Abstract
Tobacco smoking is a major risk factor for early death, and smoking cessation is an important public health issue as it has health benefits. Passive smoking is a significant health concern for children and non-smoking adults. Lung cancer is the world’s leading cause of cancer-related mortality, and tobacco exposure is responsible for the majority of cases of the disease. However, the incidence of lung cancer in patients that have never smoked is increasing. Smoking prevention measures aim to reduce the overall number of smokers and the age-adjusted frequency of lung cancer. The use of equipment designed to prevent smoking, including e-cigarettes and devices against passive smoking, is warranted to protect children.
Introduction
Tobacco smoking is a major risk factor for early death and disease and has killed more than 5 million people worldwide every year since 1990 [1]. In Japan, the speculated number of deaths ascribable to smoking in 2005 was 196,000, which represented 18% of all deaths. The leading cause of smoking-ascribable deaths was malignant neoplasms [2]. Lung cancer is the world’s leading cause of cancer-related mortality, and tobacco exposure is responsible for the majority of cases of the disease, but the incidence of lung cancer among people that have never smoked is increasing [3]. In this article, we will discuss recent tobacco control topics related to oncology, with particular focus on lung cancer.
Tobacco control and smoking cessation
Many types of cancer and respiratory or cardiovascular disease are associated to smoking, which brings more medical matters than alcohol, drugs, and high cholesterol levels. Smoking cessation is an important public health issue, as it has intermediate and long-term health benefits. Thus, smoking cessation measures that were widely available, accessible, and cost-effective would have great public health benefits. In addition, passive smoking is an important health issue for young and non-smoking adults and develops to cause bronchial asthma, meningitis, sudden infant death syndrome, and inflammation of the middle ear [4]. Thus, smoking prevention remains as a matter of most important matters and needs aggregative policy and personal procedure [5], it is high priority that exact data on the health effects about smoking sufficiently valuable to statespersons and the general public. A large global study (the Global Burden of Disease 2015 Tobacco collaborators’ study) was recently reported [6]. It found that of all global deaths 6.4 million (11.5%) were attributable to smoking. Furthermore, it was demonstrated that the number of smokers worldwide had decreased to 933.1 million (males: 768.1 million, females: 165 million), 52.2% of which were living in one of four countries (China, India, the USA, and Russia). The top 10 countries in terms of the sizes of their smoking populations are shown in (Figure 1). The age-standardized worldwide prevalence of daily smoking was 25.0% for males and 5.4% for females in 2015, which represented 28.4% and 34.4% reductions, respectively, on the numbers seen in 1990 [6]. In Japan, the prevalence of smoking was 29.7% for males and 9.7% for females in 2016, which represented 52.6% and 6.0% reductions, respectively, since 1965 (Figure 2) [7].
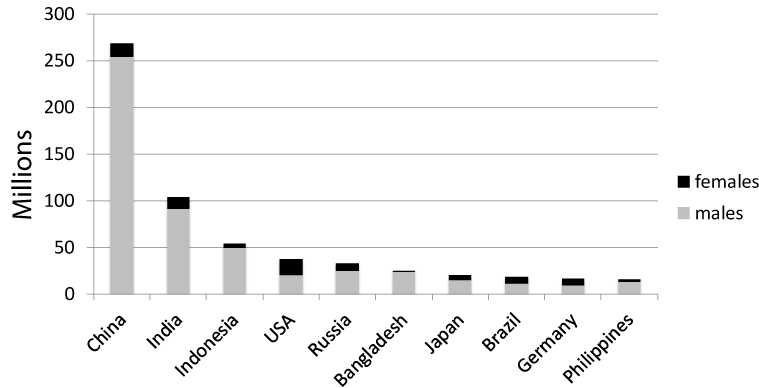
Figure 1. Top 10 countries ranked according to the sizes of their smoking populations.
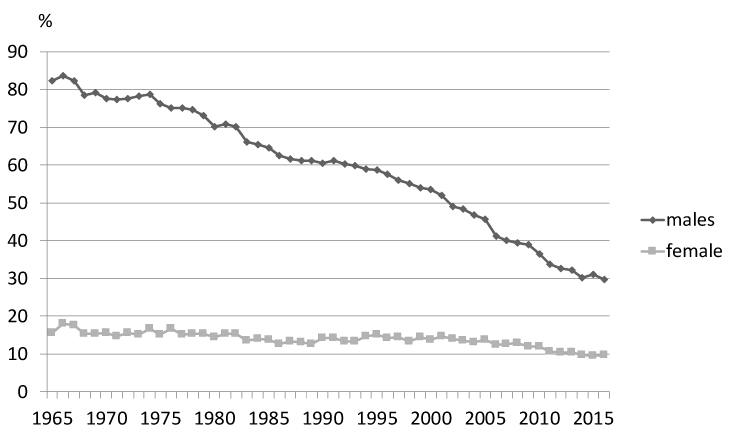
Figure 2. Adult tobacco habit (%) transition in Japan.
Frequency of smoking and the lung cancer mortality rate
“How can the reduction in the frequency of smoking and the concomitant increase in the lung cancer mortality rate be explained? The reason is that there is no relationship between tobacco smoking and lung cancer”. This is the view of the pro-smoking lobby. Is it true? Certainly, the frequency of male smokers is decreasing, as shown in (Figure 2), and the lung cancer mortality rate is increasing (from 11.201 per 100,000 people in 1965 to 87.194 per 100,000 people in 2015), as shown in Figure 3. However, the frequency of cancer generally increases in old age. In developed countries around the world, such as Japan, populations are becoming markedly older, and the number of patients with lung cancer has consequently increased. Therefore, age-adjusted cancer-related mortality per 100,000 people is used for epidemiological comparisons of the numbers of cancer patients. The age-adjusted lung cancer mortality rates for each sex in Japan are shown in (Figure 3) [8]. The frequency of lung cancer peaked in 1996, and the subsequent reduction was considered to have been due to a drop in the number of tobacco smokers. As people who smoke tobacco will not develop cancer for many years after they start smoking, the lung cancer mortality rate is expected to peak about 30 years after the frequency of smoking peaks.
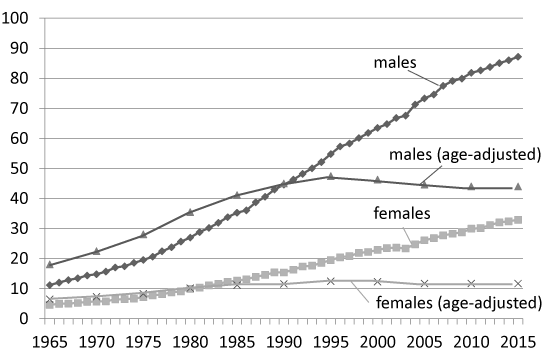
Figure 3. Mortality rate and age-adjusted mortality rate of patients with lung cancer per 100,000 people in Japan.
Passive smoking
Passive inhalation of smoke pouring from cigarette has various harmful actions to children. The children of tobacco smokers are attacked by nicotine and other baleful chemicals both in the uterus and in their home environments. Exposure during intrauterine life to smoke pouring from cigarette brings poor birth offspring and affects lung, brain, and cardiovascular growth, which places such children at enlarged risk of harmful consequences later in life, disorders like adiposeness, actionable disturbance, and cardiovascular-related disorders [9]. In addition, most smokers initiate smoking in early adulthood, when they are at increased risk of becoming addicted to nicotine. It is warranted that development of biomarkers which express tobacco chemical influence to health in childhood and rest of life.
To produce a pooled estimate of the relative risk of lung cancer associated with exposure to passive smoking in women who have never smoked, but whose spouses smoke, Taylor et al. conducted a meta-analysis of 55 studies, including cohort, population-based case-control, and non-population-based case-control studies [10]. The pooled relative risk of lung cancer for these women was found to be 1.27 (95% confidence interval [CI]: 1.17-1.37), and a causal relationship between passive smoking and lung cancer was demonstrated to exist. A systematic review by Hori et al. clarified the relative risk of lung cancer in secondhand smokers in Japan. The latter review identified nine epidemiological studies encompassing 12 populations, and the pooled relative risk of lung cancer associated with secondhand smoke exposure was estimated to be 1.28 (95% CI: 1.10-1.48) [11]. Recent meta-analyses of secondhand smoke exposure and lung cancer are listed in Table 1 [10-13]. Almost all of these meta-analyses reported an overall relative risk of lung cancer of 1.2-1.3 [10], whereas the overall relative risk of small cell lung cancer was 3.09 [13]. In the Liberal Democratic Party, the governing party of Japan, there is now debate about whether to strengthen legal measures to curb secondhand smoke in public places before the 2020 Tokyo Olympic and Paralympic Games.
Table 1. Recent meta-analyses of secondhand smoke exposure and lung cancer.
Author | Year | Studies | Relationship | Relative risk |
Taylor16 | 2007 | 12 | Yes | 1.3 |
Kim18 | 2014 | 18 | Yes | 1.31* |
Jayes17 | 2016 | 13 | Yes | 1.41 |
Hori15 | 2016 | 9 | Yes | 1.28 |
The estimated association with secondhand smoke exposure was greater for small cell lung cancer than for non-small cell lung cancer (odds ratio=2.11).
*1.26 for adenocarcinoma, 1.41 for squamous cell carcinoma, 1.48 for large cell lung cancer, 3.09 for small cell lung cancer
E-cigarettes
Electronic cigarettes (e-cigarettes) are aggressively merchandised in tune with Internet, TV, magazines, sport contents, and advertising displays, etc. E-cigarettes are generally promoted as a harmless replacement to traditional cigarettes and/or as a quitting smoking adjuvant [14, 15]. Although e-cigarettes appear to cause less harm as a nicotine delivery device than conventional cigarettes, they seem to be a gateway to traditional cigarette smoking in adolescents. E-cigarettes described easier to stop using, a cut down or the complete quitting of traditional cigarette smoking and an improved subjective health status and smokers who used e-cigarettes would encourage e-cigarettes to other smokers [16]. The British Medical Association [17], Public Health England [18], and the American Association of Public Health Physicians [19] consider that e-cigarettes have a role to play in reducing the harm of tobacco smoking. By contrast, The World Health Organization [20], World Lung Foundation [21], and World Medical Association give a caution to using of e-cigarette.
Very few controlled studies have investigated the acute toxicities associated with e-cigarettes. Exposure to the primary component of the e-liquid, propylene glycol, is generally considered safe, but this chemical can irritate the upper and lower respiratory tract. When heated, it produces formaldehyde and acetaldehyde, which are both toxic [22]. Several case reports have described suicide attempts involving the liquid found in e-cigarettes. Two of these individuals had very high levels of nicotine in their venous circulation after they intravenously injected or ingested the liquid [23, 24], and two other cases involved individuals who had ingested nicotine in much higher quantities than the immediately dangerous to life or health concentration (IDLH) level, but did not die [25, 26]. E-cigarettes can cause increases in blood pressure and heart rate and have the potential to cause cardiac events and arrhythmias in people who have or are at risk of cardiac disease [27]. Most of the suggested cardiac effects of e-cigarettes are secondary effects of the nicotine, rather than being caused by the other components of the e-liquid [28]. Carnevale et al. conducted a crossover, single-blind study of the effects of e-cigarettes vs. conventional cigarettes on oxidative stress and endothelial function in healthy smokers and non-smoking adults [29]. Although e-cigarettes seemed to have less impact, oxidative stress increased, and nitric oxide levels and vasodilation decreased after e-cigarette use. Vardavas et al. studied whether using an e-cigarette for 5 minutes has an impact on pulmonary function or the fraction of exhaled nitric oxide in healthy adult smokers [30]. As a result, a reduction in the nitric oxide level and acute increases in respiratory flow resistance and overall peripheral airway resistance were detected. These two studies demonstrated that e-cigarettes rapidly alter vascular function; i.e., they reduce nitric oxide synthesis and small airway function while increasing airway resistance. Lanza et al. studied the frequencies of e-cigarette and conventional cigarette use in US middle and high school students aged 11-19 (n=22,007) [31]. The frequency of e-cigarette use increased faster than the frequency of conventional cigarette use from the ages of 13-16. E-cigarette use was strongly associated with conventional cigarette use, particularly during early adolescence [odds ratio: >40 before age 12]. The recent increase in e-cigarette availability has resulted in many individuals using these devices without knowing about the possible adverse consequences. Healthcare providers should educate e-cigarette users, especially children, about the risks associated with accidental exposure to the liquid found within them.
The increase in the number of lung cancer patients that have never smoked
Measures aimed at reducing the frequency of smoking have decreased smoking prevalence rates for both males and females and lung cancer mortality rates in many countries [32]. Although tobacco smoking is a major risk factor for lung cancer, it is estimated that 25% of lung cancer cases involve patients that have never smoked, and it is the seventh most common cause of cancer-related mortality [33]. Lung cancer in people that have never smoked is now regarded as a distinct disease entity that is separate from smoking-related lung cancer, as there are differences in the histological characteristics, age at diagnosis, stage at presentation, and survival outcomes of the two forms of the disease [34-36], and actionable driver oncogene mutations are more frequent in cases of non-small cell lung cancer (NSCLC) involving patients that have never smoked [37-39]. Two of the most common actionable driver mutations associated with lung cancer in patients that have never smoked are an activating mutation in the kinase domain of the epidermal growth factor receptor gene (EGFR) and chromosomal rearrangement involving the anaplastic lymphoma kinase gene (ALK) [40-43]. The etiologies of these actionable driver mutations in lung cancer patients (regardless of smoking status) remain unknown. Estrogen has been reported to transactivate growth factor signaling pathways, including the EGFR axis, and these effects appear to be reciprocal [44, 45]. In addition, the effects of passive tobacco smoking and the aging of society remain potential etiologies of lung cancer in individuals that have never smoked. The absolute number [the number of affected individuals per 100,000] of lung cancer patients who have never smoked is actually increasing. We do not know the exact reason why the frequency of lung cancer is increasing among patients that have never smoked and so this issue needs further study.
Low-tar filter cigarette smoke
Cellulose acetate filters brought more porous cigarette papers and decreased 37mg to 22mg for the tar of a cigarette during 1950 and 1960s [46]. Moreover, the development of air infiltration bores in the filter tip resulted low tar (commonly 8-14 mg) and very low tar (equal to or less than 7 mg) cigarettes in the late 1960s. At the same time, the mean tar volume per cigarette reduced to 13 mg in US by 1990 [46]. Close directional movement in standardized tar blew up to the UK [47, 48] and other countries. In Japan, shifting from nonfilter to filter cigarettes observed around 1965 [49, 50]. Indeed, the WHO Framework Convention on Tobacco Control (FCTC) has recommended the removal of tar and nicotine numbers from packages [51]. Low-tar filters mostly cart off the bigger particles in cigarette smoke that have tendency to be caught in the middle thick bronchus which is the area squamous cell carcinoma occur. By contrast, low-tar filters do not cart off smaller particles that have tendency to arrive the peripheral of the lung which is the area adenocarcinomas occur. It is hypothesized that the temper adenocarcinoma increase compare with squamous cell carcinoma in various countries due to the spread of low-tar filter cigarettes. In addition, low-tar filter smokers have tendency to inspire deeply compare with those of nonfilter cigarettes, resulting in cigarette smoke arriving the peripheral area. This hypothesis is supported by the several studies. It is documented that the risk of lung adenocarcinoma is many times higher for current smokers than non-smokers, i.e. , 19.0-fold for men and 8.1-fold for women in the Cancer Prevention Study (CPS) II (1982-1984) from US, while in CPS I (1959-1961) corresponding estimates were 4.6-fold for men and 1.5-fold for women [52]. European countries also documented that the risk of lung adenocarcinoma was much higher for current smokers than non-smokers, i.e. , 8.0 times higher for men and 4.1 times for women in a case-control study conducted in 6 countries (1988-1994) [53], while in a case-control study in 5 countries conducted previously (1976-1980) corresponding estimates were 3.5 and 1.8 for men and women, respectively [54].
Tobacco smoking and lung cancer histologic type
Lung cancer come into existence by smoking, however, there is an attitude it for single disease but capture and research independent disorder separated by histological types. Kreyberg et al. [55] divided lung cancer into two groups based on the result of case study separated by histological types of patients with lung cancer which compare the tobacco smoking rates, and advocate group I tumors (squamous, large, and small cell carcinoma) which related tobacco smoking tightly and group II tumors (adenocarcinoma) which have uncertain etiological relationship with tobacco smoking. Analytical epidemiology researches which confirm Kreyberg hyposis include the reports of Doll et al [56] and Shimizu et al [57]. Doll et al. [56] conducted hospital based case control study that research relationship with 872 male patients with lung cancer (832 cases in Kreyberg group I and 40 cases in group II) and tobacco smoking. The result, dose-response relationship was observed between daily tobacco smoked number and lung cancer risk in Kreyberg group I, however, in Kreyberg group II it was small sample size and not observed obvious relationship to tobacco smoking. Although Doll also studied with female patients with lung cancer and observed tendency of dose-response relationship, the conclusion was sustained because group II was small sample size (13 cases). Shimizu et al [57] selected the patients visited Aichi Cancer Center Hospital Respiratory Department who diagnosed lung cancer afterward and control cases and conducted case control study by matched pair analysis. The result, relative risk of smokers was high as 7.0 in Kreyberg group I (63 pairs) and confirmed dose-response relationship, however, confirmed only slightly higher rate of 1.3 (or 1.5 for current smokers) in group II (36 pairs).
By contrast, Stayner et al. [58] and Weiss et al [59] reported relationship between adenocarcinoma which included in Kreyberg group II and tobacco smoking in a proactive manner. Stayner et al [58] conducted case control study and research relationship with tobacco smoking about between male patients with lung cancer (152 squamous cell, 50 adenocarcinoma, and 45 small cell carcinoma) which selected 9 areas of The Third National Cancer Survey (TNCS) and population-based control. In the result, age-adjusted relative risk of smokers was all significantly higher as 5.1, 3.1, and 3.1 in small cell carcinoma, squamous cell carcinoma, and adenocarcinoma, respectively. Although the dose-response relationships were observed all histological types, it was not statistical significant in adenocarcinoma. Weiss et al. [59] identified smoking status of 6,136 male participant ≥ 45 years old at the beginning of a study of Philadelphia Pulmonary Neoplasm Research Project (PNRP), and follow up over 10 years, and calculated lung cancer affect risk by person-year method. In the results, dose-response relationship between number of tobacco smoked and lung cancer affect risk was observed in not only well-differentiated squamous cell cancer (23 cases) and small cell carcinoma (8 cases) but also adenocarcinoma (14 cases), and was not observed in poorly differentiated squamous cell carcinoma (13 cases) and large cell carcinoma (4 cases). Lubin et al. reported the relationship of smoking and lung cancer histology as part of large scale hospital base case control study in Europe (7,804 cases and 15,207 control cases). It was strongly suggested the positive significant dose-response correlation between lung cancer risk and duration of smoking, amount of smoking and degree of inhale, and negative significant dose-response correlation between length of smoking cessation not only in squamous cell carcinoma (3,708 males, 272 females) and small cell carcinoma (1,172 males, 199 females) alone, but in adenocarcinoma (716 males, 223 females) in both male and female, and strongly suggested causal association between smoking. Sobue et al conducted a population-based cohort study of 91,738 men and women, and 422 lung cancer incident cases were analyzed during 1990-1999 [60]. The relative risk for all incident cases associated with current smokers versus non-smokers was 4.5 and 4.2 for men and women, respectively. When separated by histologic type, relative risk for small cell carcinoma and squamous cell carcinoma were 17.5 and 12.7, while for adenocarcinoma it was 2.8 and 2.0 for men and women, respectively. This result suggested that current cigarette smoking is related with an increased lung cancer risk about 10- to 20-fold for small and squamous cell carcinoma and 2- to 3-fold for adenocarcinoma.
Tobacco smoking and immune checkpoint inhibitors
Immunotherapy has become as a new treatment choice for patients with advanced NSCLC. Several comparative clinical trials clearly articulated that immune checkpoint inhibitors leaded superior survival outcomes compared to chemotherapy in patients with advanced NSCLC [61-66]. Some studies with immune checkpoint inhibitors in NSCLC suggested that smoking history was associated with improved survival outcomes. Kim et al. conducted meta-analysis to investigate if survival benefits of immune checkpoint inhibitors in patients with advanced NSCLC are different according to smoking status and 2,389 ever-smokers and 413 never-smokers were included from 6 studies [67]. In first-line treatment setting, immune checkpoint inhibitors tended to improve PFS in patients with smoking history (HR = 0.85, P=0.07), however, chemotherapy was significantly associated with improvement of PFS (HR = 2.30, P=0.009) for never-smokers. In more than second-line setting, immune checkpoint inhibitors significantly prolonged OS over that with chemotherapy in ever-smokers (HR= 0.70, P<0.00001), however, immune checkpoint inhibitors failed to significantly improved OS for never-smokers with NSCLC (HR=0.79, P=0.12). Nishio M, et al. recently reported phase II study of nivolumab for patients with advanced or recurrent non-squamous non-small cell lung cancer and current/former smokers were more responsive to treatment than non-smoker (ORR 29.1% vs 4.8%) [68]. Smoking status may will be a predictive marker for survival to immune check point inhibitors.
Conclusion
In conclusion, smoking prevention measures aim to reduce the overall number of people who smoke and the age-adjusted frequency of lung cancer. The use of equipment designed to prevent smoking, including e-cigarettes and devices against passive smoking, is warranted in order to protect children.
Conflict of interest
The authors report that no potential conflicts of interest exist.
References
- GBD 2015 Risk Factors Collaborators (2016) Global, regional, and national comparative risk assessment of 79 behavioral, environmental and occupational, and metabolic risks or clusters of risks, 1990-2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet 388: 1659-1724. [Crossref]
- Katanoda K, Marugame T, Saika K, Satoh H, Tajima K, et al. (2008) Population attributable fraction of mortality associated with tobacco smoking in Japan: a pooled analysis of three large-scale cohort studies. J Epidemiol 18: 251-264. [Crossref]
- Pelosof L, Ahn C, Gao A, Horn L, Madrigales A, et al. (2017) Proportion of never-smoker non-small cell lung cancer patients at three diverse institutions. J Natl Cancer Inst 109. [Crossref]
- Inoue M, Tsuji I, Wakai K, Nagata C, Mizoue T, et al. (2005) Evaluation based on systematic review of epidemiological evidence among Japanese populations: tobacco smoking and total cancer risk. Jpn J Clin Oncol 35: 404- 411. [Crossref]
- Zhong L, Goldberg MS, Parent ME, Hanley JA, et al. (2000) Exposure to environmental tobacco smoke and the risk of lung cancer: a meta-analysis. Lung Cancer 27: 3-18. [Crossref]
- GBD 2015 Tobacco Collaborators (2017) Smoking prevalence and attributable disease burden in 195 countries and territories, 1990-2015: a systematic analysis from the Global Burden of Disease Study 2015. Lancet 389: 1885-1906. [Crossref]
- Japan health promotion a fitness foundation (2017) http://www.health-net.or.jp/gaiyou/.
- Cancer statistics in Japan -2016 (2017) Foundation for Promotion of Cancer Research (FPCR) http://ganjoho.jp/data/reg_stat/statistics/brochure/2016/cancer_statistics_2016.pdf.
- Farber HJ, Groner J, Walley S, Nelson K, et al. (2015) Protecting children from Tobacco, nicotine, and Tobacco smoke. Pediatrics 136: e1439-1467. [Crossref]
- Taylor R, Najafi F, Dobson A (2007) Meta-analysis of studies of passive smoking and lung cancer: effects of study type and continent. Int J Epidemiol 36: 1048-1059. [Crossref]
- Hori M, Tanaka H, Wakai K, Sasazuki S, Katanoda K (2016) Secondhand smoke exposure and risk of lung cancer in Japan: systematic review and meta-analysis of epidemiologic studies. Jpn J Clin Oncol 46: 942-951. [Crossref]
- Jayes L, Haslam PL, Gratziou CG, Powell P, Britton J, et al. (2016) SmokeHaz: Systematic Reviews and Meta-analyses of the Effects of Smoking on Respiratory Health. Chest 150: 164-179. [Crossref]
- Kim CH, Lee YC, Hung RJ, McNallan SR, Cote ML, et al. (2014) Exposure to secondhand tobacco smoke and lung cancer by histological type: a pooled analysis of the International Lung Cancer Consortium (ILCCO). Int J Cancer 135: 1918- 1930. [Crossref]
- Cobb NK, Brookover J, Cobb CO (2015) Forensic analysis of online marketing for electronic nicotine delivery systems. Tob control 24: 128-131. [Crossref]
- Dockrell M, Morrison R, Bauld L, McNeill A (2013) E-cigarettes: prevalence and attitudes in Great Britain. Nicotine Tob Res 15: 1737-1744. [Crossref]
- Berg CJ, Barr DB, Stratton E, Escoffery C, Kegler M (2014) Attitudes toward e-cigarettes, reasons for initiating e-cigarette use, and changes in smoking behavior after initiation: a pilot longitudinal study of regular cigarette smokers. Open J Prev Med 4: 789-800. [Crossref]
- British Medical Association (2016) E-cigarettes Available from: http://www.bma.org.uk/working-for-change/improving-and-protecting-health/tobacco/e-cigarettes.
- McNeill A, Brose LS, Calder R (2015) E-cigarettes: an evidence updates. A report commissioned by Public Health England. London: Public Health England.
- American Association of Public Health Physicians (2013) Principles to Guide AAPHP Tobacco Policy: American Association of Public Health Physicians. Available from: http://www.aaphp.org/tobacco.
- World Health Organization (2014) Electronic nicotine delivery systems.
- World Lung Foundation (2014) WHO right to call for e-cigaretteregulation. Available from: http://www.worldlungfoundation.org/ht/d/ReleaseDetails/i/32757.
- Bekki K, Uchiyama S, Ohta K, Inaba Y, Nakagome H, et al. (2014) Carbonyl compounds generated from electronic cigarettes. Int J Environ Res Public Health 11: 11192-11200. [Crossref]
- Bartschat S, Mercer-Chalmers-Bender K, Beike J, Rothschild MA, Jübner M (2015) Not only smoking is deadly: fatal ingestion of e-juice - a case report. Int J Legal Med 129: 481- 486. [Crossref]
- Thornton SL, Oller L, Sawyer T (2014) Fatal intravenous injection of electronic nicotine delivery system refilling solution. J Med Toxicol 10: 202-204. [Crossref]
- Eberlein CK, Frieling H, Kohnlein T, Hillemacher T, Bleich S (2014) Suicide attempt by poisoning using nicotine liquid for use in electronic cigarettes. Am J Psychiatry 171: 891. [Crossref]
- Schipper EM, de Graaff LC, Koch BC, Brkic Z, Wilms EB, et al. ( 2014) A new challenge: suicide attempt using nicotine fillings for electronic cigarettes. Br J Clin Pharmacol 78: 1469-1471. [Crossref]
- Lippi G, Favaloro EJ, Meschi T, Mattiuzzi C, Borghi L, et al. (2014) E-cigarettes and cardiovascular risk: beyond science and mysticism. Semin Thromb Hemost 40: 60-65. [Crossref]
- Middlekauff HR, Park J, Moheimani RS (2014) Adverse effects of cigarette and noncigarette smoke exposure on the autonomic nervous system: mechanisms and implications for cardiovascular risk. J Am Coll Cardiol 64: 1740-1750. [Crossref]
- Carnevale R, Sciarretta S, Violi F, Nocella C, Loffredo L, et al.(2016) Acute impact of Tobacco vs electronic cigarette smoking on oxidative stress and vascular function. Chest 150: 606-612. [Crossref]
- Vardavas CI, Anagnostopoulos N, Kougias M, Evangelopoulou V, Connolly GN, et al. (2012) Short-term pulmonary effects of using an electronic cigarette: impact on respiratory flow resistance, impedance, and exhaled nitric oxide. Chest 141: 1400-1406. [Crossref]
- Lanza ST, Russell MA, Braymiller JL (2017) Emergence of electronic cigarette use in US adolescents and the link to traditional cigarette use. Addict Behav 67: 38-43. [Crossref]
- Islami F, Torre LA, Jemal A (2015) Global trends of lung cancer mortality and smoking prevalence. Transl Lung Cancer Res 4: 327-338. [Crossref]
- Ferlay J, Shin HR, Bray F, Forman D, Mathers C, et al. (2010) Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 127: 2893-2917. [Crossref]
- Rudin CM, Avila-Tang E, Harris CC, Herman JG, Hirsch FR, et al. (2009) Lung cancer in never smokers: molecular profiles and therapeutic implications. Clin Cancer Res 15: 5646-5661. [Crossref]
- Sun S, Schiller JH, Gazdar AF (2007) Lung cancer in never smokers--a different disease. Nat Rev Cancer 7: 778-790. [Crossref]
- Toh CK, Gao F, Lim WT, Leong SS, Fong KW, et al. (2006) Never-smokers with lung cancer: epidemiologic evidence of a distinct disease entity. J Clin Oncol 24: 2245-2251. [Crossref]
- Pao W, Girard N (2011) New driver mutations in non-small-cell lung cancer. Lancet Oncol 12: 175-180. [Crossref]
- Li C, Fang R, Sun Y, Han X, Li F, et al. (2011) Spectrum of oncogenic driver mutations in lung adenocarcinomas from East Asian never smokers. PLoS One 6: e28204. [Crossref]
- Sun Y, Ren Y, Fang Z, Li C, Fang R, et al. (2010) Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 28: 4616-4620. [Crossref]
- Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, et al. (2004) Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 350: 2129-2139. [Crossref]
- Song X, Wang Z (2017) Clinical efficacy evaluation of tyrosine kinase inhibitors for non-adenocarcinoma lung cancer patients harboring EGFR-sensitizing mutations. Onco Targets Ther 10: 3119-3122. [Crossref]
- Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, et al. (2007) Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 448: 561-566. [Crossref]
- Tao H, Cai Y, Shi L, Tang J, Liu Z et al. (2017)Analysis of clinical characteristics and prognosis of patients with anaplastic lymphoma kinase-positive and surgically resected lung adenocarcinoma. Thorac Cancer 8: 8-15. [Crossref]
- Pietras RJ, Marquez-Garban DC (2007) Membrane-associated estrogen receptor signaling pathways in human cancers. Clin Cancer Res 13: 4672-4676. [Crossref]
- Nose N, Sugio K, Oyama T, Nozoe T, Uramoto H, et al. (2009) Association between estrogen receptor-beta expression and epidermal growth factor receptor mutation in the postoperative prognosis of adenocarcinoma of the lung. J Clin Oncol 27: 411-417. [Crossref]
- Hoffmann D, Djordjevic M, Brunnemann K (1996) Changes in cigarette design and composition over time and how they influence the yields of smoke constituents. In: Shopland D, ed. The FTC cigarette test method for determining tar, nicotine, and carbon monoxide yields of US cigarettes: report of the NCI expert committee. NCI smoking and tobacco control monograph No 7. Bethesda MD: US National Institutes of Health, National Cancer Institute, 15-37.
- Wald N, Doll R, Copeland G (1981) Trends in tar, nicotine, and carbon monoxide yields of UK cigarettes manufactured since 1934. Br Med J (Clin Res Ed) 282: 763-765. [Crossref]
- Kiryluk S, Wald N (1989) Trends in cigarette smoking habits in the United Kingdom, 1905-1985. In: Wald N, Froggatt P, eds. Nicotine, smoking and the low tar programme. Oxford: Oxford University Press.
- Burns DM, Major JM, Shanks TG (2001) Smoking lower yield cigarettes and disease risks. In: National Cancer Institute, smoking and tobacco control, monograph 13: risk associated with smoking cigarettes with low machine-measured yields of tar and nicotine. Washington DC: NIH-NCI, 65-158.
- Tominaga S (1986) Smoking and cancer patterns and trends in Japan. In: Zaridze DG, Peto R, eds. Tobacco: a major international health hazard. IARC: 103-13.
- First Report of Committee A (2008) Conference of the Parties to the WHO Framework Convention on Tobacco Control. Third Session, Durban, South Africa. http://www.tobaccolabels.ca/fctcandh/fctcarticl .
- Thun MJ, Lally CA, Flannery JT, Calle EE, Flanders WD, et al. ( 1997) Cigarette smoking and changes in the histopathology of lung cancer. J Natl Cancer Inst 89: 1580-1586. [Crossref]
- Simonato L, Agudo A, Ahrens W, Benhamou E, Benhamou S, et al. (2001) Lung cancer and cigarette smoking in Europe: an update of risk estimates and an assessment of inter-country heterogeneity. Int J Cancer 91: 876-887. [Crossref]
- Lubin JH, Blot WJ (1984) Assessment of lung cancer risk factors by histologic category. J Natl Cancer Inst 73: 383-389. [Crossref]
- Kreyberg L (1955) Lung cancer and tobacco smoking in Norway. Br J Cancer 9: 495-510. [Crossref]
- Doll R, Hill AB, Kreyberg L (1957) The significance of cell type in relation to the aetiology of lung cancer. Br J Cancer 11: 43-48. [Crossref]
- Shimizu H (1983) Case control research of lung cancer separated by histology. Lung Cancer 23: 127-137.
- Stayner LT, Wegmar DH (1983) Smoking, occupation, and histopathology of lung cancer: a case-control study with the use of the Third National Cancer Survey. J Natl Cancer Inst 70: 421-426.
- Wiss W, Boucot KR, Seidman H, Carnahan WJ (1972) Risk of lung cancer according to histologic type and cigarette dosage. JAMA 222: 799-801. [Crossref]
- Sobue T, Yamamoto S, Hara M, Sasazuki S, Sasaki S, et al. (2002) Cigarette smoking and subsequent risk of lung cancer by histologic type in middle-aged Japanese men and women: the JPHC study. Int J Cancer 99: 245-251. [Crossref]
- Herbst RS, Baas P, Kim DW, Felip E, Pérez-Gracia JL, et al. (2016) Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet 387: 1540-1550. [Crossref]
- Brahmer J, Reckamp KL, Baas P, Crinò L, Eberhardt WE, et al. (2015) Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 373: 123-135. [Crossref]
- Borghaei H, Paz-Ares L, Horn L, Spigel DR, Steins M, et al. (2015) Nivolumab versus Docetaxel in Advanced Non-squamous Non-Small-Cell Lung Cancer. N Engl J Med 373: 1627-1639. [Crossref]
- Fehrenbacher L, Spira A, Ballinger M, Kowanetz M, Vansteenkiste J et al. (2016) Atezolizumab versus docetaxel for patients with previously treated non-small-cell lung cancer (POPLAR): a multicenter, open-label, phase 2 randomised controlled trial. Lancet 387: 1837-1846. [Crossref]
- Rittmeyer A, Barlesi F, Waterkamp D, Park K , Ciardiello F, et al. (2017) Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a phase 3, open-label, multicentre randomized controlled trial. Lancet 389: 255-265. [Crossref]
- Reck M, RodrÃguez-Abreu D, Robinson AG, Hui R, CsÅ‘szi T, et al. (2016) Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375: 1823-1833. [Crossref]
- Kim JH, Kim HS, Kim BJ (2017) Prognostic value of smoking status in non-small-cell lung cancer patients treated with immune checkpoint inhibitors: a meta-analysis. Oncotarget 8: 93149-93155. [Crossref]
- Nishio M, Hida T, Atagi S, Sakai H, Nakagawa K, et al. (2017) Multicentre phase II study of nivolumab in Japanese patients with advanced or recurrent non-squamous non-small cell lung cancer. ESMO Open 1: e000108. [Crossref]



