A titrimetric technique has been successfully employed to measure esterase activities in different tissues. We have applied this technique to investigate the distribution of the esterase enzymes in spinal cord and five different brain areas of the rat (telencephalon, mesencephalon, diencephalon, cerebellum and medulla oblongata). The presence of non-specific esterase activity has been determined using alpha-napthyl acetate, a widely employed substrate for these enzymes, whereby the possible existence of "heroin-esterases" has been tested. Our results suggest that the activity of cerebral esterase’s on heroin can be studied selectively at pH 8.5, showing quantitative differences in the distribution of these so-called " heroin-esterases" in spinal cord and in the five brain areas studied.
In parallel, the efficacy of such "heroin-esterases" has been verified using voltammetric analysis of the influence of the brain enzymes upon the substrate heroin resulting in the evidence of an oxidation signal due to the selective oxidation of uprising morphine.
Furthermore, the localization of these enzymes seems to overlap the distribution of brain morphine receptors, suggesting a possible role for these "heroin-esterase" in the metabolism and subsequently in the pharmacological activity of heroin, i.e., by converting this drug into morphine
Heroin, morphine, titrimetric measurements, voltammetric measurements, rat brain areas
Hydrolase activity has already been demonstrated in serum and liver, kidney, muscle and cerebral tissues and it is currently well established that such hydrolysis enzymes act on different chemical species such as glucosides, starches and aldehydes, endogenous and exogenous e-stems [1-8]. In the nervous tissue, hydrolysis-esterase activity exists in isoenzymic forms, for example 3 and 5 acetyl and aryl esterase, respectively [6]. Chemically, heroin is a diacetylmorphine, characterized by the presence of two ester groups and therefore susceptible to hydrolysis to morphine by esterase enzymes. Therefore, it has been suggested that in vivo metabolism of heroin depends on the action of cholinesterase that seems to have partial affinity for such substrate, or of various aryl esterases already demonstrated to be present in the rat brain [6-9]. Based on this suggestion, and to define an eventual distribution specificity of such type enzymes active on heroin, the spinal cord and the remaining five large regions of the rat brain were prepared i.e., telencephalon, mesencephalon, diencephalon, cerebellum and medulla oblongata. The similarity of the activity of non-specific esterase enzymes within the whole encephalic preparations of male and female rats was previously verified by means of alpha-napthyl acetate. This substrate is the most common ester compound employed to study such enzymes [10-11] and it has also been exploited to verify the presence of esterases within the different brain tissues analyzed herein.
In parallel, in vitro and ex vivo voltammetric analyses have been carried out. Indeed, it is known that morphine is easily oxidized at relatively low potentials, while most of the other opiates such as codeine, heroin, thebaine, or papaverine are not readily detectable electrochemically [12]. This selectivity for morphine has been used to advantage for the direct determination of morphine in poppy straw extracts [13].
Here in parallel to the titrimetric measurements, in vitro differential pulse voltammetric and ex vivo chronoamperometric analysis have been performed by means of Nafion coated micro electrodes [Nafion-mCFE] prepared as described earlier [14].
The use of such Nafion biosensors was selected as it is reported that morphine is positively charged, therefore it is attracted by the negatively charged polymer, thus improving the interaction between the sensor and the opioid. This causes an enhancement in peak current due to more accumulation of morphine on the electrode surface [15]. It appeared that the addition of the homogenate to the heroin substrate was follow by a modification of the electrochemical signal with an increased level of current that indicates the rising of an electroactive molecule such as morphine then supporting the titrimetric measurement of the chemical action of the heroin esterases upon heroin.
Twelve male and six female CD-COBS rats, obtained from Charles River (Italy) and weighing 200-250 g, were used in this study. After their sacrifice, the brain and the spinal cord were quickly removed, then for six male and for the six female rats each brain was prepared as a whole. Instead, each brain of the remaining six male rats was divided into the following areas: telencephalon, mesencephalon, diencephalon, cerebellum and medulla oblongata following the rat brain atlas by Swanson [16].
Titrimetric analysis
Every single brain as well as each of the individual cerebral areas, was then quickly homogenized in 1.15% KCl, at zero degrees (° C), in a ratio of 1: 4 weight / volume using a glass-glass homogenizer potter (SAVI Milan, Italy). All the homogenates were then centrifuged at 11,000 g at 4 ° C and each clear supernatant was collected and the enzymatic activity was analyzed.
This activity was measured by titrimetric method, as described by Ecobichon and Israel [17] using an automatic pH titrator (TTT 60 Radiometer, Copenhagen). Briefly in a constant volume of 3.5 ml containing the substrate 0.15 ml of each single extract (supernatant) was added. It has been observed that within an incubation period of 45 min at 37 ° C and under positive nitrogen pressure, the studied enzyme - substrate reaction
(i.e., hydrolysis in alcohol and acid of the substrate under study) was developing in linear mode as observed by the recording of the concomitant maintenance of neutral pH, via automatically operated infusion of NaOH. Accordingly, to Ecobichon and Israel [17] NaOH was used at predetermined concentration depending on the concentration of the substrate, and therefore the recording of the concomitant maintenance of neutral pH is index of the enzymatic activity developed.
The results obtained are thus expressed as hydrolyzed μmoles of substrate per minute and per mg of proteins (± SD). The proteins present in each extract were measured using the method described by Lowry [18]. Tukey's test was finally used for statistical data evaluation.
Here in particular the catalytic activity of cerebral esterases in function of pH was determined in a 1 mM solution of α-napthyl acetate which hydrolysis was studied with the titrimetric analysis carried out every 0.5 pH step. As shown in Figure 1, the catalytic efficiency of the so called "whole brain esterases" upon such substrate was active in a range of pH from 6.5 to 8.5, attaining the highest level at pH 7.5.
Similarly, the hydrolysis efficiency of brain esterases active on the substrate heroin [1mM] was monitored and it appeared that the pH range varies in this case from 6.5 to 10.5, with the highest hydrolysis efficiency obtained at pH 8.5 (Figure 1). Thus, the definition: "heroin esterases" was arbitrarily adopted herein to indicate a specific activity of such enzymes on this particular substrate.
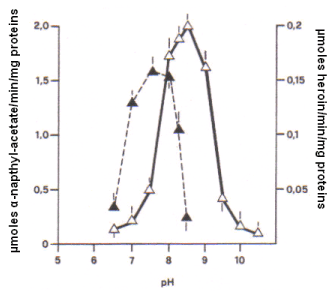
Figure 1. Profile of the esterase activity measured every 0.5 unit of pH in the whole brain tissue of six male rats incubated at 37 ° C in the presence of: α-naphtyl acetate 1 mM [ ⮝ ] , or in the presence of heroin 1 mM [ Δ ]. An identical profile was observed with the six cerebral preparations of the six females.
In addition, the direct comparison of the optimal activity of "whole brain esterases" on α-napthyl acetate (1 mM) at pH 7.5 versus that of "heroin esterases" on heroin (1 mM) at pH 8.5, shows that the latter exhibit an activity approximately 8 times lower i.e., 0.2 μmoles heroin/min/mg proteins versus 1.6 μmoles α-napthyl esterase/min/mg proteins. This fact, together with the difference in the optimal pH value and the elevated non-specificity of α-napthyl esterases, suggests a specificity and selectivity of action for the "heroin esterases" on heroin.
Then, the "heroin esterases" activity was studied upon the spinal cord and the five different brain areas mentioned above. In particular, since the activity levels of "heroin esterases" detected in the whole brain of six male rats appear to be very similar to those measured in the whole brain of six females, only the brains of male rats (n = 6) were dissected into the different brain regions cited above for the analysis of such enzymes. It appeared that medulla oblongata is the area showing the highest enzymatic activity against heroin 1 mM (0.22 + 0.026 μmoles / min / mg protein ± SD), followed by the spinal cord (0.16 ± 0.018), while the lowest "heroin-esterase" activity was measured in the mesencephalon (0.05 ± 0.007). Intermediate and comparable enzymatic activity was also highlighted in diencephalon (0.14 ± 0.007), telencephalon (0.13 ± 0.0018) and cerebellum (0.11 ± 0.009) (Figure 2).
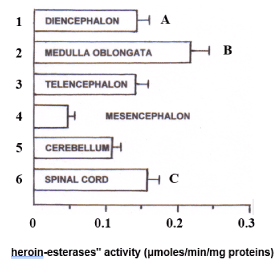
Figure 2. Quantitative distribution of "heroin-esterases" activity (μmoles/min/mg proteins), in the spinal cord and in the five male brain areas considered. Each value corresponds to the mean ± SD obtained from six individual experiments.
A: Diencephalon versus 2, 4, 6, p <0.01;
B: medulla oblongata versus 1, 3, 4, 5, 6, p <0.01;
C: spinal cord versus 2, 4, 5, p <0.01; Versus 3, p <0.05, Tukey test.
The homogenates were used as such without adjusting the pH, so that the quantitative distribution of "heroin-esterases" activity was assessed at biologically relevant pH.
Voltammetric analysis
In vitro differential pulse voltammetry was used in association with Nafion coated micro-carbon fibre electrodes [DPV- Nafion mCFE] prepared as described earlier [14]. DPV - mCFE measurements were performed in 100 μM solutions of morphine in pH 7.5 phosphate buffer (PBS) as described earlier [19] so that to confirm the feasibility of monitoring the voltammetric oxidation of such substrate with the association DPV-Nafion mCFE [14]. Accordingly, an oxidation peak was obtained at approximately 480mV as shown in Figure 3 TOP.
Ex vivo chronoamperometry was then applied as described [20] at 500mV potential and with the Nafion-mCFE placed in 3.5 ml PBS solution of the substrate heroin [1 mM, pH 8.5] to which was added either 1.15% KCl [blank solvent] or 0.15 ml of each single tissue extract (supernatant). In the first case, no change of the current value recorded was observed, while when the supernatant was added, current value increased within 15-20min, reaching then a plateau (Figure 3) [21].

Figure 3. Voltammetry was applied by means of a μAutolab polarograph (EcoChemie, The Netherlands) linked to an IBM PC computer equipped with a General-Purpose Electrochemical System Software(GPES) package [20] Measurements were performed with a three-electrode potentiostat system made of a electrochemicallycoated in HCl 0.1M silver wire (Clark Instruments, diameter100μm) to obtain a silver/silver chloride (Ag/AgCl) reference electrode, a silver wire auxiliary (counter, diameter 100μm) electrode and a 30μm diameter mCFE (working electrode) constructed and treated as described earlier [14]. In particular, here the use of short range differential amperometry applied within +500 and +550mV helps avoiding detection of ascorbic acid, dopamine, serotonin, their metabolites uric acid, i.e. the compounds monitored with DPV at oxidation potentials between −100mV and +450mV [21].
TOP: Differential pulse voltammogram (DPV) of a 100 μM solutions of morphine in pH 7.5 phosphate buffer. The DPV scan was applied from -100mV to +1000 mV versus Ag/AgCl reference at 10 mV/s with a pulse amplitude of 40 mV.
MIDDLE: amperometric scan (n=1) performed in the substrate heroin [1mM, pH 8.5] and
BOTTOM: mean of the amperometric results obtained in the substrate heroin [1mM, pH 8.5] to which was added either 1.15% KCl [blank solvent, open histograms] or 0.15 ml of each single tissue extract (supernatant i.e., here that of medulla oblongata: grey histogram). Data are presented as % of control pretreatment values ± SD, *p<0.05, Tukey test.
This indicates the insurgence of a long lasting oxidizable compound such as morphine, possibly due to hydrolysis of heroin as already proposed by Way et al. [22]. The pH 8.5 was chosen here in order to obtain the best metabolic conditions of brain areas homogenates versus heroin substrate.
Metabolic efficacy of human esterases upon drugs of abuse has been studied [23]. In the present work, a titrimetric technique has been successfully employed to investigate and to measure the distribution of esterase activities in different rat brain regions such as spinal cord, telencephalon, mesencephalon, diencephalon, cerebellum and medulla oblongata. The presence of non-specific esterase activity has been determined using α-napthyl acetate, a widely employed substrate for these enzymes. Furthermore, the putative existence of "heroin-esterases" has been tested using heroin as substrate. In particular, the recorded data suggest that the activity of cerebral esterases on heroin can be studied selectively at pH 8.5, showing quantitative differences in the distribution of these so-called "heroin-esterases" in spinal cord and in the five rat brain areas studied.
In parallel, the efficacy of such "heroin-esterases" has been verified by means of voltammetric analysis that confirmed the influence of these brain enzymes upon the substrate heroin resulting in the detection of an oxidation signal due to the selective oxidation of insurgent morphine from hydrolisate heroin. Accordingly, with earlier studies [19] the present electrochemical data support the observation that heroin is rapidly metabolized to morphine, and that the latter is probably responsible for most of the pharmacologic effects of heroin.
In previous studies carried out in the same cerebral areas we have analyzed here, a particular distribution of morphine receptors has been observed [24,25]. In particular, such distribution appears to be well correlated to the specific quantitative distribution of the "heroin esterases" monitored here. It is well known that the hematoencephalic barrier tends to prevent morphine to enter the cerebral tissue from the blood stream more efficiently than it does for heroin, given the greater liposolubility of the latter [26,27]. Once penetrated into the cerebral tissue then heroin is rapidly hydrolyzed first to mono-acetylmorphine and finally to morphine [22] and in this sense, one has to consider the suggested definition of heroin as the most rapid morphine-carrier in the brainstem [26,27], where this opioid will finally bind at the opiate receptors [24-26].
In light of these premise and of the precise quantitative correlation observed here between morphine receptors and "heroin-esterases" activity in the brain regions studied, it seems possible to suggest a role for such enzymes in the metabolism and hence the cerebral pharmacological activity of heroin, i.e., its rapid hydrophilic conversion to morphine. Indeed, once in the brain, heroin is rapidly metabolized into morphine by removal of the acetyl groups, therefore, it is known as a prodrug. It is the morphine molecule that then binds with opioid receptors and produces the subjective effects of the heroin high. Further analysis is, of course, necessary to explore this possibility; that could lead to the formulation of new therapeutic approaches as well as novel pharmacological indications towards situations of dependence from drugs. For instance, future experiments with non hydrolyzable analogs of heroin as negative control will further establish the specificity of heroin esterase towards heroin substrate. Nevertheless, these findings confirm the titrimetric technique as a quick and simple instrument for the investigation of hydrolytic enzymatic functions, i.e., able [or not] to activate particular drugs or chemicals in general, and this in biological preparations such as, for example, in selected brain areas. Indeed, while the voltammetric investigations have been performed at the selected optimal pH in order to obtain the best metabolic conditions of brain areas homogenates versus heroin substrate, for titrimetric measurements the homogenates were used as such without adjusting the pH, so that the quantitative distribution of "heroin-esterase" activity was assessed at biologically relevant pH.
- Arndt R, Schlaak H, Junge W, Uschtrin D, Südi D, et al. (1978) Investigations on the functional role of rat liver carboxylesterase isoenzymes. Hoppe-Seylers Z. Physiol Chem 359: 641. [Crossref]
- Augustinsson K (1959) Electrophoresis studies on blood plasma esterases I. Mammalian plasmas. Acta Chem Scand 13: 571.
- Crespi F, Pagliacci E, Rocchini GM, Bianchi R, Salmona M, et al. (1979) Esterase activity of rat muscle. Eur J Drug Metab Pharmacokinet 4: 175-177. [Crossref]
- Bianchi R, Garbagna L, Crespi F, Pocchiari F, Mussini E (1980) Further studies on rat, mouse and guinea pig muscle esterase activity. Biomedicine 33: 109-112. [Crossref]
- Bianchi R, Paro M, Ghidotti G, Mussini E (1982) Changes in muscle esterases in genetically dystrophic and control littermate mice. Muscle Nerve 5: 485-489. [Crossref]
- Holmes R, Masters C (1967) Developmental multiplicity and isoen-zyme status of rat esterases. Biochim Biophys Acta 146, 138.
- Salmona M, Saronio C, Bianchi R, Marcucci F, Mussini E (1974) In vitro hydrolysis of oxazepam succinate half-ester by a stereospecinc soluble esterase from different animal species. J Pharm Sci 63: 222-225. [Crossref]
- Junge W, Krisch K (1973) Current problems on the structure and classification of mammalian liver carboxylesterases (EC 3.1.1.1). Mol Cell Biochem 1: 41-52. [Crossref]
- Bernsohn J, Barron KD, Hess AR, Hedrick MT (1963) Alterations in properties and isoenzyme patterns of esterases in developing rat brain. J Neurochem 10: 783-794. [Crossref]
- Aldridge WN (1953) Serum esterases. I. Two types of esterase (A and B) hydrolysing p-nitrophenyl acetate, propionate and butyrate, and a method for their determination. Biochem J 53: 110-117. [Crossref]
- Kingsbury N, Masters CJ (1970) Molecular weight interrelationships in the vertebrate esterases. Biochim Biophys Acta 200: 58-69. [Crossref]
- Garrido J, Delerue-Matos C, Borges F, et al. (2004) Electrochemical Analysis of Opiates-An Overview. Analytical Letters 37: 831.
- Schwartz RS, Benjamin CR (1982) Voltammetric determination of morphine in poppy straw concentrate at a glassy carbon electrode. Anal Chim Acta 141: 365.
- Crespi F, Martin KF2021 Copyright OAT. All rights reservtracellular basal levels of serotonin in vivo using nafion-coated carbon fibre electrodes combined with differential pulse voltammetry measure 5HT release in vivo. Neuroscience 27: 885-896. [Crossref]
- Sanghavi BJ, Wolfbeis OS, Hirsch T, Swami NS (2015) Nanomaterial-based electrochemical sensing of neurological drugs and neurotransmitters. Mikrochim Acta 182: 1-41. [Crossref]
- Swanson LW (2004) Structure of the rat brain, Brain Maps III Academic Press, Elsevier Inc. Netherlands: pp 203.
- Ecobichon DJ, Israel Y (1967) Characterization of the esterases from electric tissue of Electrophorus by starch-gel electrophoresis. Can J Biochem 45: 1099-1105. [Crossref]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193: 265-275. [Crossref]
- Garrido J, Delerue-Matos C, Borges F, Macedo TRA, Oliveira-Brett AM (2004) Voltammetric Oxidation of Drugs of Abuse I. Morphine and Metabolites. Electroanalysis 16: 1419.
- Crespi F (2009) Anxiolytics antagonize yohimbine-induced central noradrenergic activity: a concomitant in vivo voltammetry-electrophysiology model of anxiety. J Neurosci Methods 180: 97-105. [Crossref]
- Crespi F (1991) In vivo voltammetry detection of neuropeptides with micro carbon fibre biosensors: possibile selective detection of somatostatin. Anal Biochem 194: 69-76. [Crossref]
- Way E, Young J, Kemp J (1965) Metabolism of heroin and its pharmacological implications. Bull Narc 17: 25-33.
- Meyer MR, Schütz A, Maurer HH (2015) Contribution of human esterases to the metabolism of selected drugs of abuse. Toxicol Lett 232: 159-166. [Crossref]
- Kuhar MJ, Pert CB, Snyder SH (1973) Regional distribution of opiate receptor binding in monkey and human brain. Nature 245: 447-450. [Crossref]
- Miller J, Fimar E (1973) Distribution of stereospecific binding of the potent narcotic analgesic etorphine in the human brain. Chem. Pathol Pharmacol 6: 1052-1062. [Crossref]
- Jaffe J, Martin WR (1980) Opioid analgesic and antagonists. In: Goodman Oilman A, Goodman LS (eds) The Pharmacological Basis of Therapeutics, Macmillan, New York, USA, pp: 507.
- Olendorf W, Hyman S, Olendorf S (1972) Blood-brain barrier: penetration of morphine, codeine, heroin and methadone after carotid injection. Science 178: 984-986. [Crossref]
- Pert CB, Snyder SH (1973) Opiate receptor: demonstration in nervous tissue. Science 179: 1011-1014. [Crossref]



