Introduction
To treat peripheral neuropathic pain by prescribing systemic acting drugs such as amitriptyline, duloxetine and gabapentinoids is actually quite old fashioned; none of these drugs are targeted to the exact pathogenesis of the problem, and thus the entire system is flooded with these compounds after oral intake. This is the main reason for the wide array of adverse events induced by these drugs and the low patient compliance. Especially in frail elderly patients, which are also prone to drug-drug interactions, more targeted therapies are welcomed. Clearly the search for innovative and new peripheral acting analgesics is driven by this goal. This commentary serves to take the discussion one step further. To the field of peripheral acting analgesics in topical formulations.
Sodium channels and the pathogenesis of peripheral painful neuropathy
Increasingly sodium channels are recognized as the pathogenetic base of many peripheral painful neuropathies. For instance, Painful Diabetic Neuropathy (PDN) can already manifest itself very early during the course of diabetes, even in patients with only slight signs of impaired glucose tolerance. Some patients with chronic diabetes however, never suffer from DPN [1]. DPN might therefore not solely be a complication of diabetes but could also be based on mutations at certain sodium channels, these mutations have now been identified both in nerve cells as well as in pancreatic beta cells. It is even suggested that these mutations increase independently the risk for diabetes and the risk for painful DPN [1]. Especially the sodium channels Nav1.7, 1.8, and 1.9 are key for activating the pain-mediating nociceptors [2]. Disturbances in the structure or function of these channels can lead to painful small-fiber neuropathy (SFN), a pathological state frequently found in PDN, chronic idiopathic axonal neuropathy (CIAP) and a number of internal disorders, such as sarcoidosis.
Some years ago, new findings of a gain-of-function mutation on the Nav 1.8 and Nav 1.9 channel provoked some scientist to claim Nav 1.8 or Nav 1.9 blockers were an interesting new inroad for treating peripheral neuropathic pain syndromes. After the first wave of presumed selective Nav 1.7 blockers, such as CNV1014802 (also known as BIIB074, vixotrigine, raxatrigine), new selective blockers are now under development, such as the Nav 1.8 blockers from Vertex, VX-150 and VX-128. The selectivity of these blockers however, remains to be seen, as in the past selective Nav 1.7 blockers sometimes appeared to be less selective than was initially indicated by the inventors [3]. This wave of new Nav blockers and the importance of the sodium channels for the pathogenesis of chronic pain syndromes, even in fibromyalgia, provoked some authors to speak about ‘nav-igating pain’ [4].
Navigating of peripheral neuropathic pain: the quest for topical sodium channel blockers
The oldest known sodium channel blocker used in medicine for its analgesic and anesthetic properties is cocaine. [5]. By using topical cocaine solutions, influenced by the work of the young Dr. Sigmund Freud, physicians were inducing local analgesic and anesthetic effects. The ophthalmologist Carl Koller discovered this topical analgesic effect of cocaine in 1884 [6]. We can therefore state that nav-igating the sodium channel to obtain clinical relevant effects of nociceptor inhibition is one of the oldest pharmacological pain-reducing techniques. Interestingly, this application of cocaine was topical. Sadly enough, due to the excellent absorption of cocaine via the cornea, dependency and abuse limited its use. Systemic side effects after topical application of course needed and needs to be avoided. One of the quests of our time therefore is therefore to find non-skin penetrating sodium channel blockers, which can reach the epidermal targets in peripheral neuropathic pain syndromes (the nociceptors, small fibers, but also cross-talking cells such as keratinocytes and immune-competent cells).
In the epidermis, the small nerve fibers and their nociceptors express a number of subtypes of sodium channels, but we still are in the explorative phase about the exact function of all these channels. Nine subtypes of sodium channel have been identified on mammalian cells, and most, apart from the Nav1.2 channel, expressed in the central nervous system, and Nav1.4 and Nav1.5 channels, expressed in muscle, play possibly an important role in the genesis of peripheral neuropathic pain, as these other channels are expressed in the dorsal root ganglion neurons (DRGs) and the nociceptors in the skin. Phenytoin is a non-selective (broad-acting) sodium channel blocker, because its binding-site is situated at the inner cytoplasmic membrane, at the inner vestibule of the pore. This binding site is present in all members of sodium channel family. [7] A new insight makes this channel blocker even more interesting, after being in the clinic for 80 years! Recently it is also recognized that anti-epileptics of the classes of voltage-gated calcium channel (VGCC) blockers such as gabapentinoids, and of the voltage-gated sodium channel (VGSC) blockers such as phenytoin, also might have their use in inflammatory pain conditions. The density and distribution as well as the expression of both types of channels influence afferent fiber excitability, not only in neuropathic pain states, but also in inflammatory pain states [8,9]. New approaches try to target both. For instance, one can suppress Inflammatory and neuropathic pain quickly after a peripheral block of hyperpolarisation-activated cyclic nucleotide-gated ion channels by a compound as ivabradine [7]. Interestingly phenytoin has been document to possess clear anti-inflammatory characteristics too [10-11]. Furthermore, phenytoin also has neuroprotective properties, and these might be for a part dependent of its anti-inflammatory mode of action, see figure 1 [12-13].
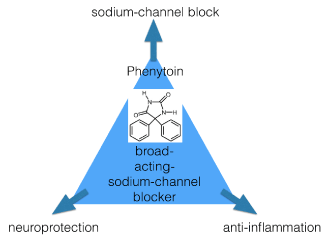
Figure 1. mechanisms of action of phenytoin as a topical analgesic.
Administrating topical phenytoin cream in peripheral neuropathic pain
The administration of a topical phenytoin formulation on the skin in patients suffering from painful SFN is therefore a logical step. We recently developed a cream, containing a range of 5%-20% of phenytoin [14]. The choice of phenytoin is further supported by recent findings of elevated local proinflammatory cytokines in length-dependent small fiber neuropathy [15]. Another symptom, regularly present in patients suffering from SFN, such as muscle cramps is also possibly related to local mediators of inflammation released by damaged small nerve that excite intramuscular nerves [16].
From 2015 onwards, we have gathered information on the treatment of peripheral painful neuropathies with a special developed topical phenytoin-formulation. We have pointed out that especially such topical formulation based on the broad acting sodium channel blocker phenytoin is more fit to induce sufficient analgesia that topical formulations containing highly selective sodium channel blockers [17]. This is especially so, because there is no clinical relevant absorption of phenytoin through the skin: its mechanism of action is purely intra-epidermal [18]. In 16 patients we sampled plasma after 1-2 hours after applying cream, after at least 10 days of application (steady state situation), and in none of these patient’s plasma levels were detectable [19]. Meanwhile we have gathered safety data and preliminary efficacy data of more than 100 patients. There are no dose-limiting side effects; we could dose up to 30% without local irritation being a reason for discontinuation of therapy. Only in around 10% of all patients there were slight local side effects, which mostly resolved after some days. We also concluded that there is a dose-effect relationship; the analgesic effects of 10% phenytoin cream lasts around twice as long as cream containing 5% phenytoin [19].
Navigating the sodium channels, in case of peripheral neuropathic pain, where the pathogenesis is residing in small fibers and nociceptors, is possible with phenytoin cream. This is therefore a new and interesting treatment option. We are currently at the start of a phase III program, after consultation with the Dutch Medicines Evaluation Board [20].
Conflict of interest
The author is a patent holder of two patents related to the topical formulations of phenytoin in the treatment of neuropathic pain.
References
- Hoeijmakers JG, Faber CG, Merkies IS, Waxman SG (2014) Channelopathies, painful neuropathy, and diabetes: which way does the causal arrow point? Trends Mol Med 20: 544-550. [Crossref]
- Huang J, Han C, Estacion M, Vasylyev D, Hoeijmakers JG, et al. (2014) Gain-of-function mutations in sodium channel Na(v)1.9 in painful neuropathy. Brain 137: 1627-1642. [Crossref]
- Keppel Hesselink JM (2017) Moving targets in sodium channel blocker development: the case of raxatrigine: from a central NaV1.3 blocker via a peripheral NaV1.7 blocker to a less selective sodium channel blocker". J Med Therap 1: 1-3.
- Erickson A, Deiteren A, Harrington AM, Garcia-Caraballo S, Castro J, et al. (2018) Voltage-gated sodium channels: (NaV)igating the field to determine their contribution to visceral nociception. J Physiol 596: 785-807. [Crossref]
- Grin J, Bueno EJ (1973) Effect of cocaine on Na channel in toad skin. Can J Physiol Pharmacol 51: 516-522. [Crossref]
- Goerig M, Bacon D, van Zundert A (2012) Carl Koller, cocaine, and local anesthesia: some less known and forgotten facts. Reg Anesth Pain Med 37: 318-324. [Crossref]
- Mantegazza M, Curia G, Biagini G, Ragsdale DS, Avoli M (2010) Voltage-gated sodium channels as therapeutic targets in epilepsy and other neurological disorders. Lancet Neurol 9: 413-424. [Crossref]
- Rahman W, Dickenson AH (2013) Voltage gated sodium and calcium channel blockers for the treatment of chronic inflammatory pain. Neurosci Lett 557: 19-26. [Crossref]
- Tomic M, Pecikoza U, Micov A, Vuckovic S, Stepanovic-Petrovic R (2018) Antiepileptic drugs as analgesics/adjuvants in inflammatory pain: current preclinical evidence. Pharmacol Ther S0163-7258: 30104-30109. [Crossref]
- Young GT, Emery EC, Mooney ER, Tsantoulas C, McNaughton PA (2014) Inflammatory and neuropathic pain are rapidly suppressed by peripheral block of hyperpolarisation-activated cyclic nucleotide-gated ion channels. Pain 155: 1708-1719. [Crossref]
- Shapiro M (1958) Acceleration of gingival wound healing in non-epileptic patients receiving diphenylhydantoin sodium (dilantin, epanutin). Exp Med Surg 1958; 16: 41-53. [Crossref]
- Lodha SC, Lohiya ML, Vyas MC, Bhandari S, Goyal RR, et al. (1991) Role of phenytoin in healing of large abscess cavities. Br J Surg 78: 105-108. [Crossref]
- Chiosi F, Keppel Hesselink J, Rinaldi M, Di Staso S, Bartollino S, et al. (2018) Phenytoin: its potential as neuroprotective and retinoprotective drug. Br J Clin Pharmacol 84: 195-196. [Crossref]
- Keppel Hesselink JM (2018) How to Define Neuroprotection: The Case of Phenytoin. Open Access J Neurol Neurosurg 7: 555719.
- Keppel Hesselink JM, Notermans NC (2018) Topical phenytoin formulations for pain in small fiber neuropathy, a pathogenetic approach. Gen Int Med Clin Innov 3: 1-4.
- Uçeyler N, Kafke W, Riediger N, He L, Necula G (2010) Elevated proinflammatory cytokine expression in affected skin in small fiber neuropathy. Neurology 74: 1806-1813. [Crossref]
- Lopate G, Streif E, Harms M, Weihl C, Pestronk A (2013) Cramps and small-fiber neuropathy. Muscle Nerve 48: 252-255. [Crossref]
2021 Copyright OAT. All rights reserv
- Keppel Hesselink JM (2018) Topical NaV1.7 channel blocker failed in postherpetic Neuralgia (PHN): lessons to learn. Gen Med Open 2: 1-2.
- Keppel Hesselink JM (2018) Topical phenytoin in painful diabetic neuropathy: rationale to select a non-selective sodium channel blocker. Clinical research in Neurology 1: 1-5.
- Kopsky DJ, Keppel Hesselink JM (2018) Phenytoin Cream for the Treatment for Neuropathic Pain: Case Series. Pharmaceuticals (Basel) 11. [Crossref]

