Tuberculosis (TB) continues to be a major health problem causing enormous morbidity worldwide despite introduction of effective and affordable chemotherapy more than 50 years ago. Among adults of most economically productive age groups and people affected with HIV TB, tuberculosis remains a leading killer, and even cured TB cases can be left with lifetime post TB sequelae, thus substantially reducing their quality of life. M. tuberculosis has shown extraordinary capabilities to subvert and resist bactericidal response of their infected host. These capabilities have enabled the bacillus to colonize about one third of the world’s population of which 1.5 million people die annually. Further development of drug resistant strains poses a serious problem for the TB control programs. These alarming facts and figures call for an urgent concern to focus our efforts against tuberculosis. However, with the currently existing case finding and treatment policies, it seems far way to eliminate the deadly pathogen in near future. With many concentrated efforts to tackle this infection, we still look for the ‘magic bullet’ which can defeat this ‘Captain of death’. There is a need for an effective vaccine to strengthen our fight in elimination of this challenging disease. Thus, the need of the situation is to tackle the disease through vaccination programme. For a nation of the size of India, therapy and prevention needs to go side by side to be able to achieve the ambitious goals of achieving tuberculosis elimination by 2025. Thus, in the present review an attempt has been made to review the status and efforts underway for vaccine development against tuberculosis and discussed the limitations of vaccines.
tuberculosis, vaccines, clinical trials, bacillus calmette guerin, immunology
Tuberculosis is a global health emergency and needs urgent attention. This deadly infectious disease has killed more people than HIV, malaria, influenza, cholera, plague and small pox combined together.[1,2] Even today, it kills more people globally as compared to any other infectious disease alone and affects more than 10 million people per year.[2-5] Rising drug resistant tuberculosis makes the situation more complex. Tuberculosis epidemic has become a matter of grave concern and current tuberculosis control programs are severely strained. An efficacious vaccine to prevent the disease has become an urgent global public health priority. [6-8]
A potent tuberculosis Vaccine can be a game changer to tackle the present TB epidemic. Thus, developing newer TB vaccines is considered a huge public health priority by the World Health Organization (WHO).[8] However, production of a successful vaccine is compromised by an incomplete understanding of immunology of tuberculosis, lack of reliable biomarkers and immune correlates, less duration of protection provided by existing vaccination, cost of production, and further magnified by social and political hurdles for vaccine commitment. All these issues need to be considered in successful vaccine design. BCG has been the age old vaccine but has several limitations. [9,10]
Encouragingly, proof of evidence from some of the vaccine studies in initial phases may pave a way for a successful candidate. Several candidates are presently in clinical trials. These preparations are based on - whole cell vaccine, live attenuated or killed vaccine; DNA or protein subunit; or virally vectored vaccines. Of these, VPM 1002, Mycobacterium indicus pranii (MIP) [earlier known as Mw] and M72/AS01 vaccines have advanced further and phase III studies are underway. India has taken a landmark initiative to synthesize the existing proof of evidence to conduct a large multi-centric phase III vaccine trial for evaluating the safety and efficacy of Indian indigenous product MIP manufactured by Cadila Pharmaceuticals and other potential vaccine candidate VPM1002 manufactured by Serum Institute of India. World is also awaiting the results of Phase III trial on M. vaccae to throw more insights on this vaccine. (Figure 1) briefly summarizes the journey of some TB vaccine candidates in pipeline.
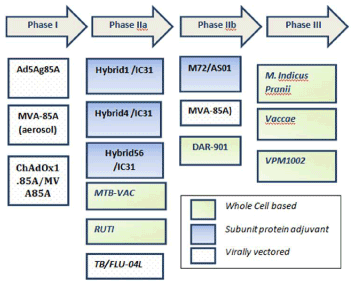
Figure 1. Representation of TB vaccine candidates in pipeline
World Health Organization (WHO) has laid special emphasis on novel approaches and the development and evaluation of a TB vaccine as a need to progress ahead in our path for TB elimination. The blue print for the same emphasizes on improving technologies and discovery for understanding TB immunology, establishing better preclinical models, building reliable biomarkers and immune correlates and formulating good clinical trials that will follow a uniform pattern.[8,9]
Unfortunately, there are no reliable correlates in TB, to define vaccine‐induced protection, although researchers have commonly used cell‐mediated immune (CMI) responses like interferon‐and gamma (IFN‐γ) production to determine vaccine's immunogenic potential. Recent literature has also suggested the role of local secreted IgA and central memory T cells as correlate of TB protection. However, the immune reaction that is indicative of actual prevention of the development of TB has not been identified. More research in this area can pave the way for identifying an effective vaccine easier.[3-6] Search for a more reliable animal models have suggested newer models like rabbit skin model which may be more insightful as a TB challenge model. Reliable TB biomarkers are another challenge in this endeavour. Focus on TB immunology and immunopathology, search for better modes of delivery are important thrust areas of focus to move ahead.[7-10] A comprehensive dedicated effort with strong social, political, and economic commitment is the need of the hour. The other caveat for the new vaccine trials is that they may have to be studied as an adjunct to or an additive for BCG as the large population in India is already pre immunized with the BCG vaccine. The vaccines evaluated both in light of pre-immunization with BCG and those focussing BCG naïve population needs to be studied individually. The benefits of the universal immunization programme in the area of child health may make the latter population difficult to identify. With a pipeline of vaccine candidates in various stages of development, trials both failed and successful are both a treasure resource to enhance our understanding and guide new breakthroughs. This article summarizes the journey of TB vaccines in brief.
For decades, the Bacille Calmette Guerin (BCG) has been the sole tuberculosis vaccine, and it is considered to be the most widely and commonly used vaccine. Despite its highly variable efficacy (ranging 0-80%) in different trials, children at many places across the world, still receive a BCG immediately after birth. Although it has been shown to prevent disseminated and severe form of tuberculosis in children, [11-14] its clinical efficacy is far from what we desire. More trials have shown lack of protection against adult forms of TB and very few trials, such as the BNRC Trial in England have shown protection. Further, in those trials that have reported a long duration of follow-up protection wanes with time.
Strain and batch variability, prior exposure to environmental mycobacteria, waning memory in adolescence, prior helminthic exposure and various other factors may have limited the success of BCG vaccine. Definite proof one way or other, does not exist for any of these hypotheses. Moreover, none of the studies have identified an immunological correlate or a biomarker as indicative of protection. Thus, it is not possible to say which biomarker can be seen in those that do not develop TB in the long run as opposed to those that do develop TB.
Although imperfect, BCG remains a proof of concept, that vaccine mediated protection against TB is possible and Insights into our experience and understanding the pitfalls with the BCG vaccine may show us direction towards a better vaccine! rBCG strains or modified BCG delivery are being evaluated for their efficacy and safety. [8,9,13,14]
TB vaccine community has a vibrant pipeline of different TB vaccine candidates, including genetically modified BCG or M. tuberculosis, antigens delivered with proteins as adjuvant or by viral vectors, and other immune-modulatory vaccines which could work as therapeutic vaccines. Killed vaccines have the advantages of having comprehensive antigen repertoire and having similarity to natural infection. The killed bacteria like RUT1, detoxified and fragmented M.tb cells, or heat‐killed/irradiated Mycobacterium vaccae are being investigated for therapeutic as well as prophylactic treatment. Sub unit vaccines are in pipeline and offer the promising benefit to boost the BCG-primed immunity, decrease bacterial loads and provide efficient protection against progressive TB-infection, and also showing a possible promise in latent tuberculosis. Many trials with single antigens have shown initial disappointment, but vaccines with combination of antigens with newer adjuvants and better delivery systems can bring the breakthrough discovery! We discuss some of the promising candidates below:
VPM1002 (SII, TBVI): VPM1002 is a live recombinant form of BCG developed by Vakzine Projekt Management in Germany and also licensed by the Serum Institute of India (SII). This recombinant vaccine has the listeriolysin gene added in the BCG genome, and urease gene is deleted, which allows it to escape macrophage lysosome.[15,16] The vaccine is being looked upon as a prospective candidate which can replace BCG vaccination in newborns and also as a potential TB vaccine in adult and adolescents. The results of the Phase II clinical trial from South Africa, reconfirmed safety and immunogenicity of this vaccine VPM1002 in HIV- unexposed newborn infants in South Africa. VPM1002 single vaccination have shown to induce poly-functional CD4 and CD8 T-cell profiles which is comparable to that of BCG. Also, the proportions of CD8 IL-17 T cells were seen to be increased at month 6, post-vaccination within the VPM1002 group.[15-17] The vaccine is viewed as a promising candidate in path towards TB elimination and is currently in Phase III trials. The large multicentre - phase three trial for its efficacy in household contacts of pulmonary tuberculosis patients in India is expected to give us short term results by 2024. Another Phase III trial with VPM1002 for prevention of recurrence in cured TB patients is also underway.
MTBVAC (BioFabri, TBVI): MTBVAC is a promising candidate which can replace BCG as the main immunization against TB. It is live attenuated M.tb vaccine which has been designed with 2 stable deletions in M.tb genome from clinical isolate. The vaccine isolate has a deletion of the phoP gene, (gene which is required to regulate the transcription of key Mtb virulence genes and thereby allowing its survival in cells of the host); and fadD26 gene, (which is required for synthesis of the cell surface lipids known to play a critical role in M.tb pathogenicity) .The protection related to a T-cell mediated response in MTBVAC is hypothesized to be superior to the existing BCG vaccine. Mtb-specific antigens i.e. CFP10 and ESAT6, which are found in MTBVAC provide enhanced immunogenicity.[18, 19]
A phase 1a trial, done in the adults who were BCG-unvaccinated and living in non-TB endemic areas, revealed that this vaccine is safe and has immunogenic potential.[19] Further studies from endemic areas are underway. The clinical trial results of HIV-unexposed newborns from TB-endemic areas of Sub-Saharan Africa will be of great interest to understand more about the role this vaccine.
DAR-901 (Dartmouth, Aeras): DAR -901 is an inactivated whole cell vaccine.[20] This vaccine is derived from a Cell Bank of agar-grown SRL172 using a novel, broth-grown manufacturing method. Animal models have shown significant protection with TB challenge. Recent studies have shown lower magnitude of memory T cells with the vaccine which is an important finding to have a better understanding of TB immunity. A Phase 1 trial of this vaccine as a booster dose established its safety and immunogenicity.[20, 21] Phase 2b trial with the vaccine to prevent infection is underway in Tanzania. The results of the study are expected to be released by end of year 2020.
Mycobacterium Vaccae (Anhui Zhifei Longcom): Inactivated M. vaccae (MV), which is a heat-killed vaccine and is obtained from non-tuberculosis mycobacterium (NTM). This immunotherapeutic agent is the one which has been recommended by the WHO within their Tuberculosis Strategic Development Plan of 1991. [22,23] Immunotheraupetic potential of MV has been demonstrated in several studies. It can restrain MTB through activation of Th1-cytokine mediated immune responses, improvement of Th1/Th2 dynamics, and activation of the macrophage phagocytosis of the mycobacterium. When it is combined along with chemotherapy, it can lead to enhancement of the efficacy of treatment as adjunct treatment for TB. Few studies have also reported that MV can also protect against MDR-TB. [22-26]
Mycobacterium indicus pranii (Cadila): The MIP (Mycobacterium indicus pranii; previously called Mycobacterium w) is a heat killed suspension of Mycobacterium w, (a non-pathogenic, cultivable atypical mycobacterium) and is a strong immunomodulator. The vaccine is already approved and is marketed by Cadila Pharma as Immuvac for treatment of leprosy cases and has shown promise of protection in contacts of index leprosy cases.[27,28] The finding that the contacts who received vaccine also showed a lower incidence of pulmonary TB makes it a promising potential candidate.[28]
Preclinical efficacy and safety of the vaccine has been demonstrated, both as stand-alone as well as its use as an adjunct immunotherapeutic agent in treating experimental animal with tuberculosis. Further clinical trials proved the same. A multicentric clinical randomized ,placebo controlled landmark study from India also proved the immunotherapeutic potential of this vaccine. MIP, when given as an adjunct to ATT in about 890 CAT II pulmonary TB patients who were sputum smear positive, revealed that more number of cases (67.1%) in the vaccine group had achieved sputum culture conversion at 4th week in comparison to placebo group (57%) (p = 0.0002), suggesting its significant role in early clearance of the bacilli,[27] and thus in the limiting of transmission. Another clinical study, using two doses vaccine, one month apart, showed good immunogenicity of the vaccine.[28-32] Based on the current evidence, a phase III double-blind, multicentric, randomized, placebo-controlled trial amongst the healthy household contacts of PTB patients is underway in India to evaluate this vaccine.
RUTI (Archivel Farma): A new strategy whereby a vaccine and the chemotherapy can be combined for augmenting the outcomes is in pipeline. [33, 34] This vaccine strategy is termed as therapeutic vaccination. RUTI is a promising candidate under this categorization. RUTI vaccine is constituted of purified and liposomal cellular fragments of M.tb bacilli which have been cultured under stressful condition to mimic the intra-granulomatous condition. This will potentially enable vaccine to induce latency antigens which would typically be hidden from body’s immune system. The proof of concept has been supported by animal models where the vaccine successfully reduces the bacillary load of M. tuberculosis when administered after chemotherapy in guinea pig and mouse models .The prophylactic role of the vaccine in animal models has also exhibited reduction in bacillary load after a challenge with virulent bacilli. The initial results with vaccine have supported its role to mount immunogenic effects against tuberculosis when given in healthy adults and to subjects with latent TB.[35-37] Phase II clinical trials have shown its effectiveness in patients having latent TB, who received the vaccine after isoniazid treatment for 1 month .The vaccine was concluded as safe with immunogenic potential in HIV infected population as well. Further studies will throw more insight on this vaccine candidate.
Sub unit TB vaccines: Sub unit TB vaccines using viral vectors or adjuvants are in pipeline for their role as potential candidates for effective tuberculosis vaccine. Although, in early phases of preclinical and clinical trials, these candidates are being focused with a lot of interest for their potential ease of manufacture and to possibly overcome the limitations of a live vaccine. These candidates are based on the dominant antigens which are generally expressed by metabolically active M.tb.[38]
Hybrid 1 with adjuvant IC31 (SSI, TBVI, Aeras) is a subunit adjuvant vaccine which is a hybrid of ESAT6 as well as Ag85B antigens with IC31.[39] Hybrid 4 + IC31 vaccine (Sanofi, Aeras) has fusion of M.tb antigens including Ag85B and TB10.4 with adjuvant IC31.[40] A phase IIa study in South Africa evaluated safety and immunogenicity of the vaccine. Hybrid 56 + IC31 (Sanofi, Aeras), a protein adjuvant vaccine made of H56, is a fusion protein which consists of ESAT6, Ag85B and Rv2660c incorporated with the adjuvant IC31.[41] IDR83 and IDR 93 (IDRI, Aeras) are other promising subunit vaccines in the group which combined 4 new sets of antigens that also included Rv2608, Rv1813, Rv3620, and Rv3619 (in IDR93) in addition to synthetic TLR4L-containing adjuvant . Preclinical studies with mice have shown good efficacy and results from guinea pig models showed mortality benefit when used in combination with BCG.
M72/AS01E (GSK, Aeras): M72/AS01E is a promising recombinant fusion protein vaccine. It is derived from highly immunogenic antigens Mtb32A and MtB39A. AS01 adjuvant system was used to promote the immunogenicity.[42] This adjunct has also shown promising results in in malaria and recombinant zoster vaccine .The vaccine can mount a successful T –cell response, Ag-specific antibody responses, cytokine release and stimulate levels of co-stimulatory molecules. The M72/AS01E vaccine has shown encouraging results in adults and adolescents having M. tuberculosis. Phase 2b trial at Kenya, South Africa and Zambia revealed 54% (90% CI 14%–75%) protective efficacy in vaccine group against pulmonary TB in subjects with LTBI during follow up for an average 2.3 years during the interim analysis and 49.7% efficacy at 36 months of follow up after final analysis[43,44]. The results also concluded that in this cohort vaccine exhibited good immunogenicity but a higher incidence of local site reaction. This potent vaccine candidate is a hope for large magnitude of TB infected population worldwide. Results of its efficacy and safety in HIV population will add further excitement to the potential benefit and scope of this vaccine.
Viral Vectored Sub unit Vaccine: Viral vectored vaccine is another upcoming candidate in the endeavor to look for a promising effective vaccine.[45] Ad5Ag85A is a viral vectored adenovirus serotype 5 vector vaccine which expresses Ag85A and has shown successful results from Phase I trial in 24 adults in Canada with and without BCG exposure (McMaster). It is being viewed as potential candidate which will serve as booster to BCG experienced population. MVA85A combined with Crucell Ad35 was evaluated in a phase I trial at Oxford University [46]. TB/FLU-04L is an influenza virus vectored vaccine in clinical trials. ChAdOx1.85A, is yet another adenovirus vaccine which also expresses M.tb antigen Ag85A is undergoing Phase 1 clinical study in BCG experienced population in United Kingdom.[8,45,47]
DNA vaccines: DNA vaccines in various preclinical models have shown promising results, and may especially be valuable as a heterologous prime-boost strategy with existing BCG vaccine.[48-50] The enhanced immunogenicity and potential to induce a robust MHC class I-restricted cytotoxic T lymphocyte (CTL) response and enhanced memory response makes them particularly suited for further evaluation. The relative low cost and ease of manufacture makes them a lucrative option for high burden settings. Some of the DNA vaccines under study are Heat shock protein 65 (hsp65) DNA vaccine, ESAT-6 DNA vaccine, mpt64 DNA vaccine, ag85a and ag85b DNA vaccines, ag85a/b chimeric DNA vaccine, ag85a/esat6 chimeric DNA vaccine, rv2190c DNA vaccine, rv1419 DNA vaccine and IFN-γ and IL-12 DNA vaccine. In Phase I clinical trial done in South Korea evaluated the tolerability, safety, and immunogenic potential of the DNA vaccine GX-70 within pulmonary TB cases having risk factors that could cause treatment failure or relapses.[51,52] Many DNA vaccines trials are underway in India including the prime boost strategy (using BCG-Acr1L) which has shown that liposomized alpha-crystalline protein 1 can reinvigorate BCG potency.[53]
As India aims toward elimination of tuberculosis by 2025, 5 years ahead of Global target, a successful vaccine candidate can be the game changer. Significant research and promising studies have been done in India to develop safe and effective vaccines to target tuberculosis. Several candidates in preclinical studies [54-64] in India are summarized in (Table 1). After a detailed landscape analysis of these studies, two most advanced promising potential candidates have been identified. MIP has shown proof of concept in previous landmark studies in India. VPM1002 is a promising vaccine candidate and has already completed Phase II study for prevention of recurrence in India (results not yet published). In a coordinated attempt to meet the demands of producing an efficacious tuberculosis vaccine to target disease prevention, Indian Council of Medical Research (ICMR) under its flagship program Indian Tuberculosis Research Consortium (ITRC) has taken a lead and initiated a Phase III prevention of disease vaccine trial. Taking these two vaccine candidates forward based on proof of concept, a large phase III, Randomized, Double-blind, Three arm Placebo controlled multicentric Trial to Evaluate the Efficacy and Safety of two vaccines VPM1002 and Immuvac (Mw) in Preventing Tuberculosis (TB) in Healthy Household Contacts of Newly Diagnosed Sputum Positive Pulmonary TB Patients is underway in India. The trial proposes to include 12000 healthy household contacts of newly diagnosed PTB patients at six states in India and hopefully will open
Table 1. Promising Tuberculosis vaccine candidates in preclinical studies in India new avenues in our march to fight tuberculosis.

