Abstract
Periodontal disease is a complex inflammatory disease characterized by loss of the supporting structures cause of the elicited response of the microbial accumulation on the tooth surfaces. To arrest the disease progression and regenerate the lost tissue structures are the definitive goal of periodontal therapy. Platelets play an important role in periodontal regeneration as they are rich in growth factors (PDGF, VEGF, and TNF-β) and leukocytes (95%). L-PRF has a strong fibrin architecture and superior mechanical properties which distinguishes it from other kinds of platelet concentrates. PRF is autologous platelet concentrate obtained after processing a whole blood sample through a process called centrifugation, which is a cost-effective and easy to handle procedure. The literature suggests the potential and the benefits of the role of L-PRF in periodontal regeneration.
Key words
platelet,PRF, PRP, regeneration
Introduction
Periodontitis is a complex inflammatory disorder characterized by loss of connective tissue attachment and destruction of alveolar bone, root cementum, periodontal ligament and gingiva as a response to insults stimulated by microbial accumulations of tooth surfaces. This leads to the initiation of intraosseous defects [1]. The definitive goal of periodontal therapy includes arresting of periodontal disease progression and the regeneration of structures lost due to the pre-existing disease process.
Periodontal regeneration refers to the complete restoration of functional and supporting tissues, hence defined as the reproduction or reconstruction of loss or injured part with form and function of loss structures restored .Periodontal regeneration is a complex procedure including the biologic events like cell adhesion, migration , proliferation , and differentiation in an planned sequence[1].Periodontal regenerative procedures include soft tissue grafts, bone grafts, root biomodifications, guided tissue regeneration, and combinations of these procedures[2]. The current perspective is that regenerative periodontal therapies to date can only restore a fraction of the original tissue volume [2] and have a limited potential in attaining complete periodontal restoration [3].
An arrangement of interaction is required between the epithelial cells, gingival fibroblast, periodontal ligament cells and osteoblasts. The disruption of vasculature during wound healing leads to fibrin formation, platelet aggregation, and release of several growth factors into tissues from platelets, [4] through molecular signals which are predominantly mediated via the cytokines and growth factors. It has been evident that the presence of growth factors and cytokines play an important role in inflammation and wound healing. Platelets also secrete fibrin, fibronectin, and vitronectin, which act as a matrix for the connective tissue and as adhesion molecules for more efficient cell migration [5]. This lead to the concept of using platelets as therapeutic tools to improve tissue repair and wound healing.
The rich sources of autologous growth factors are the various generations of platelet concentrates that include Platelet Rich Plasma (PRP), the first generation concentrate used in combination with grafting materials and barrier membrane in managing periodontal defects. But the effects of Platelet rich plasma on bone regeneration have been inadequate. The second generation of platelet concentrates is Platelet Rich Fibrin (PRF) which is considered to be the latest. It is an autologous leukocyte platelet concentrate which is successfully used in various fields of dentistry and medicine. It shows efficacious effects when used in the treatment of periodontal intrabony defect.
Platelets
Platelets are un-nucleated fragments of bone marrow megakaryocytes which circulate in blood for 8-10 days [6]. Platelets adhere together to form a platelet plug in a severed vessel and hence thought to contribute to the hemostatic process where it has been seen they actively extrude several initiators for the activation of the coagulation cascade.
In1974, Ross et al introduced the regenerative potential of platelets by discussing their role in wound healing. The alpha granules of platelets contain various mitogenic factors such as Platelet derived growth factor (PDGF), vascular endothelial growth factor (VEGF) and transforming growth factor –β (TGF-β) [7]. These growth factors are vital for initial wound healing.
Platelet concentrates
Fibrin is the activated form of a plasmatic molecule called fibrinogen [8]. This soluble fibrillary molecule is massively present both in plasma and in the platelet a-granules and plays a determining role in platelet aggregation during hemostasis. It is transformed into a kind of biologic glue capable of consolidating the initial platelet cluster, thus constituting a protective wall along vascular breaches during coagulation. In fact, fibrinogen is the final substrate of all coagulation reactions. Being a soluble protein, fibrinogen is transformed into an insoluble fibrin by thrombin while the polymerized fibrin gel constitutes the first cicatricial matrix of the injured site [9-11] in surgical management of hemostasis it is well documented the application of fibrin glue in early 1900. Thus, in the former times fibrin adhesives paved a way for the present-day platelet concentrates. PCs are blood extracts, obtained after processing a whole blood sample, mostly through centrifugation (Dohan et al. 2014a) [12]. The two emerging platelet concentrates are platelet rich plasma (PRP) and platelet rich fibrin (PRF). They are fibrin matrices which are enmeshed with morphogenic proteins (growth factors) and leucocytes.
Fabbro et al [13] summarized the ideal role of platelet concentrates as:
1. Augmentation of tissue healing: By increased proliferation of connective tissue progenitors that stimulate fibroblast and osteoblast activity and enhance osteogenesis [14].
2. Anti-microbial activity: Against bacterial species involved in oral infections [15, 16].
3. Modification of host defense mechanism: By delivery of signaling peptides that attract macrophage cells [17].
4. Modification of immune reaction: By releasing leukocytes that synthesize interleukins [6].
Platelet rich plasma
The 1st generation of platelet concentrate consisted of limited volume of plasma which was enriched with platelets obtained from the patient’s own blood which was called platelet rich plasma (PRP). If a normal human blot clot contains 5% platelets according to Sunitha et al [18] PRP blood clot contains 95% platelets. PRP is known to contain growth factors such as PDGF and TGF –β that stimulus the regenerative process [19,20,21]. Creeper et al conducted in-vitro studies that reported proliferation of PDL and osteoblastic cells under the influence of PRP. PRP contains growth factors and their release in wound site tends to be rapid and for a short duration of time. Also, complex production protocol involving use of bovine thrombin and other biochemical agents has limited the benefits of platelet rich plasma [22].
The technical and regenerative limitations of platelet rich plasma led to the discovery of a better, completely autologous fibrin matrix called Platelet Rich Fibrin.
Preparation of platelet rich plasma (PRP):
Technique:
Many different protocols can be applied to the cPRP concept. But we can schematically divide them into 2 families: complex techniques using hematology cell separators, and simplified techniques with ready-to-use commercially available kits and 2-step centrifugation to concentrate platelets. These commercial systems are being increasingly automated to simplify clinical use. Therefore, we will describe a general concept rather than any one particular system [23]:
a) Venous blood is taken with anticoagulant (sodium citrate 3.8%) to avoid platelet activation and degranulation.
b) The first centrifugation (‘‘soft spin’’) at 1200 rpm for 10 minutes, allows the blood separation in 3 distinct layers (Figure 1): At the bottom of the tube, the red blood corpuscles constitute 55% of total volume At the top of the tube, the acellular plasma layer is mainly made up of circulating plasmatic molecules (in particular, fibrinogen) and low in platelets. It is designated platelet-poor plasma (PPP) and constitutes 40% of total volume. Between the 2, an intermediate layer is where platelets concentrations are largely increased. It constitutes only 5% of total volume and presents a characteristic buffy aspect that led to it being called ‘‘buffy coat.’’ It will compose the major part of the future cPRP, but at this stage, there is still no easy scientific process allowing its separation from the other layers.

Figure 1. Technologic concept of cPRP processing
c) Using a sterile syringe, the practitioner aspirates PPP, PRP, and some red blood corpuscles (which are systematically attracted during the operation). Then the material is transferred to another tube, without anticoagulant.
d) This second tube will then undergo another centrifugation at 2400 rpm for 10 minutes , purported to be longer and faster than the first (‘‘hard spin’’). This makes it possible to concentrate platelets at the bottom of the tube and subsequently to obtain once again 3 distinct layers (Figure. 1): some residual red blood corpuscles trapped at the bottom of the tube acellular plasma (PPP) for 80% of total volume between the 2, a buffy layer, or PRP
e) At this stage, it becomes easy to collect the PRP. With a syringe, the practitioner can discard the major part of the PPP, leaving just enough serum to place the concentrated platelets in suspension. The unit is then gently shaken to obtain a ready-to-use cPRP. Note that the red blood corpuscles trapped at the bottom of the tube are also suspended by this last operation, which explains the rosy aspect of the final cPRP.
f) cPRP is then mixed with bovine thrombin and calcium chloride at the time of application, with the help of a mixing syringe. Gelling of platelet concentrate will then quickly occur: Fibrinogen is also concentrated during the cPRP preparation, and its polymerization will constitute a fibrin matrix with particularly interesting hemostatic and adhesive properties. Moreover, cPRP application can be accomplished in gel or spray form (according to the syringe nozzle used). In both cases, fibrin polymerization is completed in a few minutes. Note that to obtain a denser gel, or even a cPRP membrane, it is possible to add Tisseel to the mixture [23].
Platelet rich fibrin (PRF):
PRF (platelet rich fibrin) was first developed in France for use in the field of oral and maxillofacial surgery [24]. Platelet rich Fibrin (PRF) preparation protocol was developed by Choukroun et al [25]. Choukroun’s platelet-rich fibrin (PRF) is a leukocyte and platelet rich fibrin biomaterial with a specific composition and three-dimensional architecture [26]. PRF is classified as a second-generation platelet concentrate as it is prepared as a natural concentrate without the addition of any anticoagulants [27, 28]. PRF is frequently called has Choukroun’s PRF. Leucocytes which are concentrated in PRF play an important role in growth factor release, immune regulation and matrix remodeling in wound healing, the physiologic architecture of wound healing was created by a slow polymerization mode and cicatricial capacity of the PRF.
Procedure for (platelet rich fibrin) prf preparation:
The classical technique for PRF preparation was invented by Dr. Choukroun in 2000 [22]. It is the current PRF technique authorized by the French Health Ministry in which PRF is prepared without using an anticoagulant during blood harvesting or bovine thrombin during gelling [29]. A standard protocol for PRF preparation should be followed to obtain proper quantity and quality of the fibrin matrix, leukocytes, platelets, and growth factors.
a. The equipment required for PRF preparation includes a PC-O2 centrifuge and a blood collection kit consisting of a 24 gauge butterfly needle and 9 ml blood collection tubes.
b. A sample of blood is collected from patient without anticoagulant in 10 ml tubes which are immediately centrifuged at a rate of 3000 rpm for 10 min.
c. During the centrifugation process, when the blood gets in contact with the test tube wall the platelet gets activated leading to the initiation of coagulation cascade.
The most important parameter for the success of this procedure is the duration of time between the blood collection and centrifugation process. There is diffuse polymerization of fibrin if there is slow handling of blood to the centrifugation process which in turn leads to the formation of small blood clots of irregular consistency.
Figure 2 and Figure 3

Figure 2. Blood centrifugation immediately after collection allows the composition of a structured and resistant fibrin clot in the middle of the tube, just between the red corpuscles at the bottom and acellular plasma at the top.
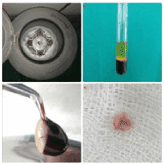
Figure 3. Blood processing with a PC-O2 centrifuge for PRF (A; Process, Nice, France) allows the composition of a structured fibrin clot in the middle of the tube, just between the red corpuscles at the bottom and acellular plasma at the top. After collection of the PRF itself, resistant autologous fibrin membranes are easily obtained by driving out the serum from the clot. After centrifugation, the resultant product consists of three layers. The topmost layer consisting of acellular PPP (platelet poor plasma), PRF clot in the middle and RBCs at the bottom of the test tube (B). The fibrin clot obtained after centrifugation is removed from the tube and the attached red blood cells scraped off from it and discarded (C). PRF can also be prepared in the form of a membrane by squeezing out the fluids present in the fibrin clot (D) [30].
Advantages of prf:
The various advantages of PRF include (Dohan et al.) [31,32,33,34]
1. Completely Autogenous.
2. Extended growth factor release for 7 days.
3. Simple and faster technique.
4. In-expensive.
5. No requirement of any additive constituent such as bovine thrombin.
6. No biochemical handling involved.
7. No associated immune reactions.
8. No associated infections.
9. Acts as an ‘immune regulation node’.
10. Has anti- inflammatory effects.
Significance:
Platelet rich fibrin allows continuous release of growth factors for over 300 minutes following its preparation a study done by Su et al in 2009[35], hence it must it immediately used after preparing. The progressive release of cytokines and leukocytes continues for a period of 7-11 days, as the fibrin network disintegrates [36].
Drawbacks of prf:
1) Main drawback is preparation and storage.
2) Time interval between speed of handling, blood collection and centrifugation as PRF is prepared without any addition anticoagulants.
3) The disadvantage of PRF is its storage after preparation also PRF membranes should be used immediately after preparation as it will shrink resulting in dehydration altering the structural integrity of PRF.
4) The risk of bacterial contamination of the PRF membrane can occur if stored in the refrigerator.
5) Dehydration also results in the decreased growth factor content in PRF and leukocyte viability will be adversely affected altering its biologic properties [37].
Limitations:
1) Connell et al in 2007 raised a concern regarding the safety issue of PRF methodology, he commented on the types of tubes used to produce PRF and the possible hazards of silica containing glass tubes [38].
2) However, Dohan et al. [39] in the same year conducted a cytotoxicity analysis of PRF on wide range of human cells and concluded that silica microparticles coating these tubes are not cytotoxic for the tested human cells. They also reported improved mitotic proliferation and suggested that contact with silica is necessary to start the polymerization process as silica behaves as clot activator. Thus, to produce PRF either dried glass tubes or glass coated plastic tubes must be used.
PRF vs. PRP:
According to Mosesson et al. who described the structural and biological features of fibrinogen and fibrin in detail, the 3-dimensional organization of fibrin network depends on activation mechanism.
1) In PRP, bilateral junctions are constituted with strong thrombin concentrations and allow the thickening of fibrin polymers; this leads to the constitution of a rigid network, not very favorable to cytokine enmeshment and cellular migration.
2) In PRF, weak thrombin concentrations imply a very significant percentage of equilateral junctions. These connected junctions allow the establishment of a fine and flexible fibrin network able to support cytokines enmeshment and cellular migration.
Moreover, this 3-dimensional organization will give great elasticity to the fibrin matrix: It is what we observe in a flexible, elastic, and very strong PRF membrane. These 3 fibrin biotechnologies therefore use different polymerization modes which imply very different biologic integration mechanisms [5].
Role of platelet rich fibrin in regeneration:
2021 Copyright OAT. All rights reserv
It enhances both soft and hard tissue healing cause of its advantages over PRP which includes ease of handling, preparation and minimal expense with the lack of biomechanical modification i.e. no anticoagulants required which reduces biomechanical handling of blood and risk associated with the use of bovine-thrombin. PRF is enriched with platelets, growth factors (PDGF, TGF- β, and VEGF) and cytokines. PDGF plays a role in regulation, migration, proliferation along with mitogenic potential ,promotes angiogenesis, TGF- β which is an inflammatory regulator ,produces new capillaries by activation of endothelial cells ,VEGF it is a most powerful vascular growth factor it initiates angiogenesis and the role of leukocyte is to stimulate the migration of neutrophils, acts as an immune regulation node has they contain all key immune cytokines like IL-1β,IL-6,IL-4 and TNF they have the aptitude to control inflammatory response at the wound site.
PRF is considered as a healing biomaterial and is commonly used in implant and plastic periodontal surgery procedures to enhance bone regeneration and soft-tissue wound healing Choukroun et al used PRF firstly in implant surgery to boost the healing properties of bone [40, 41].Diss et al. in a 1 year prospective study on osteotome sinus floor elevation using Choukroun’s platelet-rich fibrin grafting material clearly demonstrated that fibrin matrix of PRF directly promotes angiogenesis [42]. Sanchez et al. in an experimental study compared the influence of PRP and PRF on proliferation and differentiation of osteoblasts and he reported that the affinity of osteoblasts to the PRF membrane appeared to be superior than the affinity of osteoblasts to PRP [43].
Sharma et al. conducted a randomized controlled clinical trial for the treatment of 3-wall intrabony defects in chronic periodontitis patients with platelet rich fibrin and reported a statistically significant improvement in pocket depth reduction and bone fill in test group than in controls [44]. Thorat et al. investigated the clinical and radiological effectiveness of autologous PRF in the treatment of intrabony defects of chronic periodontitis patients and reported a greater reduction in pocket depth, more gain in clinical attachment level and greater intrabony defect fill at sites treated with PRF than those treated with open flap debridement alone [45]. A comparative evaluation between platelet-rich fibrin and platelet-rich plasma for the treatment of three-wall intrabony defects was done and showed a greater bone fill in PRF treated group than in PRP treated group [46].
Conclusion
In this review, it has been shown that Choukroun PRF has an excellent regenerative potential, is simple and a cost - effective technique with several advantages over PRP with regard to favorable effects on hard and soft tissue healing. Correct handling of L-PRF is crucial to obtain benefits from this technique.
Conflict of interest
The authors report no conflict of interest
Sources of funding
Nil
References
- [No authors listed] (1996) The potential role of growth and differentiation factors in periodontal regeneration.J Periodontol67: 545-553. [Crossref]
- Greenwell H; Committee on Research, Science and Therapy American Academy of Periodontology. (2001) Position paper: Guidelines for periodontal therapy.J Periodontol72: 1624-1628. [Crossref]
- Sander L, Karring T (1995) Healing of periodontal lesions in monkeys following the guided tissue regeneration procedure. A histological study. J Clin Periodontol 22:332–7.
- Deodhar AK, Rana RE (1997) Surgical physiology of wound healing: a review.J Postgrad Med43: 52-56. [Crossref]
- Dohan DM, Choukroun J, Diss A, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate, part I: technological concept and evolution. Oral Surg Oral Med Oral Path Oral Radiol Endod 101: E37–44.
- Dohan DM, Choukroun J, Diss A, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod b; 101: e45-50.
- Ross R, Glomset J, Kariya B, Harker L. (1974) A platelet-dependent serum factor that stimulates the proliferation of arterial smooth muscle cells in vitro. Proc Natl Acad Sci USA 71: 1207-10.
- Mosesson MW1, Siebenlist KR, Meh DA (2001) The structure and biological features of fibrinogen and fibrin.Ann N Y Acad Sci936: 11-30. [Crossref]
- Clark RA (2001) Fibrin and wound healing.Ann N Y Acad Sci936: 355-367. [Crossref]
- Collen A, Koolwijk P, Kroon M, van Hinsbergh VW. Influence of fibrin structure on the formation and maintenance of capillarylike tubules by human microvascular endothelial cells. Angiogenesis 1998; 2:153-65.
- van Hinsbergh VW, Collen A, Koolwijk P (2001) Role of fibrin matrix in angiogenesis.Ann N Y Acad Sci936: 426-437. [Crossref]
- Dohan Ehrenfest DM, Andia I, Zumstein MA, Zhang CQ, Pinto NR, et al. (2014a) Classification of platelet concentrates (Platelet-Rich Plasma-PRP, Platelet-Rich Fibrin-PRF) for topical and infiltrative use in orthopedic and sports medicine: current consensus, clinical implications and perspectives. Muscles Ligaments Tendons J. 4, 3–9. [Crossref]
- Del Fabbro M, Bortolin M, Taschieri S, Weinstein R (2011) Is platelet concentrate advantageous for the surgical treatment of periodontal diseases? A systematic review and meta-analysis. J Periodontol 82: 1100-11. [Crossref]
- Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, et al. (1998) Platelet-rich plasma: Growth factor enhancement for bone grafts.Oral Surg Oral Med Oral Pathol Oral Radiol Endod85: 638-646. [Crossref]
- Tang YQ, Yeaman MR, Selsted ME (2002) Antimicrobial peptides from human platelets.Infect Immun70: 6524-6533. [Crossref]
- Lindeboom JA, Mathura KR, Aartman IH, Kroon FH, Milstein DM, et al. (2007) Influence of the application of platelet-enriched plasma in oral mucosal wound healing.Clin Oral Implants Res18: 133-139. [Crossref]
- Choukroun J, Diss A, Simonpieri A, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101: e56-60.
- Sunitha Raja V, Munirathnam Naidu E (2008) Platelet-rich fibrin: evolution of a second-generation platelet concentrate. Indian J Dent Res 19: 42-6.
- Liu, Z., Yuan X, Fernandes G, Dziak R, Ionita C, Li C, Wang C, Yang S (2017) The combination of nano-calcium sulfate/platelet rich plasma gel scaffold with BMP2 gene-modified mesenchymal stem cells promotes bone regeneration in rat critical-sized calvarial defects. Stem Cell Res Ther, 8: p. 122.
- Fernandes G, Yang S (2016) Application of platelet-rich plasma with stem cells in bone and periodontal tissue engineering.Bone Res4: 16036. [Crossref]
- Fernandes, G, Wang C, Yuan X, Liu Z, Dziak R, Yang S (2016) Combination of Controlled Release Platelet-Rich Plasma Alginate Beads and Bone Morphogenetic Protein-2 Genetically Modified Mesenchymal Stem Cells for Bone Regeneration. J Periodontol, 87: p. 470-80.
- Sánchez AR, Sheridan PJ, Kupp LI (2003) Is platelet-rich plasma the perfect enhancement factor? A current review.Int J Oral Maxillofac Implants18: 93-103. [Crossref]
- Sonnleitner D, Huemer P, Sullivan DY (2000) A simplified technique for producing platelet-rich plasma and platelet concentrate for intraoral bone grafting techniques: a technical note.Int J Oral Maxillofac Implants15: 879-882. [Crossref]
- Sonnleitner D, Huemer P, Sullivan DY (2000) A simplified technique for producing platelet-rich plasma and platelet concentrate for intraoral bone grafting techniques: a technical note.Int J Oral Maxillofac Implants15: 879-882. [Crossref]
- Choukroun J, Adda F, Schoeffer C, Vervelle A (2000) PRF: an opportunity in perio-implantology. Implantodontie 42:55–62.
- Choukroun J, Adda F, Schoeffler C, Vervelle A. (2001) A opportunite’ in paroimplantology: the PRF. Implantodont 42: 55-62.
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T (2009) Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF).Trends Biotechnol27: 158-167. [Crossref]
- Dohan Ehrenfest DM, Rasmusson L, Albrektsson T (2009) Classification of platelet concentrates: from pure platelet-rich plasma (P-PRP) to leucocyte- and platelet-rich fibrin (L-PRF).Trends Biotechnol27: 158-167. [Crossref]
- Bowers GM, Chadroff B, Carnevale R, Mellonig J, Corio R, Emerson J, et al. (1989) Histologic evaluation of new attachment apparatus in humans. Part II.. J Periodontol 60:676–82.
- Cortellini P, Bowers GM (1995) Periodontal regeneration of intrabony defects: an evidence-based treatment approach. Int J Periodontics Restorative Dent 15:128–45.
- Dohan DM, Choukroun J, Diss A, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate, part I: techno- logical concept and evolution. Oral Surg Oral Med Oral Path Oral Radiol Endod 101:E37–44.
- Preeja chanuran and arun Sivadas (2013) platelet rich fibrin: its role in periodontal regeneration :review article.the Saudi international journal for dental research 2013
- Dohan DM, Choukroun J, Diss A, et al. (2006 b) Platelet-rich fibrin (PRF): a second-generation platelet concentrates. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101: e45-50.
- Dohan DM, Choukroun J, Diss A, et al. (2006 b) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part II: platelet-related biologic features. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101(3): e45-50.
- Dohan DM, Choukroun J, Diss A, et al. (2006 c) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part III: leucocyte activation: a new feature for platelet concentrates? Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101: e51-5.
- Dohan DM, Choukroun J, Diss A, Dohan SL, Dohan AJ, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part I: technological concepts and evolution.Oral Surg Oral Med Oral Pathol Oral Radiol Endod101: e37-44. [Crossref]
- Su CY, Kuo YP, Tseng YH, Su CH, Burnouf T (2009) In vitro release of growth factors from platelet-rich fibrin (PRF): a proposal to optimize the clinical applications of PRF. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 108: 56-61.
- Simonpieri A, Del Corso M, Sammartino G, Dohan Ehrenfest DM (2009) The relevance of Choukroun’s platelet-rich fibrin and metronidazole during complex maxillary rehabilitations using bone allograft. Part I: a new grafting protocol. Implant Dent 18: 102-11.
- Dohan Ehrenfest DM, de Peppo GM, Doglioli P, Sammartino G (2009) Slow release of growth factors and thrombospondin-1 in Choukroun's platelet-rich fibrin (PRF): a gold standard to achieve for all surgical platelet concentrates technologies.Growth Factors27: 63-69. [Crossref]
- O'Connell SM (2007) Safety issues associated with platelet-rich fibrin method.Oral Surg Oral Med Oral Pathol Oral Radiol Endod103: 587. [Crossref]
- Dohan DM, Del Corso M, Charrier JB (2007) Cytotoxicity analyses of Choukroun’s PRF (Platelet Rich Fibrin) on a wide range of human cells: the answer to a commercial controversy. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 103: 587-93.
- Choukroun J, Diss A, Simonpieri A, Girard MO, Schoeffler C, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate. Part IV: clinical effects on tissue healing.Oral Surg Oral Med Oral Pathol Oral Radiol Endod101: e56-60. [Crossref]
- Choukroun J, Diss A, Simonpieri A, et al. (2006) Platelet-rich fibrin (PRF): a second-generation platelet concentrate, part V: histologic evaluations of PRF effects on bone allograft maturation in sinus lift. Oral Surg Oral Med Oral Pathol Oral Radiol Endod 101:299–303.
- Diss A, Dohan DM, Mouhyi J, Mahler P (2008) Osteotome sinus floor elevation using Choukroun's platelet-rich fibrin as grafting material: a 1-year prospective pilot study with microthreaded implants.Oral Surg Oral Med Oral Pathol Oral Radiol Endod105: 572-579. [Crossref]
- Sanchez AR, Sheridan PJ, Kupp LI (2003) Is platelet-rich plasma the perfect enhancement factor? A current review. Int J Oral Maxillofac Implants 18:93–103.
- Sharma A, Pradeep AR (2011) Treatment of 3-wall intrabony defects in patients with chronic periodontitis with autologous platelet-rich fibrin: a randomized controlled clinical trial.J Periodontol82: 1705-1712. [Crossref]
- Thorat M, Pradeep AR, Pallavi B (2011) Clinical effect of autologous platelet-rich fibrin in the treatment of intra-bony defects: a controlled clinical trial. J Clin Periodontol 38:925–32.
- Pradeep AR, Rao NS, Agarwal E, Bajaj P (2012) Comparative evaluation of autologous platelet-rich fibrin and platelet-rich plasma in the treatment of three-wall intrabony defects in chronic periodontitis: a randomized controlled clinical trial. J Periodontol 2012.



