internal fixation
Case presentation
Soft tissue defects of the ventral aspect of lower leg with exposure of bone, tendon, and/or osteosynthesis plates represent a challenging therapeutic problem. The use of local pedicled flaps for coverage is one option for treatment in patients who are not willing or healthy enough to undergo free microvascular tissue transplantation. Additionally, it does not require microsurgical expertise. Negative-pressure Vacuum Assisted Closure (VAC) therapy before soft tissue coverage provides a sterile and controlled environment that can lessen the duration of wound healing, promotes better capillary circulation, and decreases the bacterial load [1].
In the first case, a 47-year-old male with a history of social desintegration, severe alcohol and tobacco abuse, chronic liver disease, and diabetes mellitus presented with a 9 cm in diameter necrotizing pre-tibial ulcus in the middle third of the right lower leg (Figure 1A, first photograph). Microbiological examination revealed massive bacterial load with Staphylococcus aureus and Enterococcus faecalis. The patient could not remember any trauma in the history. First, multiple radical debridements combined with negative-pressure VAC therapy and concomitant intravenous application of antibiotics were performed over a period of 3 weeks. Second, after consolidation of soft tissue infection (Figure 1A, second photograph), confirmed by microbiology, the defect was covered with the use of distally pedicled neurofasciocutaneous sural artery flap (NFCSAF) (Figure 1B). Eight days after coverage, the distally pedicled NFCSAF has failed due to septic venous thrombosis. Third, the necrotic distally pedicled NFCSAF was excised, followed by once more necessary multiple debridements and VAC therapies (Figure 1C). Fourth, the resulting second defect was reconstructed with the use of proximally pedicled medial hemisoleus muscle flap (HSMF) (Figure 1D). The further course was uncomplicated (Figure 1E).
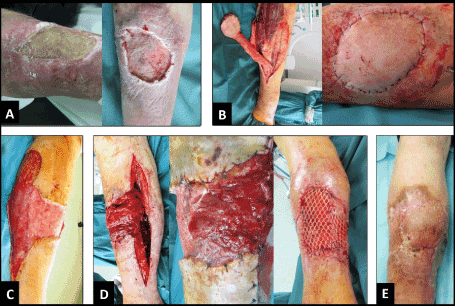
Figure 1. (First case, clinical photographs) (A) Pre-tibial necrotizing ulcer before and after multiple debridements and VAC therapies. (B) Dissection and transposition of distally pedicled NFCSAF, note that there is a sufficient arterial blood supply without venous congestion. (C) Resulting defect after excision of failed distally pedicled NFCSAF and once more necessary multiple debridements and VAC therapies. (D) Dissection, transposition with incision the skin bridge, and primary split-thickness skin grafting of proximally pedicled medial HSMF. (E) Uncomplicated healing.
In the second case, a 56-year-old male sustained a closed fracture of the proximal and middle third of the right tibia that was treated primarily with open reduction and internal fixation (ORIF), and resulting in a non-infected postoperative pre-tibial soft tissue defect associated with exposure of the osteosynthesis plate with size of 5 x 2 cm (Figure 2A). The defect was covered with the use of a distally pedicled medial HSMF, the healing was uncomplicated (Figure 2B-C). Ten weeks after injury, the patient could be mobilized with full weight-bearing on the affected leg, and there was no ankle instability nor loss of plantar flexion of his right foot (Figure 2D).
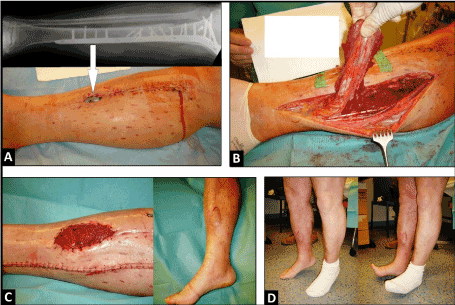
Figure 2. (Second case, antero-posterior radiograph and clinical photographs) (A) Soft tissue defect with exposure of the osteosynthesis plate (arrow) after ORIF. (B) Dissection of distally pedicled HSMF. (C) Coverage of wound with preserving the skin bridge, and uncomplicated healing after additional split-thickness skin grafting. (D) Complete mobilization of patient without loss of plantar flexion of foot.
If a primary non-infected posttraumatic or postoperative soft tissue defect is present that is usually associated with bacterial contamination, a surgical intervention should follow as soon as possible to avoid infection. As compared to the published results in 1984 with a secondary wound sepsis and required amputation rate of 42% for open fractures type IIIC, the required amputation rate of leg after infected total knee arthroplasty could be significantly decreased recently to 15% when using an adequate soft tissue management [2,3].
The distally pedicled NFCSAF, introduced in 1992 by Masquelet et al. [4], is one option for coverage of the middle and distal third of lower leg, ankle, and heel [5,6]. It is a skin island flap which is retrograde supplied by at least three perforator vessels from the peroneal artery within approximately 6 cm from the tip of lateral malleolus. However, this flap is not free of complications, mostly based on venous congestion. In a meta-analysis of 50 articles including 720 procedures, the necrosis rate or any other flap-related complications of distally pedicled NFCSAF is reported to be 18% [7]. The weakness can be the pivot point. The flap’s arterial inflow is robust and constant, but the venous congestion is susceptible occurring in up to 21.4% of cases, and it is mostly detected if the flap was used in a 180° turned manner [8,9]. To prevent early venous stasis, the pivot point of vascular pedicle including the short saphenous vein can be covered temporary with a skin substitute and covered secondary with a skin graft (Figure 3A-C). Another options to prevent venous congestion is the use of NFCSAF in a two-stage, supercharged or superdrained manner, and intermittent short saphenous vein phlebotomy [9-12]. Schmidt et al. [13] reported on a survival rate when using the NFCSAF in an adipofascial manner with additional skin grafting in 87.5% of 104 cases. In cases in which the short saphenous vein cannot be found, the flap should not be utilized; and in older, high-risk, and critically multimorbid patients including peripherial arterial disease, a considerable necrosis rate of 36% of a total of 70 procedures was found by Baumeister et al. [14]. An unacceptable failure leading to loss of flap is when the vascular pedicle was selceted too short and no sufficient arterial supply exists [15].
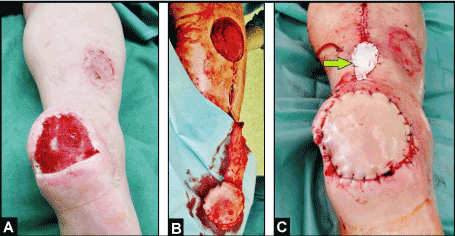
Figure 3. (Example for temporary coverage of the pivot point of a distally pedicled NFCSAF in 74-year- old male, clinical photographs): (A) Chronic ulcer of the right heel after consolidation of infection with VAC therapy. (B) Harvesting the distally pedicled NFCSAF. (C) Coverage of defect, the flap showing sufficient arterial blood supply without venous congestion, and the pivot point of flap's pedicle was temporary covered with a skin substitute (Epigard, arrow) for 5 days (after that the definitive coverage was performed by split-thickness skin grafts).
Local muscle flaps, first reported by Stark in 1946, became an established procedure for coverage of soft tissue defects of the lower leg with exposure of bone with or without osteosynthesis plates, joints with or without total knee or total ankle arthroplasties in the absence of deep infect, tendons, and can provide as well as prevent shortening of the tibial bone [6,15-20]. Muscle flaps promotes better capillary circulation leading to a decrease of bacterial load, hence, muscle flaps are not contraindicated when superficial bacterial wound contamination is present. When using muscle flaps for coverage, all surgeons need a learning curve. In 1983, Neale et al. [21] reported on major and minor complications in 32% of a total of 95 muscle flaps and they agreed that the causes were mainly technical errors, inadequate debridement, use of diseased and traumatized muscle, and unrealistic objectives. The HSMF is one option for coverage of the lower leg. The soleus muscle is located in the posterior region of lower leg, inferior to the gastrocnemius muscle, and is classified as type II according to the classification of Mathes and Nahai [22]. The proximal part of soleus is supplied by the popliteal artery (major pedicle), the main blood supply of the medial head throughout its length is from large and constant perforators (secondary pedicles) arising from the posterior tibial artery, and the main blood supply of the lateral head, its overlying skin and fibula is from large vascular pedicles of the peroneal artery mostly distributed in the upper half of the muscle which allows a composite free or pedicled transfer of the soleus muscle with the fibula [23,24]. Hence, it can be used in a proximally pedicled manner for coverage of large soft tissue defects of the proximal and/or middle third of lower leg, or in a distally pedicled manner (i.e. reversed flap), initially proposed in 1985 by Tobin, for coverage of the middle and distal third of lower leg [25,26]. When using the medial HSMF in a distally pedicled manner, it is important to know that the mean distance of the muscular branches from the medial malleolus is 14.17 ± 6.65 cm in male and 14 ± 7.02 cm in female and the range is from 6 to 24 cm [24]. The possibility for use of the HSMF as a myocutaneous flap is given as well [27]. The soleus muscle being the prime ankle plantar flexor and stabilizer of the ankle and cannot be sacrificed completely without significant morbidity. The consistently bipenniform neurovascular anatomy allows longitudinally splitting the muscle for transfer of one-half the muscle as a hemi-flap to obtain stability of the ankle and plantar flexion of foot. The lateral head has a smaller detection for coverage of pre-tibial wounds than the medial head because it must be rotated around the fibula. The HSMF is relatively easy to dissect, the donor site can be closured primarily, and it has a low complication rate. Using the proximally pedicled HSMF, Ata-ul-Haq et al. [28] reported on 100% flap survival of 10 patients, and Ahmad et al. [29] reported on complete flap loss only in 2,5% and partial skin graft loss in 12,5% of 40 patients. With our first case presentation, the proximally pedicled HSMF has proven to be successful as a revision flap for a failed distally pedicled NFCSAF. Using the HSMF in the distally pedicled manner, wound coverage can be achieved in 100% of patients with wounds smaller than 50 cm2, or wounds with a size up to 10 x 6 cm [30,31]. The use of the proximally pedicled HSMF containing a part of Achilles tendon as a rotational flap additionally allows reconstruction of chronic extensor mechanism disruption after total knee arthroplasty [32].
Acknowledgements
None.
Conflict of interests
The author declares that he has none conflict of interests concerning this article.
References
- Plikaitis CM, Molnar JA (2006) Subatmospheric pressure wound therapy and the vacuum-assisted closure device: basic science and current clinical successes. Expert Rev Med Devices 3: 175-184.
- Gustilo RB, Mendoza RM, Williams DN (1984) Problems in the management of type III (severe) open fractures: a new classification of type III open fractures. J Trauma 24: 742-746. [Crossref]
- Suda AJ, Cieslik A, Grützner PA, Münzberg M, Heppert V (2014) Flaps for closure of soft tissue defects in infected revision knee arthroplasty. Int Orthop 38: 1387-1392. [Crossref]
- Masquelet AC, Romana MC, Wolf G (1992) Skin island flaps supplied by the vascular axis of the sensitive superficial nerves: anatomic study and clinical experience in the leg. Plast Reconstr Surg 89: 1115-1121.
- Schmidt I, Schmieder A, Kilian O (2012) The simultaneous distally based sural flap. A therapeutic option for coverage of both heels. Unfallchirurg 115: 267-272.
- Schmidt I (2016) Long-term outcome in a patient with severe postoperative osteomyelitis of the critical distal third of lower leg. Int J Case Rep Images 7: 680-682.
- Follmar KE, Baccarani A, Baumeister SP, Levin LS, Erdmann D (2007) The distally based sural flap. Plast Reconstr Surg 119: 138e-148e. [Crossref]
- Hassanpour SE, Mohammadkhah N, Arasteh E (2008) Is it safe to extract the reverse sural artery flap from the proximal third of the leg? Arch Iran Med 11: 179-185. [Crossref]
- Weber O, Pagenstert G, Gravius S, Burger C, Müller M, et al. (2012) The one and two-stage distally pedicled sural flap: surgical technique and clinical results. Unfallchirurg 115: 988-993.
- Tan O, Atik B, Bekerecioglu M (2005) Supercharged reverse-flow sural flap: a new modification increasing the reliability of the flap. Microsurgery 25: 36-43. [Crossref]
- El-Diwany M, Karunanayake M, Al-Mutari S, Duvernay A, Danino AM (2015) Super-drained distally based neurofasciocutaneous sural flap: A case series and review of literature. Eplasty 12;15: e16.
- Wong CH, Tan BK (2007) Intermittent short saphenous vein phlebotomy: An effective technique of relieving venous congestion in the distally based sural artery flap. Ann Plast Surg 58: 303–307.
- Schmidt K, Jakubietz M, Harenberg P, Holzapfel BM, Rudert M, et al. (2013) The distally based adipofascial sural artery flap for the reconstruction of distal lower extremity defects. Oper Orthop Traumatol 25: 162–169.
- Baumeister SP, Spierer R, Erdmann D, Sweis R, Levin LS, et al. (2003) A realistic complication analysis of 70 sural artery flaps in a multimorbid patient group. Plast Reconstr Surg 112: 129-140.
- Schmidt I (2017) The distally pedicled peroneus brevis muscle and fasciocutaneous sural artery flap for reconstruction of the distal third of lower leg. Int J Case Rep Images 8: 17-21.
- Stark WJ (1946) The use of pedicled muscle flaps in the surgical treatment of chronic osteomyelitis resulting from compound fractures. J Bone Joint Surg Am 28: 343-350.
- Bach O, Hope MJ, Chaheka CV, Dzimbiri KM (2004) Disability can be avoided after open fractures in Africa-results from Malawi. Injury 35: 846-851. [Crossref]
- Schmidt I (2017) The role of the gastrocnemius muscle flap for reconstruction of large soft tissue defects after infected total knee arthroplasty. Int J Case Rep Images 8: 7-10.
- Schmidt I (2017) The gastrocnemius muscle flap for coverage of soft tissue defect of the proximal third of lower leg. Int J Case Rep Images 8: 168-170.
- Schmidt I (2016) The Distally Pedicled Peroneus Brevis Muscle Flap for Treatment of an Arthrocutaneous Fistula after Ankle Arthroscopy: Case Report. JSM Clin Case Rep 4: 1115.
- Neale HW, Stern PJ, Kreilein JG, Gregory RO, Webster KL (1983) Complications of muscle-flap transposition for traumatic defects of the leg. Plast Reconstr Surg 72: 512-517.
- Mathes SJ, Nahai F (1981) Classification of the vascular anatomy of muscles: experimental and clinical correlation. Plast Reconstr Surg 67: 177-187. [Crossref]
2021 Copyright OAT. All rights reserv
- Raveendran SS, Kumaragama KG (2003) Arterial supply of the soleus muscle: anatomical study of fifty lower limbs. Clin Anat 16: 248-252. [Crossref]
- El Zawawy EMM, El Sekily NM (2012) An anatomical study of the blood supply of the soleus muscle in humans. Alex J Med 48: 315-321.
- Schmidt I (2016) The proximally based hemisoleus muscle flap for coverage of large tissue defect of the middle third of lower leg. Int J Case Rep Images 7: 683-684.
- Tobin GR (1985) Hemisoleus and reversed hemisoleus flaps. Plast Reconstr Surg 76: 87-96. [Crossref]
- Hallock GG (1996) Getting the most from the soleus muscle. Ann Plast Surg 36: 139-146. [Crossref]
- Ata-ul-Haq, Tarar NM, Malik FS, Khalid K, Riaz A, et al. (2009) Hemisoleus muscle flap, a better option for coverage of open fractures involving middle third of tibia. J Ayub Med Coll Abbottabad 21: 154-158.
- Ahmad I, Akhtar S, Rashidi E, Khurram MF (2013) Hemisoleus muscle flap in the reconstruction of exposed bones in the lower limb. J Wound Care 22: 635, 638-640, 642. [Crossref]
- Houdek MT, Wagner ER, Wyles CC, Sems SA, Moran SL (2016) Reverse Medial Hemisoleus Flaps for Coverage of Distal Third Leg Wounds: A Technical Trick. J Orthop Trauma 30: e138-142. [Crossref]
- Pu LL (2006) The reversed medial hemisoleus muscle flap and its role in reconstruction of an open tibial wound in the lower third of the leg. Ann Plast Surg 56: 59-63. [Crossref]
- Auregan JC, Lin JD, Lombardi JM, Jang E, Macaulay W, et al (2016) The hemisoleus rotational flap provides a novel superior autograft reconstructive option for the treatment of chronic extensor mechanism disruption. Arthroplast Today 2: 49-52.



