A rapid voltammetric method was introduced and validated to determine duloxetine hydrochloride (DUL) via a newly introduced electrochemical sensor. Cyclic and square wave voltammetry modes were used to investigate the electrochemistry of DUL. Several experimental parameters as pH, electrode modifiers, scan rate, and deposition time were investigated to establish the optimum parameters required for analysis. A linear response ranged from 3.0 × 10-6 to 2.0 × 10-4 mol L-1 with a detection limit of 4.0 × 10-7 mol L-1. The proposed method has been successfully applied to determine DUL in its active pharmaceutical preparation as well as spiked urine.
voltammetry, duloxetine hydrochloride, nanoparticles, nanotubes, urine
Duloxetine hydrochloride (DUL), serotonin and norepinephrine reuptake inhibitor, is known as N-methyl-3-(napthalen-1-yloxy)-3-(thiophene-2-yl) propan-1-amine hydrochloride (Figure 1). Serotonin and norepinephrine are the two neurotransmitters whose lack at synapses cause depression therefore this drug was initially used in the treatment of depressive and anxiety disorders [1], and also it was used to treat fibromyalgia and chronic musculoskeletal pain. The U.S. Food and Drug Administration (FDA) approved (DUL) to treat depression and diabetic polyneuropathy [2], anxiety disorder [3], fibromyalgia [4], and chronic musculoskeletal pain. DUL is used also in treatment of stress urinary incontinence [5,6].
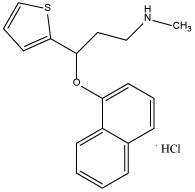
Figure 1. Chemical structure of DUL
One official analytical method was documented for determination of DUL in bulk [7]. Literature survey reveals variety of analytical methods including spectrophotometry [8-15], spectrofluorimetry [16], high-performance liquid chromatography [17-26], potentiometry [27,28], gas chromatography-mass spectrometry [29], capillary electrophoresis [30-32], and electrochemistry [33].
Attention was directed recently to chemically modified electrodes due to their important effects in improving both peak potential and current. Electrode modification by different modifiers has been reported recently with improvement of sensitivity, selectivity, and detection limit [34-37].
In the presented work, a rapid, simple and non expensive voltammetric technique is described for the determination of DUL in bulk, pharmaceutical preparation and urine at carbon paste electrode modified with titanium dioxide nanoparticles enhanced by multi-walled carbon nanotubes (TMCPE).
Materials and reagents
DUL was obtained from Eli Lilly (purity 99.99%, according to supplier certificate). Cymbalta hard gastro-resistant capsules (Batch No.C428554) labeled to contain 30 mg DUL per capsule, manufactured by Eli Lilly (Netherlands) were purchased from the local market. Britton-Robinson (BR) buffers ranged from pH 2.0 to 11.0 were prepared as described before [38], Graphite powder and paraffin oil, titanium (IV) oxide nano particles, (purity of 99.5%) and multiwalled carbon nanotubes (MWCNTs) (purity >95%) were supplied from Sigma-Aldrich. 1.0 × 10-2 mol L-1 of DUL in doubly distilled water was prepared.
Instruments
Experiments were done utlizing Bio-logic Science Instruments Pvt.ltd. (France) with EC-Lab software. A platinum wire (BASi model MW-1032, USA) and Ag/AgCl (3.0 mol L-1 NaCl) (BASi model MW-2063, USA) were used as the auxiliary electrode and reference electrode, respectively. A JENWAY 3510 pH meter (Staffordshire, England) was utilized to adjust pH. Scanning electron microscope (SEM) measurements were done utilizing a JSM- 6700F scanning electron microscope (Japan Electro Company).
Modified electrode
Carbon paste electrode (CPE) and multiwalled carbon nanotubes modified carbon paste electrode (MCPE) were prepared as described before [39].
TMCPE 1% (w/w) was prepared by the same previous manner, but with addition of 10 mg MWCNTs and 10 mg titanium (IV) oxide nanoparticles to 980 mg graphite powder in ethyl ether with continuous stirring to make them homogenous and allowed to evaporate then mixed with a suitable amount of paraffin oil in a glassy mortar until a homogenously prepared paste was resulted. The hole of the electrode was filled with the paste and smoothed on a filter paper until the shiny surface is appeared.
Procedures
Firstly, TMCPE was cycled in the potential range of (0-1.42 V) in BR buffer (pH 6.0) at a scan rate of 100 mVs-1 for many times until a reproducible response was obtained. After that, a proper amount of DUL was added and cyclic voltammograms were recorded at the same potential range and scan rate. Square wave voltammograms were recorded from 0.2-1.6 V at a scan rate 100 mVs-1.
Determination of DUL in bulk: Square wave voltammetry (SWV) was utilized to determine DUL in bulk. Aliquots of DUL (1.0 × 10-3 mol L-1) were transferred using micropipette to 5.0 mL BR buffer (pH 6.0) in the electrolytic cell. Voltammetric analyses were done and square wave voltammograms were recorded at TMCPE. Calibration graphs were obtained by plotting the anodic peak current versus final concentration of DUL.
Determination of DUL in Capsules: Ten hard capsules of Cymbalta were open; their contents are collected, finely powdered and mixed well. Amount of the powder required to prepare 1.0 × 10-3 mol L-1 DUL was added to volumetric flask (100 mL) containing 60 mL doubly distilled water. The flask was sonicated for 25 min and completed up to 100 mL with doubly distilled water, mixed well and filtered to remove all excipients. Aliquots of DUL were transferred to the cell and the procedure as for bulk was done.
Determination of DUL in Urine: Urine (1.0 mL) was added to BR buffer of pH 6.0 (9.0 mL) without any pretreatment. Aliquots of DUL (1.0 × 10-3 mol L-1) were transferred to the cell and the procedure as for bulk was done.
Electrochemistry of DUL
Figure 2A exhibits anodic peaks during the forward direction and there are no peaks during the reverse direction over the pH range (2.0-11.0), denoting to the irreversibility of DUL oxidation. The peak potential (E) has shifted less positively with increasing the pH denoting to participation of protons in the oxidation process. The peak potential varies as a function of pH in two linear parts (Figure 2B): the first one from pH 2.0 to 7.0 (E (V) = 1.282 - 0.009 pH, correlation coefficient (r2) = 0.9998) and the other from pH 8.0 to 11.0 (E (V) = 1.721 - 0.083 pH, r2 = 0.9988). pH 6.0 displays the highest and well defined oxidation peak current (I) of DUL (Figure 2C) that's why it was taken as the optimum pH for the rest of experiments.
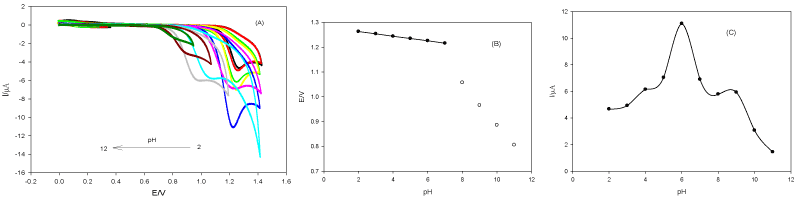
Figure 2. Cyclic voltammograms of DUL (1.0 × 10-3 mol L-1) at CPE in different BR buffers. (A), Peak potential (B) and current (C) plots as a function of pH. Scan rate of 100 mV s-1.
Influence of electrode modifications
A comparison was done between CPE, MCPE and TMCPE by applying the recommended procedure on each electrode for DUL using cyclic voltammetry to determine the electrode of the highest response and consequently the highest sensitivity which is needed to complete the work. Figure 3 shows that the oxidation peak of DUL has the following values of current and potential for CPE (11.03 µA, 1.224 V), MCPE (12.37 µA, 1.194 V) and TMCPE (15.00 µA, 1.186 V), subsequently TMCPE was selected for subsequent experiments. Figure 4 shows that the surfaces of CPE and MCPE as separate layers and protrusions, respectively, while irregular surface as moon surface of TMCPE giving a high electro-active surface area of the electrode resulted in its higher response.
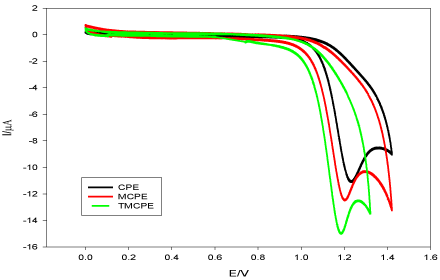
Figure 3. Cyclic voltammograms of DUL (1.0 × 10-3 mol L-1) in BR buffer (pH 6.0) at different electrodes at a scan rate of 100 mVs-1.
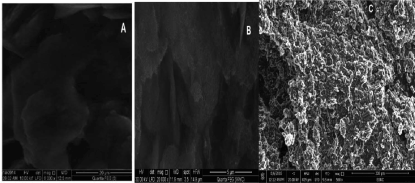
Figure 4. The pictures of SEM of CPE (A), MCPE (B) and TMCPE (C).
Influence of scan rate
Scan rate (ν) effect on oxidation current of DUL (I) was studied in pH 6.0 (BR buffer) at TMCPE (Figure 5), from which we got a linear relationship between log I and log ν. Linear relation was obtained and log I = -0.053 + 0.63 log ν, r2 = 0.9995. The slope was found to be 0.63 which indicates that the oxidation reaction taking place through diffusion controlled process with some adsorption [40].
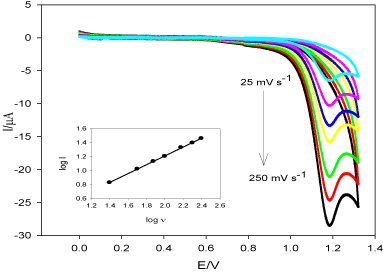
Figure 5. Cyclic voltammograms of DUL (1.0 × 10-3 mol L-1) using different scan rates (ν = 25-250 mV s-1) in pH 6.0 (BR buffer) at TMCPE. The inset: log I vs. log ν.
Figure 6 exhibits log E versus log ν, αn can be calculated from the slope of the relation (α is the transfer coefficient, n is the number of electrons) [41] based on Laviron equation E= E° + 2.303RT/αnF[log RTK°/αnF + log ν], where F is the Faraday constant, R is the gas constant and T is the temperature.
In this case and from figure 6, the slope value is 0.057; αn values were calculated to be 1.042. α ≈ 0.5, thus, n ≈ 2 assured the suggested mechanism of oxidation of DUL (Scheme 1).
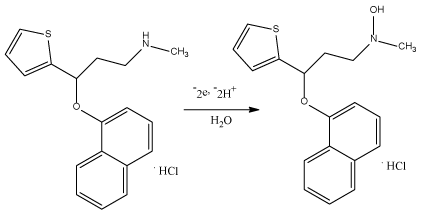
Scheme 1. The suggested mechanism of oxidation of DUL
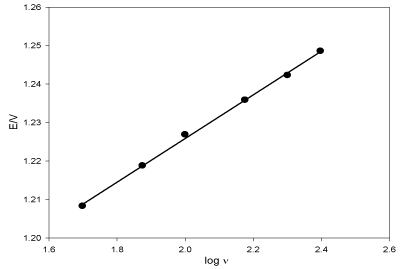
Figure 6. Relation between the oxidation peak potential of DUL versus logarithm of the scan rates.
The diffusion coefficient (Do) of DUL was calculated by Randles-Sevcik equation: I = (2.99 × 105) nα1/2 ACoDo1/2υ1/2 where A is the electrode surface area (cm2) and Co is the concentration of DUL (1.0 × 10-3 mol L-1) [42]. Do was found to be 1.76 × 10-5 cm2 s-1.
It is worthy to mention that the electro active surface area of TMCPE was calculated by Randles-Sevcik equation using 1.0 mmol L-1 K3Fe (CN)6 in 0.1 mol L-1 KCl (Do of K3Fe (CN)6 is 7.6 × 10-6 cm2 s-1) at TMCPE through applying different scan rates and found to be 0.115 cm2.
Deposition time
Deposition time (t) influences on the peak current of DUL at TMCPE (Figure 7). The current increases as deposition time increases reaching a steady state at 35 s but the broadness of the peak started to increase with increasing time after 5 s, so 5 s was taken as the optimum deposition time for subsequent measurements.
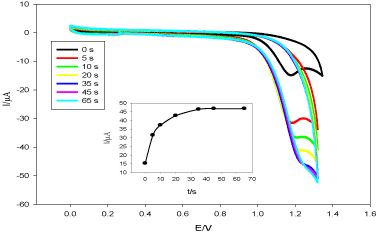
Figure 7. Cyclic voltammograms of DUL (1.0 × 10-3 mol L-1) at TMCPE in pH 6.0 (BR buffer), ν = 100 mV s-1. The inset: I versus t.
Validation
The presented method was validation according to the International Conference on Harmonization (ICH) Tripartite Guideline Q2 (R1) [43].
Determination of DUL: Figure 8 presents the linearity of DUL at TMCPE over the concentration ranges of 3.0 × 10-6 - 2.0 × 10-4 mol L-1, r2 = 0.9996. The regression data of this linear relation was tabulated and shown in Table 1.
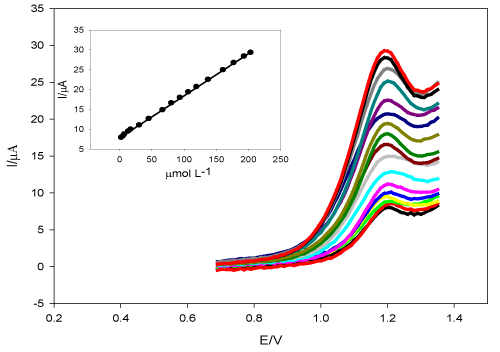
Figure 8. The influence of changing the concentration of DUL at TMCPE in pH 6.0 (BR buffer), ν = mV s-1. The inset: the peak current versus the concentration of DUL.
Table 1. Parameters of the regression equations for quantitative determination of DUL in bulk and in human urine sample.
Parameters |
DUL |
DUL in urine |
Linearity (mol L-1) |
3.0 × 10-6 - 2.0 × 10-4 |
7.0 × 10-6 - 1.1 × 10-4 |
Slope |
0.106 |
0.121 |
Intercept |
7.8 |
14.864 |
r2 |
0.9996 |
0.9994 |
LOD (mol L-1) |
4.0 × 10-7 |
6.9 × 10-6 |
LOQ (mol L-1) |
1.33 × 10-6 |
2.3 × 10-6 |
The limit of detection (LOD) and of quantification (LOQ) for DUL were found to be 4.0 × 10-7 mol L-1 and 1.33 × 10-6 mol L -1, respectively. 5.96 × 10-6 mol L-1 DUL (Five measurements) was used to determine repeatability of the suggested SWV method, recoveries range was (99.826% - 100.32%) and the relative standard deviation (RSD) was 1.95% indicating precision of the proposed method.
Robustness of the method using 1.38 × 10-5 mol L-1 DUL solution was demonstrated by repeating experimental procedure several times but with making minor change in optimum experimental parameters such as changing buffer pH by ±0.2, ν by ±5 and t by 5 ± 1. The RSD values were 0.743%, 0.688% and 0.858%, respectively. Therefore, the proposed method was unaffected by these minor changes.
Table 2 presents a comparison between the suggested method and the reported methods for DUL determination. The proposed method was found to be more sensitive than the reported spectrophotmetric methods [10-14], and chromatographic methods [16-20].
Table 2. Comparison between suggested method and some methods to determine DUL in literature.
Method |
DUL linear range |
Ref. |
Proposed voltammetry (mol L-1)
µg mL-1 |
3.0 × 10-6 to 2.0 × 10-4
(1.00-66.78) |
This work |
Spectrophotometry (µg mL-1) |
5-30 |
[10] |
|
5-50 |
[11] |
|
10-50 |
[12] |
|
5-25 |
[13] |
|
2.5-25 |
[14] |
Chromatography (µg mL-1) |
20-120 |
[16] |
|
2-10 |
[17] |
|
30-90 |
[18] |
|
8-56 |
[19] |
|
20-120 |
[20] |
Assay of DUL in dosage form: The ability of the proposed method in determining DUL in capsules without interference from the present excipients reflected and proved its selectivity. The determination of DUL in Cymbalta capsules using TMCP was done successfully using standard addition method. Neither sample pretreatment nor tedious extraction steps were required to be done before the experimental investigation. Known concentrations of DUL (2.99 × 10-6, 2.91 × 10-5, 6.54 × 10-5, 9.91 × 10-5 and 1.30 × 10-4 mol L-1) were added to sample solution (6.95 × 10-6 mol L-1) as shown in Table 3. The mean recovery and mean RSD were 100.198% and 0.2067%, respectively.
Table 3. Determination of DUL in Cymbalta capsules.
Drug |
Added |
Taken |
Found |
%Recovery |
|
2.99 × 10-6 |
|
9.91 × 10-6 |
100.10 |
|
2.91 × 10-5 |
|
3.56 × 10-5 |
99.972 |
|
6.54 × 10-5 |
6.95 × 10-6 |
7.16 × 10-5 |
100.26 |
|
9.91 × 10-5 |
|
1.05 × 10-5 |
100.14 |
|
1.30 × 10-4 |
|
1.36 × 10-4 |
100.52 |
Meana |
|
|
|
100.198 |
SDb |
|
|
|
0.2071 |
%RSDc |
|
|
|
0.2067 |
SEd |
|
|
|
0.0926 |
aMean for five determinations.
bSD is the standard deviation.
cRSD = (SD/mean) × 100.
dSE is the standard error.
Assay of DUL in Urine: Figure 9 presents the calibration graph DUL in urine. Linearity ranged from 7.0 x 10-6 mol L-1 to 1.1 × 10-4 mol L-1, r2 = 0.9994, LOD and LOQ were 6.9 × 10-6 mol L-1 and 2.3 × 10-6 mol L-1, respectively (Table 1).
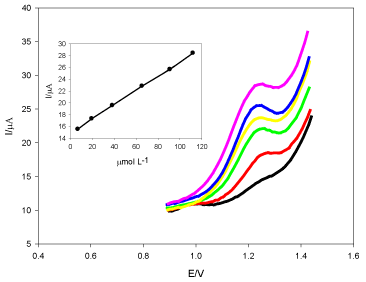
Figure 9. Square wave voltammogram of DUL spiked in urine at TMCPE in pH 6.0 (BR buffer). The inset: the peak current versus the concentration of DUL.
In the presented work, square wave voltammetric technique was utilized for fast and sensitive determination of DUL in bulk, capsules and urine through the oxidation of DUL at TMCPE. The presence of modifiers caused an increment in the peak current. SWV method has also been fully validated.
The authors thank National Organization for Drug Control and Research for supplying all solvents and tools required for completion of this work.
- Freeman MP, Hirschberg AM, Wang B, Petrillo LF, Connors S, et al. (2013) Duloxetine for major depressive disorder and daytime and nighttime hot flashes associated with the menopausal transition. Maturitas 75: 170-174. [Crossref]
- Sultan A, Gaskell H, Derry S, Moore RA (2008) Duloxetine for painful diabetic neuropathy and fibromyalgia pain: systematic review of randomised trials. BMC Neurol 8: 1-9. [Crossref]
- Ball S, Marangell LB, Lipsius S, Russell JM (2013) Brain-derived neurotrophic factor in generalized anxiety disorder: results from a duloxetine clinical trial. Prog Neuropsychopharmacol Biol Psychiatry 43: 217-221. [Crossref]
- Bennett R, Russell IJ, Choy E, Spaeth M, Mease P, et al. (2012) Evaluation of patient-rated stiffness associated with fibromyalgia: a post-hoc analysis of 4 pooled, randomized clinical trials of duloxetine. Clin Ther 34: 824-837. [Crossref]
- Leewen JHS, Lange RR, Jonasson AF, Chen WJ, Viktrup L (2008) Efficacy and safety of duloxetine in elderly women with stress urinary incontinence or stress-predominant mixed urinary incontinence. Maturitas 60: 138-147. [Crossref]
- Mihaylova B, Pitman R, Tincello D, Vaart H, Tunn R, et al. (2010) Cost-effectiveness of duloxetine: the stress urinary incontinence treatment (suit) study. Value Health 13: 565-572. [Crossref]
- USP 40-NF 35 (2017) The United States Pharmacopeia and National Formulary US pharmacopeial convention, Rockville, USA.
- Amirtha RV, Ramesh T, Phani KA (2011) A validated UV spectrophotometric determination of an antidepressant drug-duloxetine hydrochloride from capsule formulations. Int J Pharma Bio Sci 2: 716-720.
- Chadha R, Bali A (2015) Development and validation of stability indicating derivative spectrophotometric methods for determination of duloxetine hydrochloride. Br J Pharm Res 6: 402-414.
- Sagirli O, Toker SE, Önal A (2014) Development of sensitive spectrofluorimetric and spectrophotometric methods for the determination of duloxetine in capsule and spiked human plasma. Luminescence 29: 1014-1018. [Crossref]
- Yunoos M, Sankar DG, Kumar BP, Hameed S, Hussain A (2010) Simple UV spectrophotometric determination of duloxetine hydrochloride in bulk and in pharmaceutical formulations. Eur J Chem 7: 785-788.
- Kamila MM, Mondal N, Ghosh LK (2007) A validated UV spectrophotometric method for determination of duloxetine hydrochloride. Pharmazie 62: 414-415. [Crossref]
- Toker SE, Önal A (2010) Spectrophotometric determination of antidepressant drug duloxetine in pharmaceutical preparations using p-acceptors. Eur J Chem 9: 323-329.
- Ramesh T, Kumar AP, Raj RVA (2011) A validated UV spectrophotometric determination of an antidepressant drug–duloxetine hydrochloride from capsule formulations. Int J Pharma Bio Sci 2: 716-720.
- Susmitha K, Venkateswarlu G (2011) Extractive spectrophotometric methods for determination of duloxetine hydrochloride in pharmaceutical formulations using acidic triphenyl methane dyes. Int J Chem Tech Res 3: 1246-1254.
- Chadh R, Bali A (2015) Stability indicating spectrofluorimetric method for determination of duloxetine hydrochloride in bulk and in dosage form. Der Pharmacia Lettre 7: 232-240.
- Bhimanadhuni CN, Garikapati DR, Srinivas C (2012) Development and validation of RP-HPLC method for determination of Duloxetine hydrochloride in bulk and dosage form. Int Curr Pharm J 1: 98-102.
- Kumar, Bimlesh, et al. (2017) Validated reversed phase high performance liquid chromatography method for simultaneous estimation of curcumin and duloxetine hydrochloride in tablet and self nanoemulsifying drug delivery systems. J Pharm Res 11: 1166-1178.
- Sheladia S, Patel B (2016) Implementation of quality by design approach to develop and validate analytical method for simultaneous estimation of duloxetine hydrochloride and methylcobalamin in pharmaceutical dosage form by RP-HPLC method. Int J Pharm Res Rev 5: 13-26.
- Puranik M, Wadher S, Sharma K (2014) A Simple, novel validated stability indicating RP-HPLC method for estimation of duloxetine HCl in capsule pharmaceutical formulation. Indian J Pharmaceut Edu Res 48: 91-98.
- Boopathy D, et al. (2010) New RP-HPLC method development and validation determination for estimation of duloxetine HCl in enteric coated capsules. Int J Chem Tech Res 2: 239-241.
- Chhalotiya UK, Bhatt KK, Shah DA, Baldania SL (2010) Development and validation of a stability-indicating RP-HPLC method for duloxetine hydrochloride in its bulk and tablet dosage form. Scientia Pharmaceutica 78: 857-868. [Crossref]
- Patel SK, Patel NJ, Patel KM, Patel PU, Patel BH (2008) Estimation of Duloxetine Hydrochloride in Pharmaceutical Formulations by RP-HPLC Method. Indian J Pharm Sci 70: 825-827. [Crossref]
- Mercolini L, Mandrioli R, Cazzolla R, Amoreb M, Raggi MA (2007) HPLC analysis of the novel antidepressant duloxetine in human plasma after an original solid-phase extraction procedure. J Chromatogr B 856: 81-87. [Crossref]
- Raman NV, Harikrishna KA, Reddy KR, Prasad AV, Ramakrishna K (2010) Determination of duloxetine hydrochloride in the presence of process and degradation impurities by a validated stability-indicating RP-LC method. J Pharm Biomed Anal 51: 994-997. [Crossref]
- Reddy PB (2009) Validation and stability indicating reverse phase-high performance liquid chromatography for the determination of duloxetine in tablets. Int J Chem Tech Res 3: 602-605.
- Alarfaj NA, Ammar RA, El-Tohamy MF (2012) Disposable screen-printed sensors for determination of duloxetine hydrochloride. Chem Central J 6: 1-8. [Crossref]
- Ammar RA, Otaif H, Al-Warthan A (2012) Quantitative determination of duloxetine hydrochloride in pharmaceuticals and urine using prepared ion selective membrane electrode. Int J Electrochem Sci 7: 2531-2542.
- Anderson D, Reed S, Lintemoot J, Kegler S, DeQuintana S, et al. (2006) A first look at duloxetine (Cymbalta) in a postmortem laboratory. J Anal Toxicol 30: 576-580. [crossref]
- Liu L, Nussbaum M (1999) Systematic screening approach for chiral separations of basic compounds by capillary electrophoresis with modified cyclodextrins. J Pharm Biomed Anal 19: 679-694. [Crossref]
- Rickard EC, Bopp RJ (1994) Optimization of a capillary electrophoresis method to determine the chiral purity of a drug. J Chromatogr A 680: 609-621.
- Musenga A, Amore M, Mandrioli R, Kenndler E, De Martino L, et al. (2009) Determination of duloxetine in human plasma by capillary electrophoresis with laser-induced fluorescence detection. J Chromatogr B 877: 1126-1132. [Crossref]
- Hassanein AM, Moharram YI, Oraiby NF, Ebied SE (2017) Trace determination of duloxetine HCl in formulation and spiked human serum at a carbon paste electrode. Am J Anal Chem 8: 708-725.
- Zen JM, Senthil KA, Tsai DM (2003) Recent updates of chemically modified electrodes in analytical chemistry. Electroanalysis 15: 1073-1087.
- Dryhurst G, McAllister DL, Kissinger PT (1984) Carbon electrodes, in laboratory techniques in electroanalytical chemistry, WRHeineman ed. New York. Marcel Dekker Inc.
- Shams E, Babaei A, Taheri AR, Kooshki M (2009) Voltammetric determination of dopamine at a zirconium phosphated silica gel modified carbon paste electrode. Bioelectrochemistry 75: 83-88. [Crossref]
- Wang J (2000) Analytical Electrochemistry. (2nd ed) New York. Wiley-VCH.
- Rizk M, Attia AK, Elshahed MS, Farag AS (2015) Validated voltammetric method for the determination of antiparkinsonism drug entacapone in bulk, pharmaceutical formulation and human plasma. J Electroanal Chem 743: 112-119.
- Attia AK, Abo-Talib NF, Tammam MH (2017) Voltammetric determination of ivabradine hydrochloride using multiwalled carbon nanotubes modified electrode in presence of sodium dodecyl sulfate. Adv Pharm Bull 7: 151-157. [Crossref]
- Gosser DK (1993) Cyclic voltammetry: simulation and analysis of reaction mechanism. New York.
- Laviron E (1979) General expression of the linear potential sweep voltammogram in the case of diffusionless electrochemical systems. J Electroanal Chem 101: 19-28.
- Eggins BR (2003) Chemical sensors and biosensors, UK. JohnWiley & Sons Ltd.
- ICH Trapartite Guideline, validation of analytical procedures: text and methodology (2005) Q2 (R1), 1–13, http://www.ich.org/cache/compo/276-254-1.html.










