Abstract
Aim: Serum and follicular fluid ischemia-modified albumin levels and total antioxidant capacity were evaluated in patients with polycystic ovary syndrome applied ICSI-ET to clarify the association between these markers and IVF outcome parameters.
Methods: In this prospective controlled clinical trial, the study group consisted of PCOS cases (n=30) undergoing ICSI-ET cycles. The control group consisted normogonadotropic female partners of male factor infertility (n=30). The controlled ovarian hyperstimulation was performed by long protocol down regulation and recombinant FSH stimulation. Aspirated follicular fluid from the first retrieved follicle were analyzed for TAC and IMA levels. Oocyte quality, fertilization rate, embryo quality and final pregnancy outcomes were assessed.
Results: No statistically significant difference was found between the groups when compared for mean age, body mass index and cycle outcome parameters (p>0.05). FF IMA levels were found positively correlated with embryo grading (r=0.328; p=0.03). The sensitivity, specificity, positive and negative predictive values of the best cutoff value of FF IMA (1.475 ABSU) for the prediction of grade 1 embryo development were 75%, 72%, 73% and 60%, respectively (AUC:0.765;CI 95%:0,606-0,924; p=0.017). In PCOS cases with TAC ≤1.299 mmol/L compared with TAC >1.299 mmol/L, number of metaphase II oocytes, fertilization rates, embryo grading and FF IMA levels were found significantly different (p<0.05).
Conclusions: The levels of IMA and TAC in FF were higher than serum levels which inturn indicates a greater degree of oxidative stress in follicular microenvironment compared to systemic circulation. Although oxidative stres in FF of PCOS cases was found similar to non-PCOS cases when detected by IMA, a significant positive correlation between FF IMA levels and embryo grade may indicates the role of augmented oxidative stress in infertility.
Key words
polycystic ovary syndrome, ICSI-ET, follicular fluid, oxidative stress, ischemia-modified albumin, total antioxidant status
Introduction
Polycystic ovary syndrome (PCOS) is the most common endocrine abnormality of reproductive age women and has a prevalence of approximately 18% [1]. PCOS is a common cause of infertility associated with ovarian dysfunction, metabolic and hormonal impairments. The pathogenesis of PCOS is complex and its underlying basis remains unclear. Several characteristics and associations of PCOS contribute to decreased antioxidant concentrations, and thus PCOS is considered as an oxidative state [1,2].
Oxidative stress (OS) is a state characterized by an imbalance between pro-oxidant molecules and and antioxidant defenses. OS has been identified to play a key role in the pathogenesis of subfertility in both males and females [3]. For normal female physiological reactions, like folliculogenesis, a certain amount of reactive oxygen species (ROS) is needed, whereas excessive production of ROS may overpower the body’s natural antioxidant defense system, creating an unsuitable environment for reproduction [3]. Moreover, increased ROS activity in follicular fluid (FF) can be toxic to embryo formation [4]. In assisted reproductive technologies fertility outcome is adversely affected if an imbalance exists between ROS and antioxidants in the oocyte microenvironment [4]. In women with PCOS undergoing in vitro fertilization (IVF) and embryo transfer (ET), OS mediated alterations in granulosa cells have a negative impact for IVF success [5]. Supporting this data, FF total antioxidant capacity (TAC) has also been reported as a predictor of IVF outcome parameters such as fertilization, embryo quality and pregnancy rates [6-9].
Ischemia modified albumin (IMA) is a new marker of OS. During ischemia, acidosis and generation of superoxide free radicals occurs and the N-terminal portion of the human serum albumin becomes unable to bind metal ions. As a result of oxidative stress, IMA generation occurs, lacking tissue specificity [10]. The role of OS in PCOS has been demonstrated with previous reports, but underlying mechanism is not fully understood [11-13]. Previously by our group, in PCOS cases significantly higher levels of serum IMA has been reported compared to healthy controls [14]. Regarding the previous data, the analyses of serum and FF IMA and TAC levels in PCOS-ICSI cases were the main objective of this study. Additionally, the association between ICSI outcome parameters and serum/FF IMA and TAC levels were also analyzed. This is also the first study in the literature documenting FF IMA, as an oxidative stress marker.
Materials and methods
Study groups
All of the patients included in the prospective controlled clinical trial had attended IVF clinic for infertility treatment. The study protocol was approved by the Ethics Committee of the Institute (No:161/January 28, 2013). All women participating the study gave informed consent. Thirty women with PCOS constituted the study group. The diagnosis of PCOS was made as proposed at the Rotterdam Consensus Meeting [15]. The controls (n=30) were cases suffering from male factor infertility without any features of clinical or biochemical hyperandrogenism who had regular menstrual cycles. Exclusion criteria were endometriosis and tubal factor infertility known to be associated with OS, cases age under 20 and over 40 years of age, previous low responses to controlled ovarian hyperstimulation and previous history of ovarian surgery. All of the subjects were nonsmokers. Only one cycle of each patient and cycles that ended with embryo transfer were included in the statistical analyses.
Ovarian stimulation protocol
All patients received GnRH agonist long protocol. For pituitary desensitization daily subcutaneous injection of 1mg/day leuprolide acetate was started from the midluteal phase of the cycle. After down regulation was confirmed by menses, GnRH agonist dose was halved and recombinant FSH was started at a constant initial dose (150 IU/day in PCOS group and 150-225 IU/day in controls) for 5 days. Then the dose was adjusted according to the ovarian response and GnRH was continued until the day of hCG administration. Ovarian response and follicular growth was monitered by transvaginal ultrasonography and serum estradiol assays. In both groups recombinant hCG (250 µg; Ovitrelle, Serono) was given when at least three leading follicles are ≥17 mm in size. Oocyte pick up was performed with transvaginal ultrasound-guided 36 hours after injection of hCG. All patients underwent intracytoplasmic sperm injection (ICSI). The grading of day 3 embryos was performed using modified Veeck scoring system [16]. The grading of day 5 embryos was performed using Gardner blastocyst scoring system [17]. Embryo transfer was done on day 3 or 5 after oocyte pick up using soft catheter (Vitrolife, Sweden). For luteal support, all patients received 8% progesterone gel/daily starting the evening after oocyte pick up and continuing until a negative pregnancy test or a viable fetus documented by transvaginal sonography. A biochemical pregnancy was defined as β-hCG concentration > 10 IU/L on day 12 after transfer. A clinical pregnancy was defined as an intrauterine gestational sac with a heartbeat 3 weeks after positive results on hCG test.
Collection of follicular fluid
FF was obtained from all participants of the study. To avoid contamination from blood, flush medium, or mixed follicular fluid during oocyte pick up, only the follicular fluid from the first retrieved follicle which contains a single oocyte-cumulus complex was collected. Therefore, one follicular sample per patients was used for analysis. Samples of follicular fluid were centrifuged at 2,000 g for 10 minutes and the surpernatants stored at −80°C for further analysis until TAC and IMA analysis. At the beginning of oocyte retrieval, venous blood samples were obtained from antecubital vein into a non-heparinized tube from all participants for the detection of serum TAC and IMA levels. Blood samples were immediately centrifugated, and serum was separated and frozen at -80 ºC until assayed for IMA and TAC analyses.
IMA and TAC analyses
IMA concentrations were analyzed by measuring the complex composed of dithiothreitol and cobalt unbind to albumin by colorimetric method in spectrophotometer. First, a mixture of 200 μL of patients serum and 50 μL cobalt chloride (Sigma Aldrich, USA) was prepared in glass tubes. The mixture was left to incubation in room temperature for 10 minutes. After that, 50 μL of dithiothreitol (1,5 mg/ml) was added to the tubes and incubated in room temperature for 2 minutes. At the final stage 1000 μL of sodium chloride (0,9%) was added to the mixture. A blank specimen was prepared with distilled water for control. The analyses in spectrophotometer (Human Humalyzer 2000) was performed at 470 nm for detection of absorbance of the specimens and the results were given as absorbance units (ABSU). In our study, the reference values of IMA based on the study of Sinha et al [18]. A 20-µL aliquot of the samples (serum and FF) was used to measure TAC with Randox Total Antioxidant Status kit (RANDOX Total Antioxidant Status-Manual NX2332, RANDOX Laboratories, United Kingdom). Prepared tubes were incubated at room temperature for three minutes. The analyses in spectrophotometer (Human Humalyzer 2000) was performed at 600 nm at 37° C and results are given as mmol/L (Reference range: 1.30-1.77 mmol/L, RANDOX Total Antioxidant Status Manual kit).
Statistical analysis
Data analysis was performed by using SPSS for Windows, version 17 (SPSS Inc. Chicago, IL, United States). Whether the distributions of continuous variables were normally or not was determined by Shapiro Wilk test. Data were shown as mean ± standard deviation or median (minimum-maximum), where applicable. While, the mean differences between groups were compared by Student’s t test, otherwise Mann Whitney U test was applied for the comparisons of the median values. Nominal data were analyzed by Chi-square or Fisher’s exact test, where appropriate. Degrees of association between continuous variables were calculated by Spearman’s Rank Correlation Analyses. Area under Curve (AUC) and 95% confidence interval for TAC and IMA determination of embryo grade was evaluated by ROC analysis. The best cut off point of TAC and IMA and diagnostic performance such as sensitivity, specificity, positive and negative predictive values were also calculated. A p value less than 0.05 was considered statistically significant.
Results
The mean age was 30,8 ± 3,7 and 27,5 ± 3,9 years in study and control groups, respectively (p> 0.05). The mean BMI were similar in groups (26,3 ± 5,3 and 26 ± 5,4 kg/m2 in PCOS and control group, respectively;p>0.05). The cycle outcome parameters (total dose of gonadotropins, duration of induction, number of oocytes retrieved, number of MII oocytes, transferred day, number of transferred embryos, embryo grade, fertilization rate, implantation rate, pregnancy rate) of PCOS and control groups were found similar and these are given in Table 1. Although pregnancy rate in PCOS group (46%) was higher compared to control group (36%), the difference was not statistically significant (p=0.43). In 34 cases embryo transfer was performed on day 3, whereas in the remaining 26 cases embryo transfer was performed on day 5. The clinical pregnancy rate was significantly higher in participants with grade 1 embryos compared to patients with grade ≥2 embryos (p=0.029).
Serum IMA values of PCOS and control groups are 1.10 ± 0.38 and 1.15 ± 0.48 ABSU, respectively. Serum TAC levels were 1.49 ± 0.55 mmol/L in PCOS and 1.32 ± 0.31 mmol/L in controls. The FF IMA and TAC values were found similar in study and control groups. The serum and FF IMA and TAC levels of patients with grade 1 embryos were compared with participants with grade ≥2 embryos. The analysis showed a significantly higher level of FF IMA in cases with grade ≥2 embryos (p=0.021) FF IMA and TAC values regarding the embryo grading are given in Table 2. No statistically significant difference was found between FF and/or serum levels of IMA and TAC regarding the rate of clinical pregnancy (p>0.05). In PCOS group, mean FF IMA value (1.39 ± 0.32) was higher compared to mean serum IMA value (1.10 ± 0.38), the difference was statistically significant (p=0.00). In control group, mean FF IMA value (1.46 ± 0.38) was higher compared to mean serum IMA value (1.15 ± 0.48), the difference was statistically significant (p=0.00).
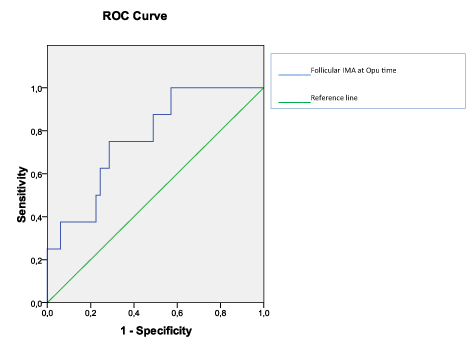
According to the Spearman’s Rank Correlation analyses performed in total group, neither the baseline characteristics (age, BMI) nor the cycle outcome parameters (given in Table 1) were correlated with serum or FF IMA and TAC levels (p>0.05). However, a positive correlation between FF IMA levels and embryo grading was found (r=0.534, p=0.004). The results of the ROC analysis showed that FF levels of IMA significantly predicted grade 1 embryo development (AUC:0.765, CI 95%: 0.606-0,924; p=0.017) (Figure 1). The cut-off point 1.475 ABSU had the sensitivity and positive predictive value in this evaluation. An IMA level of 1.475 ABSU had a sensitivity of 75%, specificity 72%, positive predictive value 73% and negative predictive value 60%. No correlation exists between FF IMA and TAC values of study groups (r=0.124, p=0.344). Moreover, serum IMA and TAC values of study groups were not correlated (r=0.171,p=0.192).
In PCOS patients, 14 patients had FF TAC levels below the reference range (≤ 1.299 mmol/L) whereas 16 cases had FF TAC levels above this value. When PCOS cases with FF TAC levels ≤1.299 mmol/L and >1,299 mmol/L were compared, significantly lower mean number of MII oocytes (11,0 vs 14,5, p=0.038) and significantly lower fertilization rate (%49,7 vs %64,75, p=0.044) were found (Table 3). Moreover, significantly higher levels of FF IMA (1.446 ABSU vs 1.365 ABSU, p=0.035) and significantly lower number of grade 1 embryos (p=0.019) was observed in PCOS cases with FF TAC levels ≤1.299 mmol/L (Table 3).
Discussion
The promising data pro2021 Copyright OAT. All rights reservOS in the FF of PCOS cases is very similar to non-PCOS cases when detected by IMA. Moreover, this is the first study in literature documenting the levels of serum and FF IMA and TAC in women with PCOS undergoing IVF.
In this study, the levels of IMA and TAC in FF were higher than serum levels which inturn indicates a greater degree of OS in FF compared to systemic circulation. Thus, it is natural that, a certain amount of ROS is needed for normal oocyte end embryo development where reactive oxygen species produced by the preovulatory follicle are considered important inducers for ovulation [3].
The results of our study also shows that FF TAC above the defined cut-off level (>1,299 mmol/L) is related with good quality embryos. In addition, a significant positive correlation between FF IMA levels and embryo grade in our study indicates the role of augmented OS in infertility. These results are in agreement with the previous data suggesting that high levels of antioxidants present in FF may be responsible for controlled generation of ROS thereby resulting in the formation of good quality of embryos [19]. Supporting this data, a positive correlation of embryo quality with TAC and a negative correlation with ROS levels in FF were recorded previously [4]. On the contrary, others reported lack of correlation between FF levels of TAC and embryo grade [7]. Although, OS in PCOS cases might be a detrimental factor in ART cycles, the results from this study does not support this hypotheses.
The meta-analysis of data of different circulating OS markers obtained from 68 individual studies, including a total of 4933 patients with PCOS and 3671 control women, showed that the concentrations of several promoters and by-products of OS are significantly increased in patients with PCOS compared with controls [2]. An approximately 20 fold increase in granulosa cell ROS generation was reported in PCOS compared to tubal factor infertility [5]. In addition, follicular fluid OS levels effecting the meiotic spindle formation in the oocyte was suggested as the related cause of infertility in PCOS cases [20]. Supporting this data, in PCOS cases, significantly higher levels of FF ROS and lower levels of FF TAC were recorded in the absence of meiotic spindle formation [21]. As previously suggested elevated ROS in the FF tends to limit the fertilization potential of oocytes, through disruption of meiotic spindle formation. Nevertheless, the results in this study indicate the importance of the balance between pro-oxidants and antioxidants in FF, supporting the previous data [8].
In general, following ovulation induction for IVF, oocyte and embryo quality in vitro are not obviously impaired in PCOS. However accompanying endocrine abnormalities, mostly insulin resistance, have been noted to be associated with reduced fertilization rates and abnormal early embryonic development [22]. We infer that the adverse fertility outcome in this subgroup of PCOS patients with insulin resistance might be explained by higher levels of OS. Supporting this data, in PCOS cases with insulin resistance higher levels of IMA was observed in serum samples compared to those without insulin resistance [22]. Even if, metabolic parameters like insulin resistance were not documented in PCOS participants in this study, further research might enlight this issue. Whether the correlation between FF IMA levels and embryo grade in our study depends on the presence of insulin resistance also needs to be clarified.
In our study, neither the TAC nor the IMA levels in serum and FF were associated with pregnancy outcome. However, recently, higher FF TAC, FF ROS-TAC scores and lower FF ROS levels were found to be associated with pregnancy after ICSI [9]. Supporting this data, high systemic blood TAC was reported as a significant factor for achieving clinical pregnancy [23]. The low number of cases in our study is a limitation for more accurate results regarding the pregnancy outcomes.
In conclusion, the results of this study adds to the currently available literature on the impact of OS in assisted reproductive technologies. Future large population based studies are necessary to elucidate the precise mechanisms through which elevated IMA levels in FF affects female reproductive outcomes in assisted reproductive technologies and PCOS.
Disclosure
No author has any potential conflict of interest.
References
- Palacio JR, Iborra A, Ulcova-Gallova Z, Badia R, Martinez P (2006) The presence of antibodies to oxidative modified proteins in serum from polycystic ovary syndrome patients. Clin Exp Immunol 144: 217-222 [Crossref]
- Murri M, Luque-Ramirez M, Insenser M, Ojeda-Ojeda M, Escobar-Morreale HF (2013) Circulating markers of oxidative stres and polycystic ovary syndrome (PCOS): A systematic review and meta-analysis. Human Reproduction Update 19: 268–288. [Crossref]
- Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, Gupta S (2012) The effects of oxidative stress on female reproduction: a review. Reproductive Biology and Endocrinology 10: 49
- Jana SK, K NB, Chattopadhyay R, Chakravarty B, Chaudhury K (2010) Upper control limit of reactive oxygen species in follicular fluid beyond which viable embryo formation is not favorable. Reprod Toxicol 29: 447-451. [Crossref]
- Karuputhula NB, Chattopadhyay R, Chakravarty B, Chaudhury K (2013) Oxidative status in granulosa cells of infertile women undergoing IVF. Syst Biol Reprod Med 59: 91-98 [Crossref]
- Das S, Chattopadhyay R, Ghosh S, Goswami SK, Chakravarty BN et al. (2006) Reactive oxygen species level in follicular fluid--embryo quality marker in IVF? Hum Reprod 21: 2403-2407 [Crossref]
- Pasqualotto EB, Agarwal A, Sharma RK, Izzo VM, Pinotti JA et al. (2004) Effect of oxidative stress in follicular fluid on the outcome of assisted reproductive procedures. Fertil Steril 81: 973-976 [Crossref]
- Oyawoye O, Abdel Gadir A, Garner A, Constantinovici N, Perrett C (2003) Antioxidants and reactive oxygen species in follicular fluid of women undergoing IVF: relationship to outcome. Hum Reprod 18: 2270-2274 [Crossref]
- Bedaiwy MA, Elnashar SA, Goldberg JM, Sharma R, Mascha EJ et al. (2012) Effect of follicular fluid oxidative stress parameters on intracytoplasmic sperm injection outcome. Gynecol Endocrinol 28: 51-55 [Crossref]
- Gaze DC. (2009) Ischemia-modified albumin: a novel biomarker for the detection of cardiac ischemia. Drug Metab Pharmacokinet 24: 333–341 [Crossref]
- Blair SA, Kyaw-Tun T, Young IS, Phelan NA, Gibney J et al. (2013) Oxidative stres and inflammation in lean and obese subjects with polycystic ovary syndrome. J Reprod Med 58: 107-114 [Crossref]
- Var A, KuÅŸcu NK, Koyuncu F, Uyanik BS, Onur E et al. (2003) Atherogenic profile in preeclampsia. Arch Gynecol Obstet 268: 45-47. [Crossref]
- Desai V, Prasad NR, Manohar SM, Sachan A, Narasimha SR et al. (2014) Oxidative stress in non-obese women with polycystic ovarian syndrome. J Clin Diagn Res 8: CC01-3 [Crossref]
- Caglar GS, Oztas E, Karadag D, Pabuccu R, Demirtas S (2011) Ischemia-modified albumin and cardiovascular risk markers in polycystic ovary syndrome with or without insulin resistance. Fertil Steril 95: 310-313. [Crossref]
- Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril (2004) 81: 19-25
- Scott RT Jr, Hofmann GE, Veeck LL, Jones HW Jr, Muasher SJ (1991) Embryo quality and pregnancy rates in patients attempting pregnancy through in vitro fertilization. Fertil Steril 55: 426-428. [Crossref]
- Gardner DK, Schoolcraft WB, Jansen R, Mortimer D, eds. Towards reproductive certainty: infertility and genetics beyond. Carnforth: Parthenon Press; 1999; In vitro culture of human blastocysts. p. 378.
- Sinha MK, Roy D, Gaze DC, Collinson PO, Kaski JC. (2004) Role of ‘‘Ischemia Modified Albumin’’, a new biochemical marker of myocardial ischaemia, in the early diagnosis of acute coronary syndromes. Emerg Med J 21: 29–34. [Crossref]
- Revelli A, DellePiane L, Casano S, Molinari E, Massobrio M et al. (2009) Follicular fluid content and oocyte quality: from single biochemical markers to metabolomics. Reprod Biol Endocrinol 7: 40 [Crossref]
- Rajani S, Chattopadhyay R, Goswami SK, Ghosh S, Sharma S et al. (2012) Assessment of oocyte quality in polycystic ovarian syndrome and endometriosis by spindle imaging and reactive oxygen species levels in follicular fluid and its relationship with IVF-ET outcome. J Hum Reprod Sci 5: 187-193 [Crossref]
- Chattopadhayay R, Ganesh A, Samanta J, Jana SK, Chakravarty BN et al. (2010) Effect of follicular fluid oxidative stress on meiotic spindle formation in infertile women with polycystic ovarian syndrome. Gynecol Obstet Invest 69: 197-202. [Crossref]
- Franks S, Roberts R, Hardy K. (2003) Gonadotrophin regimens and oocyte quality in women with polycystic ovaries. Reprod Biomed Online 6:181–184 [Crossref]
- Velthut A, Zilmer M, Zilmer K, Kaart T, Karro H et al. (2013) Elevated blood plasma antioxidant status is favourable for achieving IVF/ICSI pregnancy. Reprod Biomed Online 26: 345-352 [Crossref]