Abstract
Clinical innovations in the field of neuropathic pain have been hampered since some decades due to a lack of insight in pathogenetic mechanisms. At the EFIC 2017 in Denmark it was shocking to find out how many pharmaceutical companies stopped being active in this field due to negative experiences in the recent past. The quick and easy solutions based on the know-how of anti-epileptics and anti-depressants, capsaicin and lidocaine led to the development and registration of many drugs from these classes in peripheral neuropathic pain. Gabapentin, pregabalin, duloxetine, tapentadol and dermal formulations were registered in indications such as Painful Diabetic Neuropathy (PDN) and/or Post-Herpetic Neuralgia (PHN). However, after picking the low hanging fruit further development of new antineuropathic pain agents stagnated. Many highly selective ligands for various receptors failed in phase IIb and phase III. Clearly it is time for clinical innovations in this field, based on new insights in pathogenesis of peripheral neuropathic pain. If we can learn to better match specific targeted therapy to patient populations where the target plays a major pathogenetic role, new effective therapies will emerge. By developing a quick response test to identify responders from non-responders, based on the topical administration of placebo or phenytoin cream, we suggest being able to differentiate ex juvantibus between pain mainly induced by central sensitization versus pain of a more peripheral and dermal origin.
Furthermore, we need to reconsider our focus on disease entities, and this is not only an academic challenge, but also a challenge for authorities such as EMA and FDA. Taking Small Fiber Neuropathy (SFN) and Chronic Idiopathic Axonal Neuropathy (CIAP) as examples we will discuss some critical issues, to put emphasis on new ways to approach this matter.
Key words
pain, heterogeneity, pathogenesis, neuropathy, CIAP, peripheral
Peripheral neuropathic localized pain as for instance in PDN and SFN, is characterized by hyperexcitability of nociceptors, which can be inhibited by sodium channel blockers. Such hyperexcitability is based on reduced receptor threshold of the C nociceptor, leading to spontaneous discharges and to intensified nociceptor responses in response to stimulation. These phenomena lead to the classical symptoms of mechanical and heat allodynia and spontaneous burning or stinging pain [1]. These symptoms reduce the quality of sleep and life of patients suffering from painful peripheral neuropathies. The burning sensations in general seems to suggest involvement of small fibers, even in forms of compression neuropathy [2]. SFN is increasingly recognized as one of the major pathogenetic disturbances leading to burning and stinging pain in many peripheral neuropathic syndromes. Diabetes mellitus is the main cause of SFN. Glucose intolerance and the metabolic syndrome are also associated with SFN. In CIAP, sarcoidosis, Lyme disease, HIV and amyloidosis, SFN can develop [3]. Alcohol is the main toxic agent inducing SFN. SFN is therefore a secondary complication in many internal and neurological disorders. The diagnosis CIAP is dependent on the clinical impression, a length dependent neuropathy, including disturbances of the electromyography. In many CIAP patients there are complaints of burning pain, comparable to patients suffering from SNF. This supports a possible comparable pathogenesis in both groups. The diagnosis SFN rests on the clinical impression, a normal electromyography and a reduction of the small nerve fibers, as detected via a skin biopsy [4]. In indications as PDN, CIAP and perhaps also in PHN subgroups of patients therefore might be identified as suffering from SFN.
Peripheral neuropathic pain and sodium channel dysfunctions. Voltage-Gated Ion Channels (VGIC) are identified in the peripheral nervous system (PNS) and are identified as targets for novel therapies for neuropathic pain [5]. Interestingly, the recognition of certain genetic causes of rare forms of SFN based on the identification of gain-of-function mutations in genes encoding the sodium channels Nav1.7 and Nav1.8 stimulated the pharmaceutical industry to develop selective blockers for these channels [6]. The general idea was that these channel blockers would subsequently be promising in the therapy of other more prevalent neuropathic pain syndromes. That promise has not yet been substantiated. Furthermore, in certain cases of supposed selective sodium channel blockers, for instance related to the drug raxatrigine, the exact VGIC target remained a bit unclear [7]. Another complication is that sodium channel dysfunction due to Nav1.7 mutations have been identified in only around 25% of patients with idiopathic SFN, and clearly dysfunctions in the other 75% are related to non-Nav1.7 targets [8]. Other abnormalities, for instance of the Nav1.9 channel in certain hereditary disorders leading to pain, are meanwhile identified [9]. It remains not totally clear in how far subspecies of the Nav channel subtypes are distributed in the peripheral nervous system, let alone in the keratinocytes and the immune-competent cells of the skin. This suggests that a less selective channel blocker might be preferable for the treatment of peripheral neuropathic pain, especially developed as a topical formulation devoid of systemic side-effects.
The pharmaceutical company Teva explored the efficacy of topical 4% and 8% ointment of TV-45070 (funapride), a small-molecule inhibitor of mainly the sodium channel Nav1.7, initially developed by Xenon, in PHN, where the drug missed the primary endpoint [10]. Teva did however stratify for the Nav1.7 R1150W polymorphism and found a greater efficacy in this subgroup compared to the wild-type.
The question therefore is legitimate, whether for the topical treatment of peripheral neuropathic pain a non-selective sodium blocker might be more successful; an approach we favor and will discuss shortly. This would also be less costly, as genotyping patients before topical treatment is not required. And a second question not answered during research was whether the primary target for a selective sodium channel blocker in PHN is localized peripherally and can indeed be reached by topical treatment.
TV-45070: lessons learned
The article reporting the failed study in PHN described in detail the rationale for such development of a selective sodium channel blocker [10]. The argument was build up as follows:
- Nav1.7 is a target that has an essential role in human pain sensing.
2021 Copyright OAT. All rights reserv
- Congenital indifference to pain is caused by deficiency of Nav1.7.
- Neuropathic pain cannot be provoked in congenital indifference to pain patients and in mouse knockouts of Nav1.7.
- Gain-of-function mutations enhances the Nav1.7 firing and are the explanation for the pain experienced in paroxysmal extreme pain disorder and in inherited erythromelalgia (IEM).
- A common variant in Nav1.7, where arginine is replaced with tryptophan the protein sequence is present in around 25% of the population and leads to an overactivity of the Nav1.7 channel and increased sensitivity to pain.
These arguments were mentioned by the authors as support for inhibition of the Nav1.7 channel “as a strategy for analgesic development.” The authors stipulated that to maximize inhibition of sodium channels locally in skin and subcutaneous tissue and minimize systemic adverse events, topical TV-45070 ointment was developed, following a positive proof of principle study with oral TV-45070 in inherited erythromelalgia (IEM) [11]. This was thought to be sufficient to proceed conducting a proof-of-concept study to evaluate the safety and efficacy of topically applied TV-45070 in PHN. Now the missing link in this approach is the pathogenesis of pain in PHN; nowhere in the article this topic was mentioned. Is the major pathogenetic link situated in the nociceptors in the skin, that question was not answered. Earlier experiments suggest there is no correlation between severity of allodynia and epidermal innervation of the PHN skin [12]. The minimal absorption of the active compound through the skin is very unlikely to be the explanation for a central effect on pain modulation in PHN. The target was supposed by TEVA to reside in the skin, as there was a clear reference to high skin concentration of the compound. Histological studies in the past study correlated the presence of PHN with the severe distal nociceptive axon loss, and pain persistence with segmental atrophy of the spinal cord dorsal horn, supporting the importance of central responses to nerve injury in the pathogenesis of pain [13]. If a significant part of the pathogenesis of pain would be related to more central localized mechanisms, would a peripheral block of mainly one subtype of a sodium channel lead to sufficient efficacy? To be more specific, Peng et al recently proposed three different subtypes of PHN: patients with:
- irritable nociceptors,
- de-afferentation, and
- central reorganization.
Basically, one could differentiate between two major types of PHN, a type predominantly depending on central sensitization and a type related to peripheral sensitization. Furthermore, the authors stated that even though multiple etiological mechanisms may exist, the core pathological mechanism underlying PHN is a possible lesion somewhere in the entire afferent transmission system, and key for successful treatment is the dissection of the biological mechanism underlying PHN [14]. It is exactly this dissection which was missing in the Teva strategy so far reported and was perhaps one of the reasons for a failed study.
This is supported by data from a different study, the remifentanil infusion study in PHN, where the compound could partially reverse neuropathic pain, also in a subgroup of patients only, probably in those with relevant inhibition of the signal amplification at the spinal level (perhaps corresponding to the subgroup 3 of Peng) [15]. In a study evaluating the efficacy of oxcarbamazepine in various types of peripheral neuropathic pain, including PHN, oxcarbazepine was more efficacious in patients with the irritable vs the nonirritable nociceptor phenotype [16]. Therefore, it seems, that a dissection of a syndrome like PHN into various pathogenetic related subtypes is needed in order to enhance the likelihood for successful treatment. Such dissection will also serve to better treat patients suffering from heterogeneous indications such as CIAP and SFN.
Topical phenytoin, a non-selective sodium channel blocker used in an ex juvantibus approach to detect responders
In the last 8 years we have developed many compounded topical formulations containing active compounds such as ketamine, clonidine, amitriptyline and baclofen [17]. Since some years we optimized one specific formulation containing phenytoin, because we were impressed by its efficacy in case series, and phenytoin could be easily combined with the other (co-)analgesics in a base vehicle cream [18]. Phenytoin is increasingly recognized as a compound suited for the topical treatment of peripheral neuropathic pain [19]. Phenytoin is a co-analgesic equal in efficacy compared to carbamazepine in trigeminal neuralgia [20]. Its multiple mechanisms of action and its unselectively, with affinity for many targets of family of sodium channels (and additional targets), makes it suited to treat a variety of neuropathic disturbances, if we do not yet know precisely the pathogenetic base [21]. Three cell types in the epidermis are most probably responsible for the peripheral wind up: the nociceptors, the keratinocytes and the immunocompetent cells. All cells contain sodium channels, and one blocker might thus inhibit overactivity in all cells (Figure 1). This was the main reason why we choose to develop a topical formulation of phenytoin. Moreover, it is a small lipid molecule, easy penetrable through the outer layers of the skin.
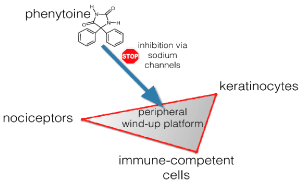
Figure 1: Phenytoin inhibits overactive cells in the skin via its sodium channel blocking properties and this mechanism leads to a reduction of the peripheral sensitization.
We subsequently analyzed many other topical developments and identified one major flaw, the selection and the evaluation in clinical trials of subclinical dosages [22]. We therefore developed topical formulations able to contain high concentrations of phenytoin, and meanwhile tested a dose-range of 5-30 % in patients suffering from peripheral neuropathic pain syndromes [23]. After application of the cream a quick ‘action of onset’, mostly within a time-frame of 15-20 minutes, was noticeable for many patients [24]. We then developed a single-blind response test based on the application of placebo and active cream, to identify responders during their first visit to our clinic. Our hypothesis is that such responder test will help to identify those patients were there is a close fit between the pathogenesis (residing in the epidermal area) and the mechanism of action of topical phenytoin. Non-responders can be identified too, and those might be patients with a significant central sensitization component. In these cases, we prefer to treat by prescribing oral (co-)analgesics. In this way, we reduce the chance that patients, after an initial positive placebo response, end up as non-responders to the cream (Figure 2).
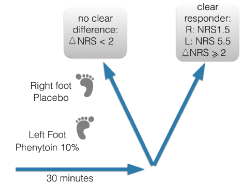
Figure 2: Quick response test to identify phenytoin responders and differentiate from placebo-responders.
The test is easy and takes only a minute to conduct. We first document the baseline Numeric (Pain) Rating Scale (NPRS) score of two localized area's (for instance both feet in SFN, DM and CIAP patients). Patients then receive a fingertip unit (0.5 gram) placebo to rub in on one foot, followed by phenytoin cream to apply on the other foot. After 10-20 minutes, we evaluate the NPRS at both sites. We define a responder as those patients noticing a difference of 2 or more points on the NPRS between placebo and the active cream. Such patients receive a prescription. Mostly we use phenytoin 10% for the response test, the cream is compounded in a specialized pharmacy in the Netherlands.
Especially patients suffering from SFN respond often quite quick, within 20 minutes, while the duration of effect might also relatively long, between 6.5 and 24 hours, as we recently reported in a small case series [25].
The EMA seems to support such approach where she stresses the importance of personalized medicine as a medical model for tailoring the right therapeutic strategy for the right person at the right time, based on an individual’s characteristics and genetic makeup. Classical RCT’s are not optimized for such personalized medicine. The aims of personalized medicine are to decrease the number of adverse drug reactions and to increase the efficacy of drug therapy. The utility of personalized medicine is defined by the EMA as to identify patients who are most likely to benefit [26]. This implies patient selection, and the response test we described above can be seen in the context of such personalized medicine.
Conclusion
In 1999 Woolf an Mannion emphasized in the Lancet that “only when we have the tools to identify the mechanism responsible for the pain in an individual, and then the capacity to reverse the mechanisms, will the management of neuropathic pain really advance [27].” This still holds true. We discussed the heterogeneity in disease entities such as SFN and CIAP and pointed out that multiple mechanisms on the level of the sodium channels are to be expected. SFN seems to be a common denominator in many prevalent neuropathic pain syndromes and its pathogenesis resides to be expected in the skin. In the absence of a clearly defined pathogenetic pathway related to one variety of a sodium channel in a specific neuropathic pain state, it seems therefore more promising to use a broad acting sodium channel blocker such as phenytoin, especially when compounded as a topical formulation containing a high concentration.
Conflict of interest
JMKH is one of the patent holders of two patents related to repurposing of phenytoin: topical phenytoin for use in the treatment of peripheral neuropathic pain and topical pharmaceutical composition containing phenytoin and a (co-)analgesic for the treatment of chronic pain.
References
- Ochoa JL, Campero M, Serra J, Bostock H (2005) Hyperexcitable polymodal and insensitive nociceptors in painful human neuropathy. Muscle Nerve 32: 459-472.
- Triki L, Zouari HG, Kammoun R, Kammoun F, Kammoun I et al. (2017) A reappraisal of small- and large-fiber damage in carpal tunnel syndrome: New insights into the value of the EMLA test for improving diagnostic sensitivity. Neurophysiol Clin 47: 427-436. [Crossref]
- De Schryver EL, van Schelven LJ, Notermans NC, de Valk HW, Oey PL (2011) Small-fibre neuropathy can be detected in patients with chronic idiopathic axonal polyneuropathy. Eur J Neurol 18: 1003-1005.
- Hoeijmakers JG, Faber CG, Lauria G, Merkies IS, Waxman SG (2012) Small-fibre neuropathies--advances in diagnosis, pathophysiology and management. Nat Rev Neurol 8: 369-379.
- Tibbs GR, Posson DJ, Goldstein PA (2016) Voltage-Gated Ion Channels in the PNS: Novel Therapies for Neuropathic Pain? Trends Pharmacol Sci 37: 522-542. [Crossref]
- Bennett DL, Woods CG (2014) Painful and painless channelopathies. Lancet Neurol 13: 587-599. [Crossref]
- Keppel Hesselink JM (2017) Moving targets in sodium channel blocker development: the case of raxatrigine: from a central NaV1.3 blocker via a peripheral NaV1.7 blocker to a less selective sodium channel blocker. J Med Therap 1: 1-3.
- Faber CG, Hoeijmakers JG, Ahn HS, et al. (2012) Gain of function Nanu1.7 mutations in idiopathic small fiber neuropathy. Ann Neurol 71: 26-39. [Crossref]
- Okuda H, Noguchi A, Kobayashi H (2016) Infantile Pain Episodes Associated with Novel Nav1.9 Mutations in Familial Episodic Pain Syndrome in Japanese Families. PLoS One 11: e0154827. [Crossref]
- Price N, Namdari R, Neville J, Proctor KJ, Kaber S et al. (2017) Safety and Efficacy of a Topical Sodium Channel Inhibitor (TV-45070) in Patients With Postherpetic Neuralgia (PHN): A Randomized, Controlled, Proof-of-Concept, Crossover Study, With a Subgroup Analysis of the Nav1.7 R1150W Genotype. Clin J Pain 33: 310-318.
- Goldberg YP, Price N, Namdari R, Cohen CJ, Lamers MH et al. (2012) Treatment of Na(v)1.7-mediated pain in inherited erythromelalgia using a novel sodium channel blocker. Pain 153: 80-85.
- Buonocore M, Gatti AM, Amato G, Aloisi AM, Bonezzi C (2012) Allodynic skin in post-herpetic neuralgia: histological correlates. J Cell Physiol 227: 934-938.
- Oaklander AL (2008) Mechanisms of pain and itch caused by herpes zoster (shingles). J Pain 9: S10-8.
- Peng WW, Guo XL, Jin QQ, Wei H, Xia XL et al. (2017) Biological mechanism of post-herpetic neuralgia: Evidence from multiple patho-psychophysiological measures. Eur J Pain 21: 827-842.
- Prosenz J, Kloimstein H, Thaler U, Drdla-Schutting R, Sandkühler J et al. (2017) A brief, high-dose remifentanil infusion partially reverses neuropathic pain in a subgroup of post herpetic neuralgia patients. J Clin Neurosci 40: 195-197.
- Demant DT, Lund K, Vollert J, Maier C, Segerdahl M et al. (2014) The effect of oxcarbazepine in peripheral neuropathic pain depends on pain phenotype: a randomised, double-blind, placebo-controlled phenotype-stratified study. Pain 155: 2263-2273.
- Keppel Hesselink JM and Kopsky DJ (2017) Topical compounded analgesic treatment in neuropathic pain: 8 years of experience. J Pain Manage Med 3: 128.
- Kopsky DJ and Keppel Hesselink JM (2017) Phenytoin in topical formulations augments pain reduction of other topically applied analgesics in the treatment of trigeminal neuralgia. J Clin Anesth 38: 154-155.
- Knezevic NN, Tverdohleb T, Nikibin F, Knezevic I, Candido KD (2017) Management of chronic neuropathic pain with single and compounded topical analgesics. Pain Manag 7: 537-558.
- Keppel Hesselink JM and Schatman ME (2017 ) Phenytoin and carbamazepine in trigeminal neuralgia: marketing-based versus evidence-based treatment. J Pain Res 10: 1663-1666.
- Keppel Hesselink JM (2017) Phenytoin: a step by step insight into its multiple mechanisms of action-80 years of mechanistic studies in neuropharmacology. J Neurol 264: 2043-2047.
- Keppel Hesselink JM, Kopsky DJ, Stahl SM (2017) Bottlenecks in the development of topical analgesics: molecule, formulation, dose-finding, and phase III design. J Pain Res 10: 635-641.
- Keppel Hesselink JM and Kopsky DJ (2017) The use of topical compounded analgesic creams in neuropathic pain by the primary care physician. J Gen Pract (Los Angel) 5: 345.
- Keppel Hesselink JM (2017) Analgesic onset of action or onset of relief in neuropathic pain. Anaesthesia, Critical Care and Pain Management 1: 48-50.
- Keppel Hesselink JM, Kopsky DJ (2017) Topical Phenytoin Cream in Small Fiber Neuropathic Pain: Fast Onset of Perceptible Pain Relief. Int J Pain Relief 1: 015-019.
- EMA news. 07/03/2017 Personalised medicines – focus on patients and healthcare professionals.
- Woolf CJ, Mannion RJ (1999) Neuropathic pain: etiology, symptoms, mechanisms, and management. Lancet 353:1959-1964. [Crossref]


