Introduction
For the past 5 decades the NBRL, Boston, MA has reported data to document the therapeutic effectiveness and safety of frozen red blood cells, frozen platelets and frozen plasma to treat patients with nonsurgical blood loss. Universal donor group O Rh positive and group O Rh negative red blood cells frozen with 40% W/V glycerol can be stored at -80 C in a mechanical freezer for at least 10 years, thawed, deglycerolized in the Haemonetics Blood Processor ACP215, and the red blood cells stored at 4 C in the additive solution AS-3 (Nutricel) for 2 weeks with 24 hour post-thaw survival of 75%, moderate increase in red blood cell affinity for oxygen, and with less than 1% hemolysis.
Group O leukoreduced apheresed 2.5 to 3.0 X 1011 platelets can be treated with 6% DMSO, the supernatant DMSO removed by centrifugation at 1250 X g for 10 minutes, frozen at 2 to 3 C per minute in a -80 C mechanical freezer for at least 2 years, thawed, diluted with 10 to 20 ml of 0.9% sodium chloride and stored at room temperature for 6 hours without agitation, have in vitro recovery of 90% of the platelets, with 5 to 8% platelet microparticles, in vivo recovery of platelets 1 to 2 hours after transfusion of 25 to 30% and normal lifespan of 7 days; group AB plasma can be frozen at -80 C for at least 10 years, thawed and stored at 4 C for 24 hours with acceptable preservation of plasma clotting proteins.
The Netherlands military has reported on the treatment of trauma patients requiring more than 10 units of red blood cells in a 24-hour period who were resuscitated with group O Rh positive and group O Rh negative frozen RBC, group O Rh positive and group O Rh negative frozen platelets, and group AB frozen plasma with excellent clinical outcomes, and no adverse effects for the past 18 years. The frozen platelets contain a bimodal population of platelets one population that exerts a hemostatic effect immediately following transfusion and a population of platelets that circulate to increase the platelet count in the recipient. We have reported that the bleeding time correlated with the volume of shed blood collected at the bleeding time site (Figure 1) [1].
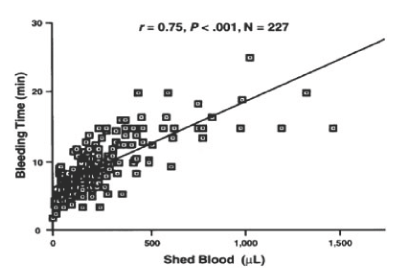
Figure 1. The relation of bleeding time to shed blood for all patients. Reprinted, with permission, from Am J Clin Pathol 1997;108:579-584
The most important observation made in our collaboration with Dr. Shukri Khuri at the West Roxbury VA Hospital between 1982 to 2008 was that the bleeding time correlated with the nonsurgical blood loss in anemic and thrombocytopenic patients (Figure 2) [1].
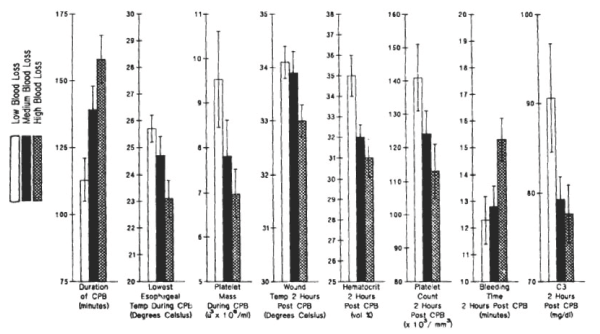
Figure 2. Preoperative, intraoperative, and postoperative variables in tierciles of blood loss during initial 4 hours after CPB. Tiercile levels designated were low: 215 to 790 ml (n=26); medium: 805 to 1140 ml (n=26) and high: 1235 to 2515 ml (n=26). Reprinted, with permission, from J Thorac Cardiovasc Surg 1992;104:94-107
NBRL has reported that the bleeding time is affected by temperature, the hematocrit, the platelet count, the platelet size, the platelet function, and the plasma clotting proteins: von Willebrand factor (vWF), factor VIII, and fibrinogen levels [1].
In 1968, the NBRL published a protocol to produce an increase in the bleeding time in male volunteers treated with 650 mg of aspirin to assess the hemostatic function of transfused platelets. The aspirin treated male volunteers produces a platelet dysfunction which increased the bleeding time and affected the platelet response to aggregation agonists in the aspirin treated volunteers. J.R. O’Brien has reported that about 10 percent normal non-aspirinated platelets corrected the aggregation patterns in vitro of aspirin treated platelets [2]. Allogeneic fresh platelets and autologous liquid preserved platelets stored at 22 C for 24 hours and 48 hours were transfused to the aspirin treated male volunteers to increase the platelet count by 15% with donor platelets to measure the reduction in the aspirin prolonged bleeding time and the correction of platelet aggregation patterns. Figures 3 and 4 report the bleeding times in aspirin treated male volunteers transfused with fresh and liquid preserved platelets stored at room temperature for 24 hours and 48 hours to increase the recipient platelet count with 15% donor platelets [3].
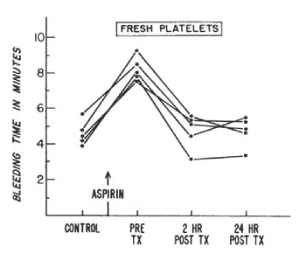
Figure 3. Bleeding times of five normal volunteers, before and after administration of 650 ml of aspirin and after transfusion of fresh allogeneic platelet concentrates (TX indicates transfusion). Bleeding time was determined by a modification of the ivy technique. Reprinted, with permission, from N Engl J Med 1971;285:538-543
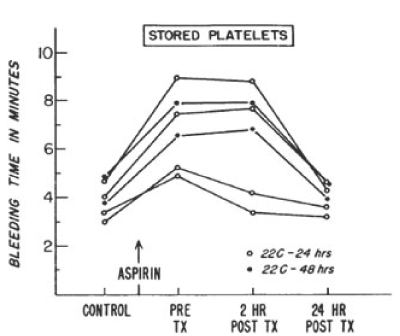
Figure 4. Serial bleeding times of six volunteers before and after administration of 650 mg of aspirin and after transfusion of autologous platelet concentrates stored for 24 to 48 hours at 22°C. Reprinted, with permission, from N Engl J Med 1971;285:538-543
Figures 5 to 9 report the bleeding time in aspirin treated male volunteers transfused with a single unit of fresh platelets and platelets preserved in the liquid state at 4 C for 24 hours and 22 C for 24 hours and in the frozen state at -80 C and at -150 C for 24 hours [4].
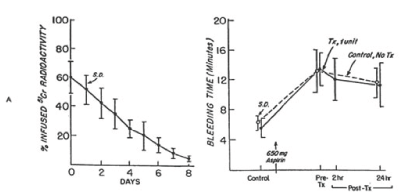
Figure 5. 51Cr survival values of allogeneic fresh platelets stored at 22°Cfor less than four hours (A) and bleeding time (B) before and after 650 mg of aspirin given to eight volunteers on two separate occasions. One unit of platelets was transfused 24 hours after the first aspirin treatment. On the other occasion no transfusion was given. The mean (±SD) recovery for the eight studies was 62.2 ±10 percent in vitro and 60.2 ± 11 percent in vivo. The mean (± SD) in vivo recovery of the original platelets was 37.0 ± 8 percent. There was no significant difference between bleeding times of subjects before (paired t-test = 0.404; p>0.05) and 24 hours after (t-test = 0.253; p>0.05) transfusion and those of subjects not given transfusion. Reprinted, with permission, from N Engl J Med 1974;290:353-358
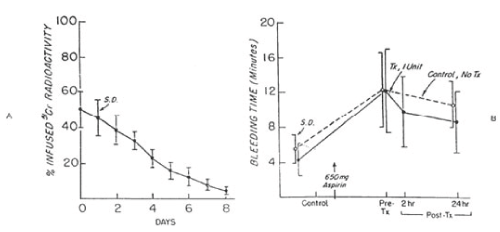
Figure 6. 51Cr survival values of autologous fresh platelets stored at 22°C for twenty-four hours (A) and bleeding time (B) before and after 650 mg of aspirin given to six volunteers on two separate occasions. One unit of platelets was transfused 24 hours after the first aspirin treatment. On the other occasion no transfusion was administered. The mean (±SD) recovery for the six studies was 60.3 ± 7.6 percent in vitro and 50.5 ± 10 percent in vivo. The mean (± SD) in vivo recovery of the original platelets was 31.0 ± 7.0 percent. There was no significant difference between bleeding times of subjects before (paired t-test = 0.226; p>0.05) and 24 hours after (t-test = 1.610; p>0.05) transfusion and those given no transfusion. Reprinted, with permission, from N Engl J Med 1974;290:353-358)
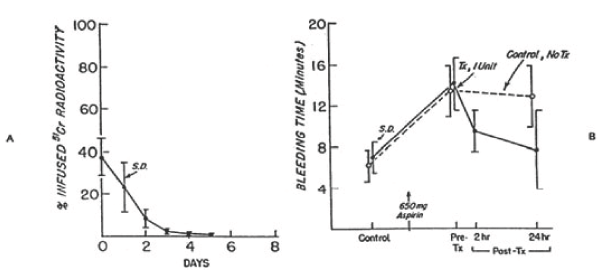
Figure 7. 51Cr survival values of autologous platelets stored at 4°C for 24 hours (A) and bleeding times (B) before and after 650 mg of aspirin given to six volunteers on two separate occasions. One unit of platelets was transfused 24 hours after the first aspirin treatment. On the other occasion no transfusion was given. The mean (±SD) recovery for the six studies was 67.0 ±10.6 percent in vitro and 37.5 ± 8.5 percent in vivo. The mean (± SD) in vivo recovery of the original platelets was 25.0 ± 8.0 percent. There was no significant difference between bleeding times of subjects before (paired t-test = 0.693; p>0.05) transfusion, but the difference between subjects transfused and those not transfused was significant (paired t-test = 2.920; p<0.05) at 24 hours. Reprinted, with permission, from N Engl J Med 1974; 290:353-358
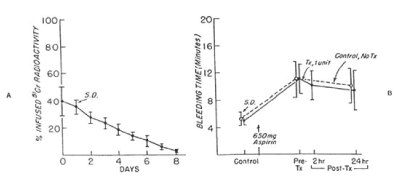
Figure 8. 51Cr survival values of washed, autologous platelet concentrate stored with 5 percent dimethylsulfoxide at -150°C for 24 hours (A) and bleeding times (B) before and after 650 mg of aspirin given to 10 volunteers on two separate occasions. One unit of platelet concentrate was transfused 24 hours after the first aspirin treatment. On the other occasion no transfusion was given. The mean (±SD) recovery for 10 studies was 62.7 ± 9.2 percent in vitro and the mean (±SD) freeze-thaw-wash 69.7 ± 14 percent in vivo. The mean (± SD) recovery was 43.0 ± 10.0 percent in vitro and 40.0 ± 9.5 percent in vivo. The mean (± SD) recovery of the original platelets was 17.5 ± 6.0 percent. There was no significant correlation between bleeding times of subjects before (paired t-test = 1.175; p>0.05) transfusion, and 24 hours (t-test = 1.809, p>0.05) transfusion and those of subjects not given transfusions. Reprinted, with permission, from N Engl J Med 1974; 290:353-358
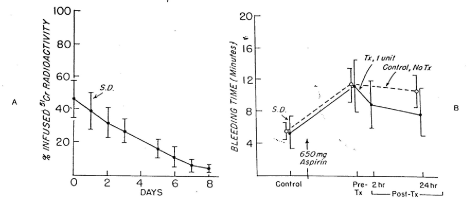
Figure 9. 51Cr Survival values of washed, autologous platelet concentrate stored with 6 per cent dimethyl sulfoxide at -80°C for 24 hours (A) and bleeding times (B) before and after 650 mg of aspirin given to 12 volunteers on two separate occasions.
Tables 1 and 2 and Figure 10 report the bleeding time and platelet aggregation patterns in nine (9) aspirin treated male volunteers transfused with a single unit of autologous platelets frozen with 5% DMSO at 2 to 3 C per minute, stored at -80 C for as long as 8 months, thawed, washed and resuspended in autologous plasma in Spector JI et al, [5].
Table 1. Bleeding times in minutes measured in normal volunteers treated with aspirin alone and normal volunteers treated with aspirin and transfused 24 hours after aspirin ingestion with a single unit of autologous frozen washed platelets. Reprinted, with permission, from Transfusion 1977;17:8-15
|
Baseline |
24hours after ASA |
48 Hours after ASA |
Controls |
x̅ |
5.43 |
11.56 |
10.57 |
S.D. |
1.42 |
3.12 |
2.78 |
S.E. |
0.20 |
0.44 |
0.39 |
n |
51 |
51 |
51 |
Transfusion of 4% DMSO Platelet Concentrates |
x̅ |
4.63 |
11.13 |
8.67 |
S.D. |
1.42 |
3.07 |
3.76 |
S.E. |
0.58 |
1.25 |
1.53 |
n |
|
|
|
Transfusion of 5% DMSO Platelet Concentrates |
x̅ |
3.08 |
9.11 |
6.22 |
S.D. |
0.64 |
1.97 |
2.65 |
S.E. |
0.21 |
0.66 |
0.88 |
n |
9 |
9 |
9 |
Table 2. Changes in platelet aggregation patterns after aspirin ingestion and freeze-preserved platelet transfusion in normal volunteers*. Reprinted, with permission, from Transfusion 1977;17:8-15
|
|
|
Post Transfusion |
|
Aggregating Agent |
Baseline |
Post Aspirin |
2Hrs |
4Hrs |
24Hrs |
Freeze-Thawed-washed Platelets Frozen with 4or 5 Percent DMSO |
Adenosine diphosphate 25 µM |
73 ± 4 |
66 ± 17 |
69 ± 9 |
56 ± 10 |
66 ± 12 |
16 ± 10 |
1-Epinephrine 7.5 µM |
70 ± 16 |
19 ± 8 |
19 ± 9 |
12 ± 5 |
16 ± 8 |
13 ± 7 |
Collagen 5x10-3 |
76 ± 10 |
44 ± 15 |
41 ± 12 |
38 ± 12 |
42 ± 18 |
11 ± 7 |
*Light transmittance (mean ± standard deviation)
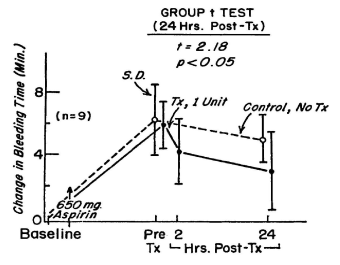
Figure 10. The change in bleeding time during the 48-hour period after treatment of 51 normal volunteers with 650 mg of aspirin. In each of 9 volunteers, 24 hours after treatment with aspirin, a unit of autologous platelets frozen with 5 per cent DMSO at 2 to 3 C per minute and washed was transfused. The bleeding time was measured 2 hours and 24 hours after the transfusion. Reprinted, with permission, from Transfusion 1977;17: 8-15
Male volunteers were treated with 650 mg of aspirin and 24 hours later were transfused with single units of allogeneic fresh platelets, autologous liquid preserved platelets, and autologous cryopreserved platelets in Valeri CR [4]. The transfusion of the fresh, liquid preserved, and cryopreserved platelets occurred 24 hours after the treatment with 650 mg of aspirin.
The 24hour period between the oral ingestion of the 650 mg of aspirin and the transfusion of the platelets was required to permit the clearance of the aspirin from the blood of the volunteer and to prevent the aspirin treatment of the donor platelets. Fresh platelets from allogeneic donors stored at room temperature for 4 hours were transfused and autologous platelets were stored at 4 C for 24 hours and at 22 C for 24 hours and autologous cryopreserved platelets were stored for at least 24 hours in the frozen state were transfused and the bleeding time and the platelet aggregation patterns were measured prior to and following the transfusions.
In reference 3 the quantity of platelets transfused to the aspirin-treated male volunteer produced about 15% donor platelets in the circulation following the transfusions (Figure 3 and Figure 4) [3]. The transfusion of 4 units of allogeneic fresh platelets stored at room temperature (22 C) for 4 hours restored the bleeding time and the platelet aggregation patterns to normal 2 hours after the transfusions. Eight units of autologous platelets stored at room temperature (22 C) for 24 hours restored the bleeding time and the platelet aggregation patterns to normal 24 hours after the transfusions. Twelve units of autologous platelets stored at room temperature (22 C) for 48 hours restored the bleeding time to normal and partially restored the aggregation patterns to normal 24 hours after the transfusions. The paper “Hemostatic effectiveness of platelets stored at 22 C” by Handin RI and Valeri CR was published in NEJM [3]. This study documented that the aspirin induced increase in bleeding time and the alterations of the platelet aggregation patterns were corrected by the 15% increase in donor platelets that were transfused. Autologous platelets treated with 6% DMSO, frozen at 2 to 3 C per minute and stored at -80 C, thawed, washed, and resuspended in autologous plasma were transfused to measure the 51Cr survival and the hemostatic function to correct the prolonged bleeding time 2 hours and 24 hours after the transfusions. The bleeding time was reduced by 50% 2 hours after the transfusion in 9 of the 12 volunteers and 24 hours after the transfusion of the autologous previously frozen, washed platelets the bleeding time in the 12 volunteers was restored to normal [6].
The 51Cr survival and the hemostatic function of a single unit of platelets in aspirin treated male volunteers were studied by Valeri CR [4]. Allogeneic fresh platelets stored at room temperature for 4 hours, autologous platelets stored at room temperature (22 C) for 24 hours, autologous platelets stored at 4 C for 24 hours, autologous platelets frozen at 2 to 3 C per minute with 6% DMSO, stored at -80 C for 24 hours, thawed, washed, and resuspended in autologous plasma and autologous platelets frozen with 5% DMSO at 1 C per minute and stored at -150 C in the gas phase of liquid nitrogen for 24 hours, thawed, washed, and resuspended in autologous plasma were transfused into aspirin-treated male volunteers and the 51Cr survival and hemostatic function were measured by Valeri CR in NEJM [4].
Since 1968, the aspirin induced increase in bleeding time in male volunteers has been utilized by the NBRL to measure the hemostatic function of fresh, liquid preserved, and cryopreserved platelets [3-6]. Slichter S and associates in Transfus Med Rev [7] have criticized the study of male volunteers treated with aspirin to measure the hemostatic function of platelets whereas Pineda AA and associates in Transfusion [8] reported the hemostatic function of washed autologous platelets to correct the increased bleeding time in aspirin-treated volunteers and Brecher ME and associates in Transfusion [9] reported the hemostatic function of filtered autologous platelets to correct the increased bleeding time in the aspirin treated volunteers.
Becker GA and associated have reported in Transfusion [10] the hemostatic function of autologous platelets stored at 4 C and at 22 C to correct the increased bleeding time in aspirin treated volunteers [10]. The hemostatic function of allogeneic cryopreserved platelets has been shown to reduce nonsurgical blood loss and to reduce the need for allogeneic red blood cells and allogeneic fresh frozen plasma (FFP) transfusion compared to the transfusion of liquid preserved allogeneic platelets stored at room temperature for 3.4 days in patients following cardiopulmonary bypass by Khuri SF in J Thorac Cardiovasc Surgery [11].
The Netherlands military have successfully utilized universal donor frozen group O RBC, frozen group O platelets, and frozen AB plasma to treat wounded casualties in war zones in Afghanistan, Iraq and Bosnia during the past 18 years [12-16].
The NBRL in a monograph published in 2007 reported that the bleeding time is affected by temperature, the hematocrit, the platelet count, the platelet volume, the platelet function and the plasma clotting proteins (Valeri CR et al [1]. In the male volunteers treated with aspirin all the factors which affect the bleeding time except platelet function are controlled prior to and following the transfusion of the platelets [1].
The function of the platelets is measured by the reduction in the prolonged bleeding time in the aspirin treated male volunteers. The in vitro testing of platelet aggregation patterns prior to and following the platelet transfusion did not correlate with the reduction in the bleeding time. There was a significant reduction in the bleeding time after transfusion of a single unit of autologous platelets treated with 5% DMSO, frozen at 2 to 3 C per minute and stored at -80 C for as long as 8 months, thawed, washed and resuspended in autologous plasma in 9 aspirin treated volunteers in 24 hours (Figure 10) [5] but no improvement in the aspirin induced aggregation patterns reported in Table 2 in Spector JI et al in Transfusion [5]. The significant reduction in the bleeding time in the aspirin treated volunteers without a change in the aggregation patterns demonstrated that the bleeding time is sensitive to change in platelet function not detected by the platelet aggregation patterns reported in Spector JI et al Transfusion [5].
O’Brien JR in Ref 2 has reported that approximately 10 percent fresh non-aspirin-treated platelets corrected the aggregation patterns in vitro of aspirin-treated platelets. We found that 1 to 2 percent non-aspirin-treated platelets frozen with 5 percent DMSO did not correct the platelet aggregation pattern in the aspirin-treated volunteers in Ref 5. Approximately 1 to 2 percent of non-aspirin-treated donor platelets circulated after transfusion of 1 unit of autologous platelets during which time the bleeding time was significantly reduced but the aggregation patterns were not corrected in Table 2 [5]. This finding indicates that there was no correlation between bleeding time and platelet aggregation patterns.
In patients the bleeding time used to measure the function of the preserved platelets is difficult to assess because of the numerous factors which affect the bleeding time. Whereas in aspirin-treated male volunteers, the function of fresh, liquid preserved, and cryopreserved allogeneic and autologous platelets to reduce the bleeding time can be measured and compared to other methods to preserve platelets.
Studies performed at the NBRL over the past 50 years have reported the correlation of the bleeding time to nonsurgical blood loss in anemic, thrombocytopenic patients after cardiopulmonary bypass surgery Valeri CR et al. [1] and the bleeding time correlated to the volume of shed blood collected at the bleeding time site in Figure 1 [1]. The bleeding time is affected by temperature, the red blood cells, the platelets, and the plasma clotting proteins.
In the study of aspirin treated male volunteers all the factors that affect the bleeding time are controlled except for the platelet function which is affected by the aspirin treatment of the volunteers, whereas all the factors that affect the bleeding time are not controlled in patients to assess the hemostatic function of the transfused platelets.
In the patients the function of transfused platelets is difficult to measure because all the factors that affect the bleeding time are not controlled. The survival and function of a single unit of fresh, liquid preserved platelets, and cryopreserved platelets in aspirin-treated male volunteers were reported by Valeri CR “The hemostatic effectiveness of liquid preserved and previously frozen platelets” in NEJM [4].
The hemostatic function of fresh platelets and platelets preserved at 22 C for 24 hours and 48 hours to reduce the increased bleeding time in male volunteers by transfusion of 15% donor platelets was reported by Handin RI, Valeri CR in NEJM [3]. Studies performed at the NBRL in aspirin-treated male volunteers [3-6] demonstrate that the hemostatic function of frozen platelets was superior to the hemostatic function of platelets in fresh whole blood and liquid preserved platelets stored at 22 C for 24 and 48 hours.
In a study of patients subjected to cardiopulmonary bypass surgery, blood loss was collected for 73 patients by Khuri SF and associates in J Thorac Cardiovasc Surgery [11]. Twenty of the patients had surgical blood loss and 53 patients had nonsurgical blood loss. The 53 patients with non-surgical blood loss were randomized and 29 patients received liquid preserved platelets and 24 patients received frozen platelets. Single donor leukoreduced apheresed platelets were frozen with 6% DMSO at 2 to 3 C per minute, stored at -80 C for as long as 2 years, thawed, washed and resuspended in autologous plasma and stored at room temperature (22 C) without agitation for 5 hours prior to transfusion. The liquid preserved apheresed platelets were stored at 22 C for as long as 5 days and the single unit of platelets were stored at 22 C for as long as 5 days and pooled prior to transfusion.
Twenty-nine (29) patients received 6.9±3.9 X 1011 liquid preserved platelets per patient and twenty-four patients received 4.5±2.1 X 1011 previously frozen platelets per patient (Table 3) [11]. The hemostatic effect of liquid preserved platelets stored at room temperature for 3.4 days was compared with platelets frozen with 6% DMSO stored at 80 C for as long as 2 years, thawed, washed, and stored in plasma for 5 hours before transfusion to patients with anemia and thrombocytopenia after cardiopulmonary bypass surgery by Khuri et al in J Thorac Cardiovasc Surgery (Figure 11) [11]. The platelets frozen with 6% DMSO and stored at -80 C for 2 years, thawed, washed, and stored in plasma at room temperature for 5 hours, had in vivo survival 2 hours after transfusion of 24% compared with the in vivo survival of 37% for liquid preserved platelets stored at room temperature for 3.4 days.
Table 3. Comparison of characteristics of liquid-preserved versus cryopreserved platelet transfusions. Reprinted, with permission, J Thorac Cardiovasc Surg 1999;117:172-184
|
Liquid-preserved |
Cryopreserved |
P |
Age (d) |
Mean |
34 ± 1.1 |
289 ± 193 |
0.0001 |
Median |
3.8 |
250 |
|
Range |
2.5 |
30.720 |
|
Volume of Platelets transfused/patient (ml) |
487 ± 278 |
184 ± 96 |
0.0001 |
No of platelets transfused per patient (1011 cells) |
6.9 ± 3.9 |
4.5 ± 2.1 |
0.008 |
Data are expressed as mean ± SD.
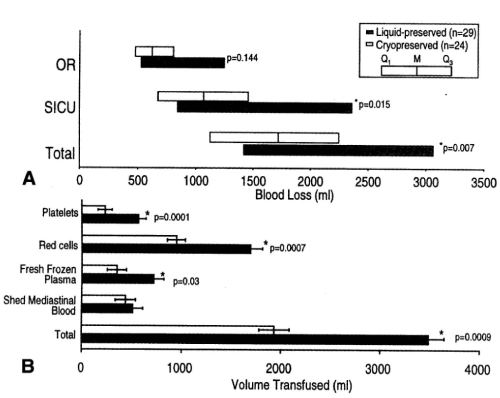
Figure 11. Blood loss measured in the operating room (OR), blood loss measured in the surgical intensive care unit (SICU), and combined total blood loss in patients who received liquid-preserved platelet transfusions and in those who received cryopreserved platelet transfusion. Data are shown as median and IQR, M, Median: Q1, 25th percentile; Q3, 75th percentile. B, volumes of transfused blood products received by the same groups. Data are shown as mean ± SEM. (Reprinted, with permission, J Thorac Cardiovasc Surg 1999;117:172-184).
Patients undergoing cardiopulmonary bypass surgery were transfused PLTs that had been frozen with 6 percent DMSO and stored at −80°C for as long as 2 years and after thawing and washing were resuspended in plasma for as long as 5 hours. These PLTs exhibited greater reduction in nonsurgical blood loss and required fewer transfusions of red blood cells (RBCs) and fresh frozen plasma (FFP) than patients who received liquid preserved PLTs that had been stored at 22°C for a mean of 3.4 days (Figure 11) [11]. The previously frozen washed PLTs produced more thromboxane A2 than did the liquid-preserved PLTs after stimulation with arachidonic acid and adenosine diphosphate (ADP) and accumulated more factor FV on the PLTs than did the liquid-preserved PLTs. The procoagulant activity and the increased thromboxane production in the previously frozen washed PLTs produced improved hemostasis in the patients after transfusion, which resulted in reduced nonsurgical blood loss and reduced requirements for allogeneic RBCs and FFP (Table 4) [11].
Table 4. Results of in vitro platelet function studies. Reprinted, with permission, J Thorac Cardiovasc Surg 1999;117:172-184
|
Liquid-preserved at 22°C |
|
|
|
Fresh |
24h |
48h |
72h |
Cryopreserved |
|
|
|
Mean ± SD |
n |
Mean ± SD |
n |
Mean ± SD |
n |
Mean ± SD |
n |
Mean ± SD |
n |
P* |
P# |
pH |
7.06 ± 0.06 |
20 |
7.25 ± 0.10 |
20 |
7.33 ± 0.12 |
20 |
7.33 ± 014 |
18 |
6.81 ± 0.15 |
24 |
<0.01 |
<0.01 |
Hypotonic stress response (%) |
70 ± 25.0 |
7 |
79 ± 18.4 |
8 |
78 ± 7.7 |
8 |
76 ± 13.1 |
6 |
28 ± 13.3 |
23 |
<0.01 |
<0.01 |
Platelet aggregation (DU) |
256 ± 59 |
20 |
201 ± 57 |
20 |
189 ± 78 |
18 |
169 ± 85 |
20 |
140 ± 31 |
25 |
<0.01 |
0.083 |
TXB2per platelet (pgx10-6) |
310 ± 188 |
20 |
191 ± 220 |
20 |
122 ± 127 |
20 |
115 ± 92 |
16 |
440 ± 254 |
25 |
<0.01 |
<0.01 |
Liquid-preserved platelets were all apheresis units. TXB2, Thromboxane B2; DU, digitizer units.
*By analysis of variance.
#Difference between 72-hour liquid-preserved and cryopreserved platelets, by t test.
In March 2000 the NBRL modified the method of freezing platelets with 6% DMSO at 2-3 C per minute by storage in a -80 C mechanical freezer. With the modified method the supernatant DMSO was removed after centrifugation at 1250 X g for 10 minutes before the platelets were frozen at 2-3 C per minute stored at -80 C, thawed, and diluted with 10-20 ml of 0.9% NaCl prior to transfusion. The previously frozen platelets were not washed prior to transfusion [15]. When platelets are washed the in vitro recovery is reduced by 25%. With the modified method, the thawed platelets are not washed but are diluted with 10 ml to 20 ml of 0.9% NaCl and stored without agitation at room temperature for 6 hours. The freeze-thaw (F-T) recovery was approximately 90%, with 5 to 8% platelet microparticles, in vivo recovery of 25 to 30% with linear lifespan of 7 days (Table 5) [15]. The diluted platelets have a bimodal population: one population is GPIb-normal with reduced annexin V-binding and the other population is GPIb-reduced with increased annexin V binding. A study was performed to assess the modification of the method to freeze PLTs with 6 percent DMSO and storage at −80°C. The modification simplified the freezing process by removing the supernatant solution containing 95 percent of the DMSO before freezing (the new method without supernatant DMSO). The PLTs were frozen at a rate of 2 to 3°C per minute by the storage of a 10-mL volume of PLTs concentrated in 6 percent DMSO solution in a 300 mL PVC plastic bags covered with a polyester plastic bag and stored in a rigid cardboard box placed at the bottom of a −80°C freezer (Figure 12) [15]. The amount of residual DMSO in the previously frozen washed PLTs was 400 mg per unit, whereas that in the previously frozen nonwashed diluted PLTs was 600 mg per unit. There is no significant difference in the safety profile between 400 and 600 mg of DMSO in the frozen PLTs processed by the old and new methods with DMSO. The time required for thawing and washing previously frozen PLTs resuspended in plasma was 1 hour 15 minutes (the old method with supernatant DMSO), whereas the time required for thawing and diluting the previously frozen nonwashed PLTs with 0.9 percent NaCl was only 10 minutes (the new method without supernatant DMSO).
Table 5. Comparison of the two methods of freezing human PLTs with 6 percent DMSO and storage at -80 °C. Reprinted, with permission, from Transfusion 45:1890-1895, 2005
|
Cryopreserved PLTs with 6 percent DMSO and storage at -80°C |
Method of preservation |
With Post thaw washing |
Without Post thaw washing |
Mean in vitro recovery (%) |
70 |
90 |
Biomodal population GPlb normal and GPlb reduced |
Yes |
Yes |
PLT annexin V microparticles (%) |
3-5 |
5-8 |
In Vivo recovery (%) 1 to 2hr after transfusion |
35-40 |
25-30 |
Life span (days) |
7 |
7 |
Residual DMSO (mg) in the unit |
400 |
600 |

Figure 12. The freezing rate of 10-mL volume of 6 percent DMSO-treated PLTS in the 300-mL PVC plastic bag placed in a polyester plastic bag and stored in a cardboard box placed at the bottom of a -80°C mechanical freezer. Thermocouples were placed on the top [□] and bottom [◊] of the PVC plastic bag. Reprinted, with permission, from Transfusion 2005;45:1890-1895
In a previous study, human and baboon PLTs that were frozen with 6 percent DMSO at −80°C and after thawing and washing were resuspended in plasma were found to have GPIb-normal and GPIb-reduced PLT populations (Figure 13) [17]. In the baboon, the survival of the autologous PLT GPIb-normal population 1 to 2-hour posttransfusion recovery was 48 percent and PLT life span was less than 6 days (Figure 13) [17].
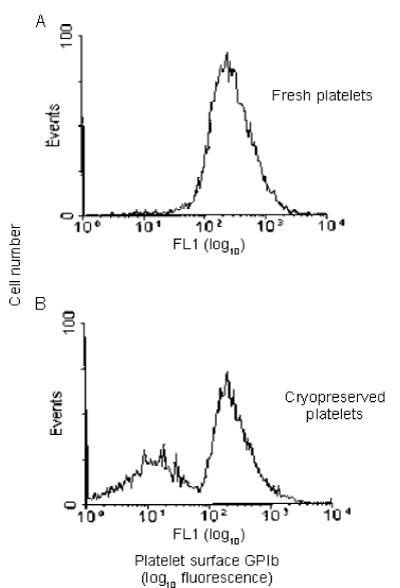
Figure 13. Representative histograms illustrating the effect of cryopreservation on the human platelet surface expression of GPIb. Fresh platelets (A) and cryopreserved platelets (B) were incubated with 6D1-FITC and analyzed by flow cytometry. Platelets were identified with CD41 antibody. Cryopreservation results in the appearance of a GPIb-reduced (left side, B) subpopulation that was distinctly separable from the GPIb-normal subpopulation (right side, B). FL1 = fluorescence channel 1. Reprinted, with permission, from Transfusion 1999;39:880-888
In a study in baboons, the GPIb-reduced PLTs with increased annexin V binding in PLTs frozen with 6 percent DMSO, stored at −80°C, thawed, washed, and resuspended in plasma were rapidly removed from the circulation within 5 minutes (Figure 14) [17].
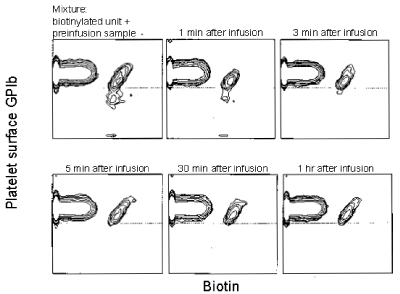
Figure 14. The circulation of autologous biotin-X-NHS-labeled, previously frozen, washed baboon platelets. The GPIb level in the unit and in the baboon’s blood during the first 60 minutes after infusion is reported. Mixture of nonbiotinylated fresh baboon platelets and the biotinylated cryopreserved platelets before infusion and nonbiotinylated and biotinylated platelets after the infusion is shown on the X axis. GPIb-specific antibody binding is shown on the Y axis. Both axes represent log fluorescence. The data are representative of two separate experiments. Reprinted, with permission, from Transfusion 1999;39: 880-888
A correlation has been observed between the improved in vivo hemostatic function of cryopreserved PLTs processed by the old method with supernatant DMSO and their procoagulant activity and their ability to produce thromboxane at the bleeding time site. The procoagulant activity of the PLTs that were frozen by the old method with supernatant DMSO has been assessed by the accumulation of FV and FX and the PLT binding of annexin V. Annexin V binding to cell surface–exposed phosphatidylserine can be used to assess the procoagulant activity of PLTs and PLT microparticles. Annexin V binding to phosphatidylserine identifies PLTs and PLT microparticles undergoing apoptosis that are rapidly removed from the circulation by tissue macrophages.
Analysis of PLTs that were treated with DMSO, concentrated to remove the supernatant solution, before freezing and after thawing, diluted with 0.9 percent NaCl had a freeze-thaw recovery of 94 percent and 7 percent PLT microparticles (Figure 15 and Figure 16) [15]. The PLTs that were frozen with 6 percent DMSO at −80°C and after thawing were washed and resuspended in plasma had a freeze-thaw-wash recovery of 74 and 5 percent PLT microparticles. The washing procedure removed 20 percent of the thawed PLTs and reduced the PLT microparticles to 5 percent (Figure 15 and Figure 16) [15].
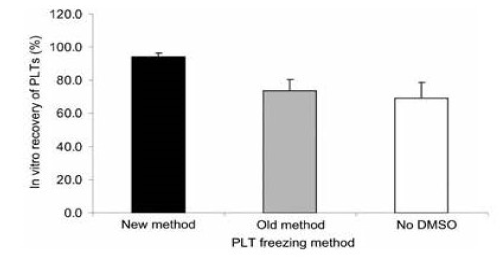
Figure 15. In vitro recovery of PLTS following the three different freezing procedures. Mean ± SD, n=7. Paired t tests: old versus new, p<0.001); old versus no DMSO, NS; new versus no DMSO, p<0.001. New method: DMSO added to 6 percent final concentration, supernatant DMSO removed before freezing at -80°C, and previously frozen PLTs thawed and diluted with 0.9 percent NaCl (not washed). Old method: DMSO added to 6 percent final concentration, frozen at -80°C and previously frozen PLTs thawed, washed with 0.9 percent NaCl-0.2% glucose, and resuspended in plasma. No DMSO: 0.9 percent NaCl added to PLTs, supernatant removed before freezing at -80°C, and previously frozen PLTs thawed and diluted with 0.9 percent NaCl. Reprinted, with permission, from Transfusion 45:1890-1895, 2005
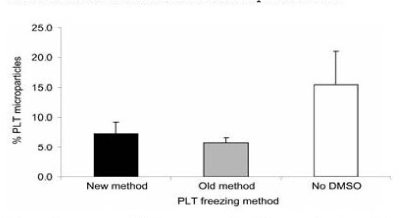
Figure 16. Percentage of PLT microparticles following the three different freezing procedures. Mean ± SD; n=7. Paired t test: old versus new, NS; old versus no DMSO, p<0.01; new versus no DMSO, NS. New method: DMSO added to 6 percent final concentration, supernatant DMSO removed before freezing at -80°C, and previously frozen PLTs thawed and diluted with 0.9 percent NaCl (not washed). Old method: DMSO added to 6 percent final concentration and frozen at -80°C and previously frozen PLTs thawed, washed with 0.9 percent NaCl-0.2% glucose, and resuspended in plasma. No DMSO: 0.9 percent NaCl added to PLTs, supernatant removed before freezing at -80°C, and previously frozen PLTs thawed and diluted with 0.9 percent NaCl. Reprinted, with permission, from Transfusion 45:1890-1895, 2005
The in vivo recovery 1 to 2 hours after the autotransfusion of the PLTs that were frozen by the new method without supernatant DMSO, diluted with 0.9 percent NaCl, and stored at room temperature for 4 hours was approximately 30 percent with a life span of 7 days compared to that of 35 to 40 percent and a life span of 7 days for the PLTs frozen by the old method with supernatant DMSO and resuspended in plasma (Table 5) [15].
PLT surface marker testing demonstrated that the PLTs that were frozen by the old method with supernatant DMSO had significantly greater numbers of normal GPIb and reduced annexin V binding PLTs than the PLTs that were frozen by the new method without supernatant DMSO which had significantly increased numbers of reduced GPIb and increased annexin V-binding PLTs (Figure17 and Figure 18) [15].
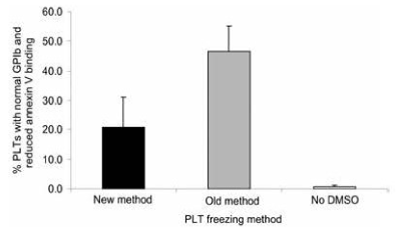
Figure 17. Percentage of PLTs with normal GPIb and reduced annexing V binding following the three different freezing procedures. Mean ± SD; n=7. Paired t test: old versus new, p<0.05; old versus no DMSO, p<0.001; new versus no DMSO, p<0.01. New method: DMSO added to 6 percent final concentration, supernatant DMSO removed before freezing at -80°C, and previously frozen PLTs thawed and diluted with 0.9 percent NaCl (not washed). Old method: DMSO added to 6 percent final concentration and frozen at -80°C and previously frozen PLTs thawed, washed with 0.9 percent NaCl-0.2% glucose, and resuspended in plasma. No DMSO: 0.9 percent NaCl added to PLTs, supernatant removed before freezing at -80°C, and previously frozen PLTs thawed and diluted with 0.9 percent NaCl. Reprinted, with permission, from Transfusion 45:1890-1895, 2005

Figure 18. The percentage of PLTs with reduced GPIb and increased annexin V binding following the three different freezing procedures. Mean ± SD; n=7. Paired t test: old versus new, p<0.01; old versus no DMSO, p<0.001; new versus no DMSO, p<0.05. New method: DMSO added to 6 percent final concentration, supernatant DMSO removed before freezing at -80°C, and previously frozen PLTs thawed and diluted with 0.9 percent NaCl (not washed). Old method: DMSO added to 6 percent final concentration and frozen at -80°C and previously frozen PLTs thawed, washed with 0.9 percent NaCl-0.2% glucose, and resuspended in plasma. No DMSO: 0.9 percent NaCl added to PLTs, supernatant removed before freezing at -80°C, and previously frozen PLTs thawed and diluted with 0.9 percent NaCl. Reprinted, with permission, from Transfusion 45:1890-1895, 2005
The PLTs frozen by the new method without supernatant DMSO, diluted with 0.9 percent NaCl, and stored at room temperature (22°C) for 6 hours without agitation had a mean pH value of 6.4. PLTs frozen by the old method with supernatant DMSO, resuspended in plasma after washing, and stored at room temperature for 6 hours had a mean pH value of 6.8. The previously frozen PLTs that were resuspended in 0.9 percent NaCl produced significantly more thromboxane A2 after stimulation with a combination of arachidonic acid and ADP than the previously frozen PLTs that were resuspended in plasma.
The in vivo survival of autologous DMSO-frozen PLTs prepared by the new method without supernatant DMSO had an in vivo recovery value 1 to 2 hours after autotransfusion that was approximately 10 percent lower than the PLTs frozen by the old method with supernatant DMSO (Table 5) [15]. The PLTs frozen without supernatant DMSO by the new method exhibited a significantly increased number of reduced GPIb and increased annexin V-binding PLTs compared to PLTs frozen with supernatant DMSO by the old method. Better in vivo recovery values 1 to 2 hours after transfusion and the PLT life span were associated with PLTs with normal GPIb and reduced annexin V binding. PLTs with reduced GPIb and increased annexin V binding were associated with improved hemostatic function, which reduced nonsurgical blood loss. The reduced GPIb and increased annexin V binding by PLTs and PLT microparticles, however, may be associated with thromboembolic events in the recipients (Figure 17 and Figure 18) [15].
Data have been reported by Khuri and associates on the therapeutic benefit of allogeneic PLTs frozen by the old method with supernatant DMSO in reducing nonsurgical blood loss without producing adverse events in patients after cardiopulmonary bypass [11]. The elimination of the postthaw washing of the previously frozen PLTs has simplified the procedure to provide PLTs with improved hemostatic function. The protocol reported in Transfusion [15] to freeze platelets has been evaluated by Dumont LJ and associates in Transfusion [18]. The in vitro recovery of the frozen platelets and the in vivo recovery 1-2 hours following transfusion and the lifespan of the frozen platelets in 24 healthy volunteers were reported. The past 50 years have seen many significant improvements in cryopreservation procedures. Removing supernatant glycerol from RBC and DMSO from platelets prior to freezing have simplified the postthaw processing. Platelets diluted with 10 ml to 20 ml of 0.9% NaCl after thawing can be stored at room temperature without agitation for 6 hours prior to use. With the functionally closed Haemonetics ACP215 instrument, deglycerolized RBC can be stored at 4 C in AS-3 (Nutricel) for 2 weeks. With this instrument, the volume of solution needed to deglycerolize RBC is now only 2.0 liters compared to 3.2 liters with the Haemonetics Blood Processor 115 and 6.8 liters with the Huggins cytoglomerator.
The Netherlands military has been actively freezing universal donor Group O Rh positive and Group O Rh negative RBC, single donor leukoreduced frozen Group O Rh Positive and Group O Rh negative platelets and frozen AB plasma in -80 C mechanical freezers. These frozen blood products have been collected from donors meeting FDA regulations that were safely transported and stored in -80 C mechanical freezers without breakage. These blood products have been obtained from screened blood donors and tested for the mandated infectious markers prior to freezing and have eliminated the need for fresh whole blood or apheresed leukoreduced platelets stored at room temperature for 5 days with agitation to treat patients suffering traumatic injuries with therapeutically effective outcomes and without adverse events.
The utilization of the -80 C mechanical freezers to freeze RBC, platelets, and plasma by the Netherlands military has demonstrated the safety and therapeutic effectiveness of these frozen blood products without the need for fresh whole blood or apheresed platelets to treat military and civilian casualties requiring more than 10 units of red blood cells within a 24-hour period in combat zones in Afghanistan and Iraq. It is of interest that the Netherlands investigators pioneered the use of liquid nitrogen to freeze blood products but now utilize -80 C mechanical freezers to freeze red blood cells, platelets and plasma to treat combat casualties.
Dr. John Badloe at the ATACCC meeting on August 16, 2010 at St. Pete, Florida reported that in Afghanistan from 2000 to 2010, 859 patients received 6,335 blood products which include 1918 units of frozen red blood cells, 841 units of liquid preserved red blood cells, 2560 units of frozen plasma and 1,016 units of frozen platelets with no transfusion reactions. Fresh whole blood was not used by the Netherlands military because the fresh whole blood could not be tested prior to transfusion for the mandated infectious disease markers whereas all the frozen blood products were tested for the mandated infectious disease markers prior to freezing from the donors who were screened prior to donation.
Dr. John Badloe reported the Netherlands military experience in the Middle East war zones using frozen blood products, i.e. frozen group O Rh positive and group O Rh negative RBC, frozen AB plasma, and frozen group O single donor leukoreduced 2.5 - 3.0 X 1011 platelets treated with 5% DMSO with removal of supernatant DMSO, all frozen and stored at -80 C in mechanical freezers at ratio of 1:1:1 increased survival of patients from 44% to 84%. No adverse events were reported and only frozen blood products without the need for fresh whole blood were safe, available, effective and efficient in the treatment of patients requiring at least 10 units of red blood cells in a 24-hour period for resuscitation.
The abstract which was reported by Dr. John Badloe and Dr. Femke Noorman from the Ministry of Defense, Military Blood Bank, Leiden, Netherland at the Annual meeting of the AABB, San Diego, CA October 22-25, 2011 confirm the procedures provided by the NBRL, Boston, MA to freeze human RBC, plasma, and platelets in -80 C mechanical freezers. This report by the Netherlands military demonstrates that fresh whole blood advocated by Colonel William Crosby and the U.S. Army is no longer used by the Netherlands military and the fresh whole blood can be replaced by frozen RBC, frozen plasma, and frozen platelets significantly improved survival of the massively transfused patients [13].
A comparison between the U.S. Army procedures and the Netherlands procedures to provide blood products to treat combat casualties needs to be performed regarding the logistic requirements, outdating of blood products, compatibility, cost of transportation, availability, safety and therapeutic effectiveness. The quality and quantity of the blood products to resuscitate casualties requiring at least 10 units of red blood cells in a 24-hour period and the mortality and morbidity utilizing the U.S. Army procedures to the Netherlands procedures in combat zones need to be compared. The Netherlands military has documented that -80 C frozen red blood cells, plasma and platelets were deployable, available, compatible, safe and effective in the treatment of trauma patients with or without massive blood loss in military theatre.
Dr. Femke Noorman and Dr. John Badloe and associates presented one oral presentation and three poster presentations at the AABB annual meeting from October 6-9, 2012 in Boston, MA [13]. Studies can now be done to compare the safety and therapeutic effectiveness of universal donor RBC frozen for at least 10 years, group O platelets frozen for at least 2 years, and AB plasma frozen for at least 10 years (all blood products frozen and stored at -80 C with a range of -65 C to -90 C) without the need for fresh whole blood to the current use of fresh whole blood, liquid preserved RBC stored at 4 C in additive solutions for as long as 42 days, single donor leukoreduced apheresed platelets stored with agitation at room temperature for 5 days, and fresh frozen plasma and cryoprecipitate stored at -20 C for one year on mortality and morbidity in the recipients.
In the study to compare the procedures utilized by the Netherlands military to the current FDA approved procedures to provide blood products; the volume and composition of the resuscitation fluids used with the blood products need to be reported. The morbidity and mortality associated with the current FDA approved blood products can be compared to the use of frozen RBC, frozen platelets, and frozen plasma without the need for fresh whole blood and apheresed platelets which Netherlands military has successfully utilized to treat combat casualties in war zones from August 2006 to February 2012; 2,175 units of frozen RBC, 1,070 units of frozen platelets, 3,001 units of frozen plasma and 879 units of liquid preserved RBC stored at 4 C were transfused to 1,011 casualties without any transfusion reactions observed. The blood products were obtained from pre-screened donors and the blood products were tested for infectious diseases prior to freezing.
In the response to the paper “Transfusion of older stored blood and risk of death” published by Wang D and associates in Transfusion [19] an editorial was written by Warkentin TE and Eikelboom JW “Old blood bad? Either the biggest issue in transfusion medicine or a nonevent” in Transfusion [20]. These authors stated, “the demonstration of an association (if indeed there is one) between the age of transfused blood and outcome would be useless if one could not do anything about it”. This editorial failed to acknowledge that freeze preservation of red blood cells, platelets and plasma has been reported to provide safe and therapeutically effective blood products from 2006 to 2012 to successfully treat 1,011 civilian and military casualties in war zones in Iraq and Afghanistan by the Netherlands military using -80 C mechanical freezers to freeze and store universal donor group O Rh positive and group O Rh negative red blood cells, single donor leuko reduced group O Rh positive and group O Rh negative frozen platelets, and group AB frozen plasma without the need for fresh whole blood and apheresed platelets.
It is imperative that civilian and military blood banking communities change their methods of collection and preservation of blood products if they are really interested in providing patients with the safest and most therapeutically effective blood products and in avoiding risks associated with transfusion.
The Food and Drug Admi2021 Copyright OAT. All rights reservrvices (HHS), the American Red Cross (ARC) and the blood banking community have focused on further testing of blood products to reduce the rate of disease transmission. Another consideration is the disinfection of red blood cells, platelets, and plasma. What is more important, they should be looking at the quality of the blood products being transfused. We know that the length of storage of blood products affects their survival and function and that transfusion of nonviable compatible RBC, antibodies to granulocytes and WBC HLA antigens, and biologically active substances affect the patient’s clinical outcome. One of the easiest ways to prevent the severe adverse effects that have been observed is to ensure that the transfused blood products survive and function at an optimum level and that the levels of antibodies to granulocytes and WBC HLA antigens and biologically active substances are reduced. The best way to ensure this is to store liquid preserved human red blood cells at 4 C in additive solutions for no more than 2 weeks and leukoreduced platelets at room temperature for no more than 2 days. These liquid preserved blood products can be used in conjunction with frozen RBC, frozen platelets and frozen plasma all stored in -80 C mechanical freezer.
The reluctance of the blood banking community to consider any changes that could improve the safety and therapeutic effectiveness of blood products bring to mind two very relevant quotations: one by Maurice Maeterlinck is that “At every crossway on the road that leads to the future, every progressive spirit is opposed by a thousand men appointed to guard the past”. The other by Winston Churchill is that “Occasionally man will stumble on the truth but will manage to pick himself up and continue on as if nothing had happened.” It is time now to investigate the current blood banking procedures and to seek ways to improve them.
Summary
For the past 18 years, the Netherlands military have utilized universal donor RBC, platelets and plasma all frozen and stored at -80 C in a mechanical freezer to treat wounded casualties with excellent clinical results and without adverse events. The data reported by the NBRL over the past 50 years and the Netherlands military over the past 18 years now recommend that the Food and Drug Administration, the American Red Cross, Health and Human Service, and the Department of Defense should support the use of universal donor frozen group O Rh positive and group O Rh negative RBC, frozen group O Rh positive and group O Rh negative leukoreduced platelets and frozen AB plasma. All the blood products frozen and stored at -80 C provide safe and therapeutic effective blood products to treat patients. The frozen blood products will eliminate or reduce the severe adverse events of mortality and morbidity associated with the current FDA approved red blood cell products, platelet products and plasma products. The universal donor group O Rh positive and group O Rh negative frozen RBC, group O Rh positive and group O Rh negative leukoreduced single donor frozen platelets, and AB frozen plasma obtained from male donors will provide blood products that are compatible with acceptable in vivo survival and function to treat patients and reduce or eliminate the severe adverse events associated with the current FDA approved blood products.
One unit of concentrate was transfused 24 hours after the first aspirin treatment. On the other occasion no transfusion was administered. The mean (± SD) in vitro recovery for 12 studies was 62.8 ± 8.8 per cent; the mean freeze-thaw-wash recovery was 75.8 ± 11.0 per cent. The mean (± SD) recovery was 47.2 ± 7.1 per cent in vitro and 46.0 ± 11.0 per cent in vivo. The mean (± SD) in vivo recovery of the original platelets was 22.0 ± 5.7 per cent. There was no significant difference between bleeding times of subjects before (paired t-test =0.153; p>0.05) transfusion, but the difference between subjects transfused and those not transfused was significant (paired t-test = 4.130; p<0.01) at 24 hours. (Reprinted, with permission, from N Engl J Med).
Acknowledgement
To the memory of Domenica Piccolomini and Angelo Valeri
References
- Valeri CR, Khuri S, Ragno G (2007) Non-surgical bleeding diathesis in anemic thrombocytopenic patients: Role of temperature, RBC, platelets, and plasma clotting proteins. Transfusion 47: 206S-248S.
- O'Brien JR (1968) Effects of salicylates on human platelets. Lancet 1: 779-783. [Crossref]
- Handin RI, Valeri CR (1971) Hemostatic effectiveness of platelets stored at 22 degrees C. N Engl J Med 285: 538-543. [Crossref]
- Valeri CR (1974) Hemostatic effectiveness of liquid-preserved and previously frozen human platelets. N Engl J Med 290: 353-358.
- Spector JI, Yarmala JA, Marchionni LD, Emerson CP, Valeri CR (1977) Viability and function of platelets frozen at 2 to 3 C per minute with 4 or 5 per cent DMSO and stored at -80 C for 8 months. Transfusion 17: 8-15. [Crossref]
- Valeri CR, Feingold H, Marchionni LD (1974) A simple method for freezing human platelets using 6 per cent dimethylsulfoxide and storage at -80 degrees C. Blood 43: 131-136. [Crossref]
- Slichter SJ, Jones M, Ransom J, Gettinger I, Jones MK, et al. (2014) Review of in vivo studies of dimethyl sulfoxide cryopreserved platelets. Transfus Med Rev 28: 212-225.
- Pineda AA, Zylstra VW, Clare DE, Dewanjee MK, Forstrom LA (1989) Viability and functional integrity of washed platelets. Transfusion 29: 524-527. [Crossref]
- Brecher ME, Pineda AA, Zylstra-Halling VW, Chowdhury S, Forstrom LA (1990) In vivo viability and functional integrity of filtered platelets. Transfusion 30: 718-721.
- Becker GA, Tuccelli M, Kunicki T, Chalos MK, Aster RH (1973) Studies of platelet concentrates stored at 22 C nad 4 C. Transfusion 13: 61-68. [Crossref]
- Khuri SF, Healey N, MacGregor H, et al. (1999) Comparison of the effects of transfusions of cryopreserved and liquid-preserved platelets on hemostasis and blood loss after cardiopulmonary bypass. J Thorac Cardiovasc Surg 117: 172-184.
- Lelkens CC, Koning JG, de Kort B, Floot IB, Noorman F (2006) Experiences with frozen blood products in the Netherlands military. Transfus Apher Sci 34: 289-298. [Crossref]
- Noorman F, Badlow JF. -80 C frozen red blood cells, plasma and platelets: efficient logistics, available, compatible, safe and effective in the treatment of trauma patients with or without massive blood loss in military theatre. AABB Oct 2012. Transfusion practices/clinical case studies/transfusion practices in emergent settings. SP383: poster presentation. Transfuison 52: SP383, 198-A.
- Valeri CR, Ragno Giorgio G (2013) Forty-five years of research at the NBRL, Boston, Massachusetts. Chaotic observations, serendipity, and patience. Lulu Publishing Services.
- Valeri CR, Ragno G, Khuri S (2005) Freezing human platelets with 6% dimethylsulfoxide with removal of the supernatant solution before freezing and storage at -80 C without post-thaw processing. Transfusion 45: 1890-1895.
- Valeri CR, Giorgio GR (2015) The safety and therapeutic effectiveness of universal donor frozen RBC, frozen platelets and frozen plasma stored at -80 C in mechanical freezers to treat wounded casualties. Trauma Cases Rev 1: 3-5.
- Barnard MR, MacGregor H, Ragno G, Pivacek LE, Khuri SF, et al. (1999) Fresh, liquid-preserved, and cryopreserved platelets: adhesive surface receptors and membrane procoagulant activity. Transfusion 39: 880-888.
- Dumont LD, Cancelas JA, Dumont DE, Siegel AH, Szczepiorkowski ZM, et al. (2013) A randomized controlled trial evaluating recovery and survival of 6% dimethyl sulfoxide-frozen autologous platelets in healthy volunteers. Transfusion 53: 128-137.
- Wang D, Sun J, Solomon SB, Klein HG, Natanson C (2012) Transfusion of older stored blood and risk of death: a meta-analysis. Transfusion 52: 1184-1195. [Crossref]
- Warkentin TE, Eikelboom JW (2012) Old blood bad? Either the biggest issue in transfusion medicine or a nonevent. Transfusion 52: 1165-1167. [Crossref]


















