According to the present alert information theory, viruses are not microorganisms external to our body, but their genetic material is already contained in the DNA/RNA of our cells, in what we know as Endogenous Retroviruses (ERVs). A virus would actually be an Exogenous Cellular Gene Secretion (ECGS) carrying alert information that would be produced by cells under stress. They are made up of DNA or RNA genes wrapped in a protein capsule and, in some cases, a protective membrane. Such coatings would allow them to withstand the conditions of displacement within the organism, or between different individuals, and possess a form of binding to transmit their information to a specific recipient cell.
Finally, the Extracellular Vesicles (EVs) secreted after the entry of these ECGS in the target cells, would perform, among other functions, that of second messengers of the viral message from abroad, defining the immune response of the receptor.
virus, exosomes, extracellular vesicles, endogenous retrovirus, genetically encoded messages
It is almost presumptuous to propose a new theory on the origin and functioning of viruses when thousands of researchers from all over the world carry out research and publish countless reports on them daily. However, an old saying goes that many times “trees do not let us see the forest”. 99% of the articles published on these “microorganisms” study very specific facts of their operation, always trying to make the results fit the official theory that viruses “hijack” a very complex cellular machinery, using it at will to create new copies of themselves. Science advances like a ladder using the rungs that its predecessors have put there to help future researchers. Each publication is like a piece of a puzzle and from time to time you should try to match the pieces to form a global image with all the information.
Many years passed since the virus was claimed to be a filterable poison until we discovered that it was in fact small units made up of genetic material wrapped in a protective capsule. Since then, dozens of articles have been published that link viruses with EVs and theories have been presented such as the “Fifth viral column” or the “Trojan exosome”, which try to give a global meaning to the information that we have accumulated over decades.
Science does not agree on whether viruses are living beings nor about their origin. Given the evidence that 8% of our genome is of viral origin, we have sought the easiest explanation, although it is not always the correct one. Proposing that they are pieces of viruses that have been “stuck” to our genome for millions of years, developing extremely important exclusive functions of multicellular organisms such as stem cell reversal, placentation, or the telomeres themselves, which define the life expectancy of cells, does not seem to support that theory. Likewise, we do not know how to explain how a few viral genes can take over the entire machinery of transcription, translation, intracellular transport, or protein folding, among many others. Little by little we are discovering that our virioma is mainly made up of viruses with beneficial effects on their hosts, that the majority of responses to viruses are mild and that only when viruses have crossed the species barrier do they carry serious diseases.
Finally, it is becoming clearer every day that there are several types of extracellular vesicles capable of transporting information between cells, including fragments of genetic material. We now know that these vesicles (which can be produced by infected or healthy cells) play important roles in modulating the antiviral immune response. We know that in addition to producing new functional virions, “infected” cells can produce virus-like particles without genetic material, produce encapsulated virions when it comes to viruses that lack membranous capsule in nature, or produce IFN capable of activating multiple immune response points. including at the genetic level.
The present Alert Information Hypothesis aims to unify and make all this new data understandable under a single operating explanation. Its rationale involves three main concepts:
1. ERVs are not viral genetic material that has been included in our genome, but are an integral and vital part of it, performing very important functions of multicellular organisms.
2. Viruses are actually a type of ECGS that carry alert information that would be produced by cells under stress and that would fulfill an intercellular communication function, which would activate a number of actions that can lead the receptor cells to develop or not a hostile reaction to the external stimulus.
ERVs can make secretable copies of part of their genetic material (DNA/RNA) when cells are subjected to toxic or stressful situations. These genes travel protected by a single or double envelope (capsid/membrane) capable of binding to specific target cell receptors.
3. The EVs perform, among other functions, that of second messengers of the message from abroad and that is contained in the ECGS. The multiple forms and contents that EVs can present (genetic microparticles, virus-like particles, whole viruses ...) in addition to many other responses mediated by various cytokines and immune cells (NK, dendritic cells, CD4+, Treg ...) define the type of receptor immune response.
Individual intercellular communication
When a cell receives a stimulus, modifications are generated in the structure of its membrane that are followed by changes in its cytoplasm, generally by the appearance of second messengers, which will produce some cellular metabolic effect. Intercellular communication by chemical messengers can be close (Autocrine, Juxtacrine, Paracrine and Neurotransmitters) and remote (endocrine and exocrine hormones).
It was only 30 years ago that we learnt of another form of intercellular communication mediated by vesicles loaded with proteins, lipids, mRNA and microRNA, which are released into the extracellular space. They are called EVs and were classified according to their size: Exosomes (30-100nm), microvesicles (100-1000nm) and apoptotic bodies (large vesicles produced during programmed cell death) [1-3].
Since all cells (eukaryotes and prokaryotes) can generate them, it is thought to be a very old type of communication and has been preserved throughout evolution. At first it was thought that they were simply carriers of waste material, but it has been shown that they are vehicles for intercellular communication and exert important functions in receptor cells, generating a huge leap in their study and understanding [4-7].
The study of EVs generated by stem, blood, immune, nerve, kidney and tumor cells has grown exponentially in recent years. It is currently known that they can regulate various physiological processes, as well as the development and progression of diseases [8-10].
Intercellular communications between individuals
Pheromones are the best-known form of communication between different individuals of the same species. These are certain chemical messengers that, voluntarily secreted abroad by exocrine glands, provide a means of alert, stimulus or signal intended to modify the behavior of the individuals who receive it. The objective of this communication, based on simple molecules, is multiple and includes the search for food, marking of a territory and reproduction.
Another form of communication between individuals is volatile chemical signals that some plants and insects secrete but are also used by complex organisms including mammals [11].
Plants can communicate by air via volatile chemical signals that warn of danger, usually the presence of predatory insects, producing defense chemicals that make their foliage less palatable to attackers. The tobacco plant has even created symbiotic relationships with insects; when it is attacked by caterpillars, it releases a chemical into the air that attracts insects that feed on them [12].
As we see the communication between individuals of the same, or other species, it is a complex reality of which we only know a minimal fraction. We propose a more sophisticated communication mechanism, capable of performing more specific and adjustable functions. In general, the possible communication between the human being and another living being through chemical or biological signals has never been seriously explored.
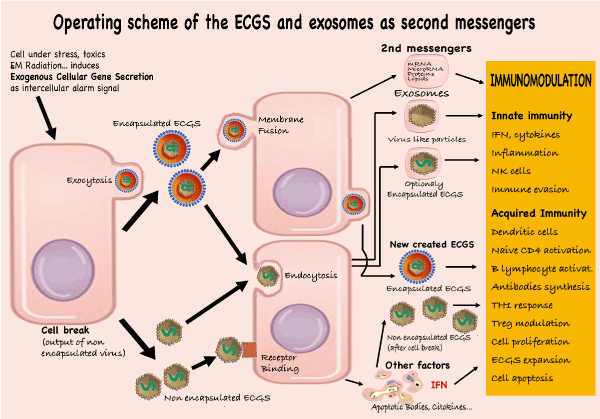
Figure 1. Operating scheme of the ECGS and Exosomes as second messengers.
If a situation is potentially harmful to an individual, their cells will produce SCEGs (encapsulated or not) to inform nearby individuals by penetrating the recipient cells by fusion of their membranes (encapsulated), endocytosis, or binding to membrane receptors (unencapsulated). These cells will secrete second messengers: exosomes, virus-like particles, and new viruses encapsulated or not. All of these will produce a plethora of immunomodulatory (activation or inactivation) reactions encompassed within innate as well as acquired immunity.
Since EVs are membranous structures that carry complex molecules (including genetic material) and are present in all body fluids that go outside (saliva, respiratory secretions, faeces and urine) they would theoretically be capable of reaching individuals of the same or other species 3-10. A virus would be (like EVs) an ECGS carrying alert information produced by cells under stress.
Are the ERVs genetic material accumulated in our genome?
It is currently known that the information of the ERVs contained in our cells is so important that without it the eukaryotic cells would not be able to perform many of their vital functions. Linear chromosomes, telomeres, transcription and translation processes originate from genes that we consider ERVs [13-17].
Up to 8% of our genome is made up of ERVs, a discovery which forced us to find a theory that explains how an important part of our genome was made up of genetic material present in viruses. The most logical reasoning, and which all scientists immediately accepted, was that retroviruses introduced their genetic material into ours when they infected us. However, it does not make sense that the remains of an infectious material were perpetuated millions of years within our genome if they did not fulfill some function. Evolutionary pressure would simply rule them out [13,14].
In the last 2 decades, it has been discovered that these genes, theoretically of viral origin, perform important cellular functions. How could it be explained that viral genes are permanently introduced into the genome of evolutionarily superior cells and produce important improvements in their physiology? [13-17].
The ERVs “Syncytin” is of vital importance in the normal architectural development of the placenta, especially in the process of fusion of the cytotrophoblasts with the syncytotrophoblasts, to the point that their dysfunction produces diseases such as pre-eclamsia or the HELLP syndrome [17].
American scientists discovered the surprising ability of ERVs to activate the totipotential state of stem cells [13]. By adding these viral genes, the cells reverted to a more plastic and more developmental state. In Lund (Sweden), they showed that when nerve cells differentiate into adult cells, they activate the ERVs that regulate the functions of neighboring genes, acting on neuronal development and configuring basic functions of our brain [18].
Among the defense functions, ERVs enhance the innate immune system. The elimination of one or more of them seriously damages the cellular capacity to carry out a correct defensive response against new microorganisms. Various ERVs distributed by our genome act as interferon inducible enhancers, including the regulation of essential immune functions, such as the activation of the inflammatory cascade through the AIM213 inflammasome. It is proposed that the ERVs never introduced their genes but are their own cellular genetic material and generate viruses as coded alarm signals in close relation to the EVs.
Could the EVs act as second messengers?
On many occasions, EVs are functionally related to viruses, acting as a second messenger that would expand or limit their message [19-23]. Secreted EVs can carry viral genes, form virus-like particles (with or without infective capacity) [24-32], or even contain whole viruses that would be non-encapsulated viruses on the outside. EVs can induce a strong humoral and cellular response by different immune pathways. Notably, the hepatitis E virus, which is normally non-encapsulated in faeces and bile, is secreted into the blood by membrane-covered “infected” cells, similar to encapsulated viruses [31]. This coating allows the virus to circulate without being attacked by the immune system. In the case of the AIDS virus, EVs are capable of reactivating latent viruses [32]. It is proposed that, like EVs, viruses (ECGS) can be secreted into any body fluid in contact with the outside such as saliva, mucus, sputum, feces, and urine.
Theoretically, when a toxic or stressful situation affects a population, the weakest individual in the community will be the first to release viruses that will reach the rest of the individuals. Depending on the state of health and immunity of the rest of the group, they will have from practically no response to even fatal clinical symptoms. Such variability will depend on the EVs and other immunomodulatory molecules that amplify or inhibit the immune response. EVs can bind to other cells using pathways independent of the specific virus receptor and further preventing the antiviral immune response. Significantly, this property could explain the formation of EVs as vectors of molecular transmission in infections by BCG and other bacteria [28].
Are viruses living beings?
It is said that viruses are “acellular” microorganisms that infect cells to produce new virions (infectious and morphologically complete viral particle) to spread their genes. However, they do not have a cellular structure, they do not have their own metabolism and they need a host cell to create new units of themselves, so they are not considered true living beings [19-32].
In order to self-copy, they must bind to the recipient cell by fusion of its membranes or by attaching to specific receptors, insert its genetic material into the correct cell compartment, use different cellular organelles, navigate through intracellular structural and mobility systems, use specific enzymes for its transcription and translation, recruit chaperones that confer the quaternary structure of its constitutive proteins, and finally form fully “infective” virions that will be secreted from the cell or cause cell disruption.
It is difficult to imagine how a virus, carrying a few genes, can “hijack” all that complex cellular machinery. The facts seem to show that the cell actively participates in this process and agrees to the production of new virions; as well as in the activation / inhibition of immune processes, or of another type (cellular repair, etc.), which occur as a consequence of their replication.
Obviously, our current knowledge does not allow us to understand when and why one response or another occurs. What we do know is that the vast majority of viruses with which we live do not produce pathological phenomena in our bodies, which when they do, are generally mild symptoms.
We should ask ourselves why viruses have evolved to create thousands of different families and species when they are not even true living things. It is hard to imagine that any kind of biological survival pressure justified such evolution.
Theories about the origin of viruses?
Three main theories explain the origin of viruses but all pose drawbacks [33-35].
Theory of cell regression: It affirms that the viruses were small parasitic cells that lost their biological structures and capacities, evolving into “inert bodies circulating in the environment” that would only re-copy themselves by binding to the receptor of a specific cell. This is not logical, not even the bacteria that became definitive intracellular organelles (mitochondria / chloroplasts) lost all the machinery necessary for their function.
Coevolution theory: It suggests that both viruses and their hosts evolved together since the first cells were formed from proteins and nucleic acids. Viruses can infect cells from all 3 domains (Bacteria, Archaea, and Eukaria), but they themselves cannot fit into any of these groups representing all living things. Analysis of the capsid proteins has revealed that at least two types of virions would have originated independently before the last universal common ancestor of cell life appeared. The simultaneous appearance of cells and microorganisms that need to hijack the most complex intracellular systems in order to divide makes little sense in my opinion.
Theory of nomadism: It argues that some viruses have evolved from fragments of DNA or RNA that “escaped” from a multicellular organism. Said genetic material would come from plasmids or transposons formerly known as “jumping genes” that also left copies of themselves in our genome as ERVs. They participate in processes as complex as placentation, cellular reversion to their totipotential origins, or the development of nerve cells [18], making their accidental inclusion in DNA highly unlikely. Furthermore, viruses cannot be DNA / RNA fragments that have “escaped” from a cell since it is impossible to explain two of their fundamental characteristics. 1.- How were they endowed with a complex protein capsid and, in enveloped viruses, with a second membranous coating with the capacity to bind to specific receptors of the target cell? and 2.-How are these fugitive fragments of genetic material able to reach a cell and take over the entire cellular production mechanism?
A new explanation for the origin of viruses would be the Alarm Message Theory. It argues that viruses are messengers to a complex genetically encoded information system, differentiating them from messages sent using simple biochemical molecules.
In this way, the ERVs, in addition to other vital biological functions, would also be involved in the production of new viruses as a way of amplifying the alarm message between the cells of the same or another organism. Furthermore, these or other genes activated during the copying process of virions, produce generally beneficial effects on the host, generally activating innate immunity.
The present theory is complemented by the “Trojan exosome hypothesis”, which proposes that retroviruses exploit the cellular capacity to manufacture exosomes to create new viral particles (containing proteins and viral genetic material) that can infect without viral capsular proteins binding to specific receptors [35]. This allows them to evade the immune system and create a mechanically important but low efficiency mode of infection [34].
Although both hypotheses correctly explain why retroviral antigenic vaccines provide little protection, and that alloimmunity is a central component of antiretroviral immunity, the “Trojan exosome” would only be true in the case of the hepatitis E virus and HIV, which make exosomes with fully infective virions. The present theory explains the formation and more or less complete viral content of exosomes as a “second messenger of the alarm message” and may be infective or immunomodulatory [35,36].
Virus-host relations: Are they always attacks?
We understand virus-host relationships simply as attacks by microorganisms that cause more or less serious diseases in infected organisms. However, these relationships are in fact bilateral and involve modifications of both the virus and host genomes.
In 1892, Dmitry Ivanovski demonstrated that the leaked sap from a diseased tobacco plant could infect a healthy one by calling it “vivum fluidum”. Years later, Martinus Beijerinck renamed this infectious substance as “virus”, which comes from the Greek and means “poison”.
Fifty years ago the first virus capable of affecting humans was discovered, it was the Epstein-Barr virus (EBV). Since then, dozens of RNA or DNA viruses, bi or single-stranded, have been discovered that are capable of “causing” diseases in man [35].
We now know that the majority of the population is infected by Anelovirus, a group discovered less than a decade ago, but which make up the majority of our “virioma” (All viruses that coexist in our body) [37]. These and most of the viruses we come into contact with are beneficial and have lived with us for millions of years.
Positive effects of virus “infections”: In 2014, Common Murine Norovirus were shown to enhance intestinal homeostasis and mucosal immunity through interferons by increasing antibodies and T cells in blood and intestinal tissue. Mice with the virus had less diarrhea, less intestinal tissue damage and survived longer [37].
It is important to note that we have more and more data that viruses can help us fight bacteria or other viruses. The HIV-1 virus has a cationic domain called Vpr that is responsible for cell penetration through an active death domain against E.Coli. Interestingly, HIV-1 Vpr, and other proteins encoded by different viruses, share similar physical properties to Cathelicidin LL [38], which is a peptide with important antimicrobial activity [23].
Another study revealed that ERVs are fundamental in the immune defense against bacteria and other common pathogens. They note that the response of B lymphocytes to type 2 independent T antigens depends on ERVs to rapidly produce protective antibodies by activating a reverse transcriptase. The researchers have highlighted its therapeutic implications since treatment of AIDS with Zidovudine (AZT) could render B lymphocytes unable to respond to various antigens and, therefore, make them more sensitive to opportunistic infections [27].
Viruses can even provide protection from others. The GBV-C virus, initially related to hepatitis C, does not attack the liver but affects defense lymphocyte function, hindering the action of the AIDS and Ebola virus, increasing its survival [38].
These, and other data led to the proposition of the “Viral Fifth Column Theory”, which predicts that cationic peptides encoded by multiple viruses have positive effects similar to Cathelicidin on innate immunity [36,39,40].
In animals there are also notable cases of this beneficial effect. Phage WO (virus that infects bacteria), has up to a third of genes of animal origin. Specifically, it has a latrotoxin gene, (black widow venom neurotoxin, “Latrodectus mactans”) [37]. If this is surprising in itself, it is even more so to know that Phage WO uses the toxin to destroy bacteria of the Wolbachia group which, curiously, attack the mentioned spider. There is undeniably a spider-virus symbiosis against the bacteria or, as proposed in this theory, the virus is only an VE with genetic material secreted by the spider to infect and destroy the bacteria.
When the offspring of the pea aphids (Acyrthosiphon pisum) grow in crowded conditions they develop wings that they do not usually show if they develop unstacked. The on and off genetic switch of said phenotypic expression belongs to a virus, known as densovirus, whose genome has been completely incorporated into that of the insect [41]. When new winged insects colonize new plants with little competition they return to the original wingless model. Although its discoverers suggest that the virus, which resides in the aphid genome over time, induces wing development in order to spread itself. The interpretation that the virus is nothing more than a messenger used by aphids to inform their peers that the excessive concentration of individuals compromises the viability of the entire group is much more logical.
The beneficial effects of viruses are seen even more clearly in the plant kingdom. There are viruses called “entomopathogens” that “naturally and spontaneously” infect pest insects that attack certain plants, which arouses interest in use on various crops [32]. Probably, the ERVs of these plants coincide with the genetic material of said viruses.
Another example that seems to support the present theory are the so-called resistance genes (GR). In plants, each GR confers resistance against a specific virus, triggering cellular apoptosis in neighboring cells, limiting infection [38]. This genetically programmed response is completely different from the expected immune response after a viral infection.
We must understand that all living beings are carriers of “low intensity” viruses that do not cause any disease [36]. According to the alarm information theory, most of these messages are received by the guest but do not generate any immune response because they “know” that it is a banal problem against which they do not need to take any important action. Consistent with this idea, most ERVs are silenced by methylation marks and are only activated when they need to generate a reaction against a stimulus [40].
It seems evident that viruses perform mostly positive functions for hosts, including immunomodulatory effects, destruction of microbes and collaboration in repairing damage to affected tissues [42].
Why can an alert signal, destined to initiate the defense of the organism, kill it or make it seriously ill?
To accept viruses as red flags, we should be able to explain viral diseases with high mortality. An alarm system that globally produced more problems than advantages would tend to disappear due to the evolutionary pressure it would generate.
The information carried by the viruses produces high mortality when the recipient individuals are in a highly toxic and/or stressful situation without the possibility of fleeing. Certain viruses decimate fish farms with inadequate conditions (low O2, low water volume, and increased debris). These stressful situations generated by a damaging environment cause highly contagious symptoms and high mortality, such as infectious pancreatic necrosis or viral hemorrhagic septicemia [43]. Even in these cases, we could not consider that said “alert information” was harmful to the group because in the end it would only be returning the biological balance and ensuring the survival of the species.
In general terms, the viruses that cause the highest mortality in humans are those that have crossed the barrier between species, as has happened with viruses as Ebola, AIDS, Zika, Dengue or SARS. These viruses were in immunological equilibrium with their habitual hosts such as bats, rats, pangolins, civets and even apes [44].
Viruses can sometimes induce cancers, liver cirrhosis, and autoimmune diseases such as multiple sclerosis. How can an alarm system generate such a negative response to an isolated individual? In today's urban society, allergic and autoimmune diseases that are based on a pathological immune reaction have increased significantly. There is increasingly solid data that stress, air pollution, heavy metals and electromagnetic radiation with which we live can alter our immune response to make it pathological.
Most likely, the unknown alarm signals generate an abnormal immune reaction, especially when there are pathological circumstances such as stress, toxins, hypoxemia or electromagnetic radiation that alter the pathophysiological response that would occur under normal conditions, generating an allergic, autoimmune or even tolerant response to neoplasms [41]. For this reason, viruses that have crossed the barrier between species induce cytokine release patterns and immunothrombosis phenomena that cause the severe pictures that we know [45].
Could viruses really be mechanisms for transmitting alerts?
For this statement to be true, viruses should comply with the six general principles of intercellular communication:
Synthesis of the messenger: If a cell under a toxic situation wanted to send an alarm signal, it would manufacture certain virions from cellular genetic information, including EVRs, in the same way that they secrete hormones, cytokines, and other mediators of communication. Currently we do not know if that is true, but we know that cells, infected or not, can manufacture complete virions and that thousands of fragments of ERVs are activated in our genome by means of interferons, indicating an active participation of the host cell in the synthesis of new virions [42-45].
Secretion and transport to the target cell: Viruses are secreted to the cell exterior by budding in a similar way to the production of EVs (exosomes/mycovesicles) although in some non-encapsulated viruses they are produced by cell disruption similar to apoptotic bodies, which are another form of EVs, and whose role in stimulating the immune response is well known [46,47]. Viruses (ECGS) can reach any cell through body fluids and can also be transferred to all individuals through air, urine, or feces [46,48]. In proposing that viruses are a type of EVs, it would be legitimate to ask why there are unencapsulated viruses. Probably to avoid degradation of the alert message; encapsulated viruses can be active for only 5 days, unencapsulated viruses can last for several weeks. Interestingly, some non-encapsulated viruses can be secreted through vesicles within the body, possibly because they facilitate their binding to target cells, they are not destroyed by circulating antibodies, and does not compromise their durability [49,50].
Detection/reception of the messenger by a cellular receptor (protein): Unenveloped viruses (ECGS) can only bind to specific receptors on the host membrane by limiting their binding to a very specific type of cell, after which they would inject their genetic material while the capsid is kept outside. However, in other cases, all viruses enter the cell by endocytosis. In enveloped viruses, entry is through fusion of the viral and host membranes, a process favored by specific fusion proteins [36,50].
Intracellular transmission or signal transduction: The virus (ECGS) unwinds its genetic material, leaving it accessible in the cytoplasm and its genome can travel to the correct cell compartment. In general, viral DNA, single-stranded or double-stranded, must enter the nucleus for its transcription to RNA. However, some single-stranded DNAs can be translated directly using a DNA polymerase enzyme without using RNA as an intermediary. Positive RNA viruses can be translated directly into ribosomes, and negative viruses must be “positivized” previously by RNA polymerase [51-54].
Change of cellular status (metabolism, gene expression ...): When viral genes (ECGS) are transcribed and translated, a myriad of effects are triggered, including the synthesis of genetic material (DNA/RNA), and structural and regulatory viral proteins. Viral proteins must “mature” by folding, using cell chaperones, to be fully functional. The “infected” cells also secrete EVs, containing mRNAs, microRNAs, proteins, and other substances, destined to act as second messengers informing neighboring cells [54,55].
Elimination of the signal and interruption of the process: After performing its function, the alarm signal (ECGS) should be canceled to avoid a pathological hyperimmune reaction. When the cell has decided to cancel the signal, considering it resolved or not relevant, it begins its deactivation by synthesizing IgM and IgG antibodies. The prompt appearance of this humoral immunity, which would be the most common against known messages, would deactivate the message, avoiding exaggerated immune reactions. Furthermore, activated CD8+ lymphocytes recognize cells that had initiated secretion of exosomes or virions by destroying it by apoptosis [56-58].
I want to thank the researcher and philosopher Yazhi, ST. for her inspiration and collaboration in writing the work.
- Yañez M, Siljander P, Andreu Z, Bedina A, Borras F, et al. (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4: 27066. [Crossref]
- Meldonesi J (2018) Exosomes and ectosomes in intercellular communication. Curr Biol 28: R435-R444. [Crossref]
- Correa R, Caballero Z, Leon L, Spadafora C (2020) Extracellular vesicles could carry an evolutionary footprint in interkingdom communication. Front Cell Infect Microbiol 10: 76. [Crossref]
- Stahl PD, Raposo G (2018) Exosomes and extracelular vesicles: The path forward. Essays Biochem 62: 119-124. [Crossref]
- Simons M, Raposo G (2009) Exosomes: Vesicular carriers for intercellular communication. Curr Opin Cell Biol 21: 575-581. [Crossref]
- Hessvik NP, Llorente A (2018) Current knowledge on exosome biogenesis and release. Cell Mol Life Sci 75: 193-208. [Crossref]
- Maas SL, Breakefield XO, Weaver AM (2017) Extracellular vesicles. Unique intercellular delivery vehicles. Trends. Cell Biol 27: 172-188. [Crossref]
- Zhang G, Yang P (2018) A novel cell-cell communication mechanism in the nervous system: exosomes. J Neurosci Res 96: 45-52. [Crossref]
- Buzas E, Gyorgy B, Nagy G, Falus A, Gay S (2014) Emerging role of extracellular vesicles in inflammatory diseases. Nat Rev Rheumatol 10: 356-364. [Crossref]
- Yañez M, Siljander P, Andreu Z, Bedina A, Borras Z, et al. (2015) Biological properties of extracellular vesicles and their physiological functions. J Extracell Vesicles 4: 27066. [Crossref]
- Fleming AJ (2005) Intercellular communication in plants. Abingdon: Taylor & Francis.
- Gowan K (2013) Quanta magazine. https://www.quantamagazine.org/the-secret-language-of-plants-20131216/ (consultado:1/5/20)
- Sentís C (2002) Retrovirus endógenos humanos: Significado biológico e implicaciones evolutivas. Arbor-CSIC 172: 135-166.
- Jern P, Coffin JM (2008) Effects of retroviruses on host genome function. Annu Rev Genet 42: 709-732. [Crossref]
- McFarlan T, Gifford W, Driscol S, Lettieri K, Rowe H, et al. (2012) Embryonic stem cell potency fluctuates with endogenous retrovirus activity. Nature 487: 57-63. [Crossref]
- Zeng M, Hu Z, Shi X, Li X, Zhan X, et al. (2014) MAVS,cGAS, and endogenous retroviruses in T-independent B cell responses. Science 346: 1486-1492. [Crossref]
- Knerr I, Beinder E, Rascher W (2002) Syncytin:A novel human endogenous retroviral gene in human placenta. Am J Obstet Gynecol 186: 210-213. [Crossref]
- Sampey G, Meyering S, Asad M, Saifuddin M, Hakami R, et al. (2014) Exosomes and their role in CNS viral infections. J Neurovirol 20: 199-208. [Crossref]
- Nolte E, Cremer T, Gallo R, Margolis L (2016) Extracellular vesicles and viruses: Are they close relatives? Proc Natl Acad Sci USA 113: 9155-9161. [Crossref]
- Arnold C (2018) Cells talks in a language that looks like virus. Quanta magazine. https://www.quantamagazine.org/cells-talk-in-a-language-that-looks-like-viruses-20180502/
- Wells W (2003) When is a virus an exosome? J Cell Biol 162: 960. [Crossref]
- Welch J, Stapleton J, Okeoma C (2019) Vehicles of intercellular communication: Exosomes and HIV-1. J Gen Virol 100: 350-366. [Crossref]
- Li S, Li S, Wu S, Chen L (2019) Exosomes modulate the viral replication and host immune responses in HBV infection. Biomed Res Int 2019: 2103943. [Crossref]
- Maemura T, Fukuyama S, Sugita Y, Lopez T, Nakao T, et al. (2018) Lung-derived exosomal miR-483-3p regulates the innate immune response to influenza virus Infection. J Infect Dis 217: 1372-1382. [Crossref]
- Fitzsimmons L, Kelly G (2017) EBV and Apoptosis:The viral master regulator of cell fate? Viruses 139: 339. [Crossref]
- Oliva A, O´Neal A, Santambrogio L, Kotsyfakis M, Pedra J (2019) Message in a vesicle:Trans-kingdom intercommunication at the vector-host interface. J Cell Sci 132: jcs224212. [Crossref]
- Margolis L, Sadovsky Y (2019) The biology of extracellular vesicles:The known unknowns. PLoS Biol 17: e3000363. [Crossref]
- Rodrigues M, Fan J, Lyon C, Wan M, Hu Y (2018) Role of extracellular vesicles in viral and bacterial infections: Pathogenesis, Diagnostics, and Therapeutics. Theranostics 8: 2709-2721. [Crossref]
- Petrik J (2016) Immunomodulatory effects of exosomes produced by virus-Infected cells. Transfus Apher Sci 55: 84-91. [Crossref]
- Qjan C, Liu X, Qjn X, Chen J, Li T, et al. (2020) Recent progress on the versatility of virus-like particles. Vaccines (Basel) 8: 139. [Crossref]
- Reyes J, Osuna J, DeJesus L, Mahely A, Farfan C, et al. (2019) Isolation and characterization of exosomes released from mosquito cells infected with Dengue Virus. Virus Res 266: 1-14. [Crossref]
- Barclay R, Schwab A, DeMarino C, Akpamagbo Y, Lepene B, et al. (2017) Exosomes from uninfected cells activate transcription of latent HIV-1. J Biol Chem 292: 14764. [Crossref]
- Forterre P (2006) The origin of viruses and their possible roles in major evolutionary transitions. Virus Res, 117: 5-16. [Crossref]
- Holmes EC (2011) What does virus evolution tell us about virus origins? J Virol 85: 5247-5251. [Crossref]
- Gould S, Booth A, Hildreth J (2003) The Trojan Exosome Hypothesis. Proc Natl Acad Sci USA 100: 10592-10597. [Crossref]
- Chahar H, Bao X, Casola A (2015) Exosomes and their role in the life cycle and pathogenesis of RNA viruses. Viruses 7: 3204-3225. [Crossref]
- Wilen C, Lee S, Leon H, Orchard R, Desai C, et al. (2018) Tropism for tuft cells determines immune promotion of norovirus pathogenesis. Science 360: 204-208. [Crossref]
- Bhattarai N, Stapleton J (2012) GB virus C: The good boy virus? Trends Microbiol 20: 124-130. [Crossref]
- Kaczorowska J, Van-der-Hoek L (2020) Human Anelloviruses: Diverse,omnipresent and commensal members of virome. FEMS Microbiol Rev fuaa007. [Crossref]
- Freer G, Maggi F, Pistello M (2019) Virome and Inflammasomes:A finely tuned balance with important consequences for the host health. Curr Med Chem 26: 1027-1044. [Crossref]
- Parker B, Brisson J (2019) A Laterally Transferred Viral Gene Modifies Aphid Wing Plasticity. Curr Biol 29: 2098-2103. [Crossref]
- Bordenstein S, Bordenstein S (2016) Eukaryotic association module in phage WO genomes from Wolbachia. Nat Commun 7: 13155. [Crossref]
- Murray A (2013) Epidemiology of the spread of viral diseases under aquaculture. Curr Opin Virol 3: 74-78. [Crossref]
- Moffett P (2009) Mechanisms of recognition in dominant R gene mediated resistance. Adv Virus Res 75: 1-33. [Crossref]
- Assil S, Webster B, Dreux M (2015) Regulation of the host antiviral state by intercellular communications. Viruses 7: 4707-4733. [Crossref]
- Horie M, Honda T, Suzuki Y, Kobayashi Y, Daito T, et al. (2010) Endogenous non-retroviral RNA virus elements in mammalian genomes. Nature 463: 84-87. [Crossref]
- Urbanelli L, Buratta S, Tancini B, Sagini K, Delo F, et al. (2019) The role of extracellular vesicles in viral infection and transmission. Vaccines (Basel) 7: 102. [Crossref]
- Wang J, Wu F, Liu C, Dai W, Teng Y, et al. (2019) Exosomes released from Rabies virus-infected cells may be involved in the infection process. Virol Sin 34: 59-65. [Crossref]
- Crenshaw B, Gu L, Sims B, Matthews Q (2018) Exosome biogenesis and biological function in response to viral infections. Open Virol J 12: 134-148. [Crossref]
- Meckes Jr. D, Raab-Traub N (2011) Microvesicles and viral infection. J Virol 85: 12844-12854. [Crossref]
- Firqiet S, Beaujard S, Lobert P, Sané F, Caloone D, et al. (2015) Survival of enveloped and non-enveloped viruses on inanimate surfaces. Microbes Environ 30: 140-144. [Crossref]
- Bird S, Kirkegaard K (2015) Escape of non-enveloped virus from intact cells. Virology 479-480: 444-449. [Crossref]
- Smith A, Helenius A (2004) How viruses enter human cells. Science 304: 237-242. [Crossref]
- Greiner T, Moroni A, Van-Etten J, Thiel G (2018) Genes for membrane transport proteins: Not so rare in viruses. Viruses 10: 456. [Crossref]
- Yao Z, Qiao Y, Li X, Chen J, Ding J, et al. (2018) Exosomes exploit the virus entry machinery and pathway to transmit alpha interferon-induced antiviral activity. J Virol 92: e01578- e01618. [Crossref]
- Khachatoorian R, French S (2016) Chaperones in hepatitis C virus infection. World J Hepatol 8: 9-35. [Crossref]
- Longatti A (2015) The dual role of exosomes in hepatitis A and C virus transmission and viral immune activation. Viruses 7: 6707-6715. [Crossref]
- Alenquer M, Amorin M (2015) Exosome biogenesis,regulation and function in viral infection. Viruses 7: 5066-5083. [Crossref]

