Abstract
Background: Cardiovascular disease is rising exponentially in Asian countries. High-sensitivity cardiac troponin-T (hs-TnT) is vital for evaluating acute coronary syndromes. Although hs-TnT has been available since 2010 and used widely in Asia-Pacific there is paucity of published literature on what constitutes expected hs-TnT concentrations as well as the 99th percentile (99P) upper reference limits (URL) in Asians. Almost all the hs-TnT data has been derived on Western populations. Besides, very few studies encompass large enough reference cohorts to mitigate against outlier distortion of the URL.
Methods: We determined the 99P URL for hs-TnT (Roche Diagnostics) in 1086 healthy Asian subjects (via questionnaire) aged 40-65 years (mean 52.2+7.24; 543 men) from 3 laboratories. There were at least 100 subjects in each 5-year age-group. Statistical analyses were performed on MedCalc 15.0 software (Ostend, Belgium). The hs-TnT assay has a limit of detection of 5 ng/L.
Results: The inter-assay precision was 2.8% and 1.9% at mean hs-TnT concentrations of 23 and 1950 ng/L respectively. The hs-TnT concentrations corresponding to the inter-assay coefficient of variation of 20% and 10% was 7.5 and 11.5 ng/L respectively. Hs-TnT concentrations were detectable in 39.4% of participants; higher in men and individuals >55 y. However, 82.0% of women had undetectable hs-TnT compared to 39.2% of men. This was also seen in 98.5% younger women aged 20-39 (n=260). Detectable hs-TnT was greater in those over 55 years versus younger subjects in women (30.3% and 11%) and men (71% and 54.8%).
The overall, male, and female 99th percentile URL were: 17.0,18.6 and 12.0 ng/L respectively.
Conclusion: This hs-TnT assay can perform with high sensitivity and exhibits gender and age differences. The 99P URL is similar to some previous reports but not in others. Users need to establish/verify their own decision limits. Young women may not be suitable as reference subjects.
Key words
High-sensitivity, Cardiac-troponin, 99th percentile, Upper-reference-limits, Multi-ethnic, Asians
Abbreviations and symbols
High-sensitivity – hs; high-sensitivity troponin T – hsTnT; high sensitivity troponin I – hsTnI; 99th percentile – 99P; Upper reference limit – URL; Acute coronary syndrome – ACS
Introduction
The burden of cardiovascular disease is increasing globally [1] with low- and middle-income nations bearing the brunt of this scourge [2] including Asia and South-East Asia [3]. Hospitals here are seeing greater numbers of patients for the evaluation of chest pain and acute coronary syndrome (ACS). In this regard troponin, especially the high sensitivity (hs) variety, is the preferred biomarker for the evaluation of ACS [4-8]. A key metric for decision-making in ACS is the 99th percentile (99P) upper reference limit (URL) for troponin in the healthy reference population. To derive the 99P URL a sufficiently large cohort (minimally 300 subjects of either gender, preferably 500) [5,9-11] is required to mitigate against outlier distortion of this cut-point. In Asia-Pacific hs-troponin-T (hs-TnT) has been available since 2010 and hs-troponin-I (hs-TnI) since 2012. Despite their wide usage there is paucity of published literature on what are expected hs-troponin concentrations and the 99P URL in Asians. For hs-troponin reference range studies involving at least 600 Asian subjects, there is a solitary publication on hs-TnI (n=1120) [12], but none for hs-TnT. We determined the 99 percentile URL in a large national multi-center Asian population for the hs-TnT assay (Roche Diagnostics Asia-Pacific, Singapore).
Methods
The study was a prospective, cross-sectional national study from 3 College of American Pathologists (CAP) accredited hospital laboratories in Singapore (Changi, Alexandra, and Khoo Teck Puat). It was approved by the respective local institutional review boards. The initial hs-TnT assay verification from Changi General in 2010 is reported here. All 3 sites contributed the data they generated for the reference range study from 2013 to 2015. Their performance for the CAP troponin-T proficiency testing program was satisfactory during this period.
Study population
All participants gave informed consent where appropriate. From our health screening programs, 1086 healthy, ambulatory multi-ethnic Asian subjects 40-65 y old (mean age: 52.2±7.24 y) comprising equal numbers of men (mean age 52.1±7.40 y) and women (mean age: 52.2±7.07 y) were enrolled. Pregnant subjects and those with any acute illness or co-morbidities (diabetes, hypertension, heart, muscle or renal disease) were excluded.
hs-TnT analyses
Details of the Roche hs-TnT assay have been published [13, 14] and will not be provided here. Blood was collected in serum vacutainer tubes with gel and clot activator (Becton-Dickinson); visibly hemolyzed or turbid samples were omitted. All samples were centrifuged at 3000g for 10 minutes and analyzed within an hour on the Cobas e601 analyzer (Roche). The assay limit of blank (LoB) and assay limit of detection (LoD) were verified as per CLSI document EP17-A [15]. We evaluated assay precision according to CLSI EP5-A2 [16] with imprecision profiling as per Sadler [17]. Between-day assay precision was assessed over 30 days using the 2 concentrations of control materials supplied which contained 20 and 2000 ng/L of hs-TnT. Between reagent lot comparisons was done according to CLSI guide EP26-A [18]. Measurable hs-TnT was considered to be any value above the LoD; values below the LoD were imputed at half the LoD. Consecutive samples that had been stored at 4oC were re-tested within 24 hours to verify the stability of hs-TnT samples should an add test be required. Hs-TnT was analyzed in consecutive paired EDTA plasma-serum samples to assess suitability of plasma as an alternative test material. Statistical analyses were performed using MedCalc 15.0 software (Ostend, Belgium). The 99P URL was determined according to CLSI guide C28-A3 using 1-tailed non-parametric statistics [19].
Results
Assay performance
The LoB (60 replicates of the zero calibrator) and LoD (40 measurements) were 3 and 5 ng/L respectively. Values below the LoD were thus assigned a concentration of 2.5 ng/L. The analytical measurement range was verified to be linear up to 10,000 ng/L (as stated in the package insert). The hs-TnT concentrations corresponding to different assay coefficients of variation (CVs %) were 7.5 (20%), 9.0 (15%), 11.5 (10%) and 17.0 (5%) ng/L respectively. Between-day assay precision (n = 30) for the QC materials were 2.8 and 1.9% for hs-TnT concentrations of 23 and 1950 ng/L respectively. Of the 148 consecutive, serum hs-TnT samples re-tested, 13 were below the LoD (11/13 concordance, and 2/13 differing by <0.3 ng/L), 130 paired results between 5-100 ng/L were very close (mean difference 0.01+1.52 ng/L) and 5 were > 100 ng/L (mean difference -0.2%, range: -6.5 to +6.2%). There was close agreement between the paired plasma-serum hs-TnT results (n=136): r=0.9994, plasma TnT = 0.99667 serum TnT – 0.775. Bland-Altman difference analysis showed a mean plasma-serum difference of 1.25 ng/L for hs-TnT concentrations < 100 ng/L and 1.47% for hs-TnT concentrations > 100 ng/L. This hs-TnT assay had good correlation (n=208) with the previous Roche TnT Gen-4 assay: R2 = 0.996845, hsTnT = 0.9787 TnT + 0.0051. In 2010, the 99P URL derived from 380 healthy adults (180 Males 31-60 y, 200 Females 41-60) for hs-TnT was 15 ng/L versus 30 ng/L for TnT [20].
Reference population
In our current study, serum hs-TnT concentrations (range 2.5-34.0 ng/L) were positively skewed (Kolmogorov–Smirnov test: p < 0.0001) (Figure 1). The median hs-TnT concentrations by age and gender are given in Table 1.
Table 1. hs-TnT concentrations (ng/L) by Age and Gender
Age Group (y) |
N |
Minimum |
25th percentile |
Median |
75th percentile |
Maximum |
F 40-49 |
212 |
2.5 |
2.5 |
2.5 |
2.5 |
11.0 |
F 50-59 |
231 |
2.5 |
2.5 |
2.5 |
2.5 |
21.0 |
F 60-65 |
100 |
2.5 |
2.5 |
2.5 |
6.0 |
17.0 |
M 40-49 |
212 |
2.5 |
2.5 |
2.5 |
6.0 |
11.0 |
M 50-59 |
226 |
2.5 |
2.5 |
6.0 |
8.0 |
34.0 |
M 60-65 |
105 |
2.5 |
2.5 |
7.0 |
10.0 |
19.0 |
*F – female, **M - Male
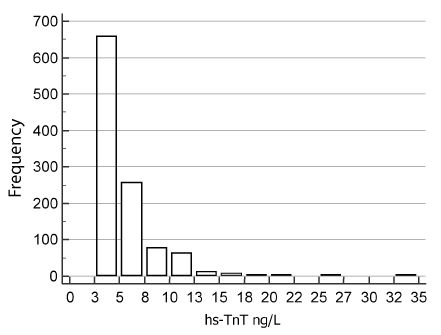
Figure 1. All-subject distribution of hs-TnT concentrations in the reference population
The overall, male and female 99P URL (90% CI) were 17.0 (14-19), 18.6 (17-25) and 12.0 ng/L (11-17) respectively. The hs-TnT assay CVs at these URL concentrations were all <10%. Men had higher serum hs-TnT than women (p < 0.0001, Mann–Whitney U test). There were 658 samples comprising 445 women and 213 men which contained TnT concentrations below the LoD. Thus, TnT was detectable (> LoD) in 39.4% (428/1086) of all subjects (18.0% female, 60.8% male). The female preponderance of undetectable hs-TnT was confirmed (98.5%) in a further cohort of young women (n=260) aged 20-39.
There is a step-wise increase in hs-TnT values with age. Non-parametric analysis of variance shows differences in hs-TnT between the various age groups in both men and women (p < 0.000001, Kruskal-Wallis test). These differences remain significant (p < 0.05) even when multiple pair-wise comparisons were made between the different age-groups (Kruskal-Wallis post hoc analysis by the method of Conover) other than that between 2 age groups - females 60-65 y and males 40-49 y (Table 2).
Table 2. Pair-wise comparison of sub-groups of hs-TnT concentrations*
Age Group (y) |
Factor |
N |
Average Rank |
Different from factor nr (p<0.05) |
F 40-49 |
(1) |
212 |
361.63 |
(2)(3)(4)(5)(6) |
F 50-59 |
(2) |
231 |
421.39 |
(1)(3)(4)(5)(6) |
F 60-65 |
(3) |
100 |
549.96 |
(1)(2)(5)(6) |
M 40-49 |
(4) |
212 |
565.43 |
(1)(2)(5)(6) |
M 50-59 |
(5) |
226 |
708.46 |
(1)(2)(3)(4)(6) |
M 60-65 |
(6) |
105 |
773.85 |
(1)(2)(3)(4)(5) |
* Kruskal-Wallis post-hoc analysis by the method of Conover
Discussion
Prior to its adoption at Changi General in 2011, we verified the performance of this hs-TnT assay and found the overall 99P URL to be 15 ng/L [20]. The assay LoB and LoD is similar to that in previous publications [13, 14]. The analytical precision of the assay and its measuring range of up to 10,000 ng/L are quite acceptable. Serum samples re-analyzed within 24 hours show stability when stored at 4oC permitting us to allow doctors to request adding an hs-TnT test from a previously drawn blood sample. In addition, the close agreement between paired EDTA plasma and serum hs-TnT enables us to use EDTA plasma as an alternative sample type.
All the current literature on TnT has reported gender differences. In a 2012 study Apple [21] reported an overall 99P URL of 15 ng/L for 524 subjects (age 18-64) without biomarker pre-qualification. This value is akin to what we found in 2010 and close to that in our current study (17 ng/L); our 99P decision limit for men and women (18.6 and 12.0 ng/L) was quite similar to that quoted by Apple – 20 and 13 ng/L. In one of the largest hs-TnT reference range investigations (n=1157) involving 565 US and 592 Vietnamese volunteers (mean age 41.2 y) screened by questionnaire, a higher Asian male-female hs-TnT cut-points (25 and 19 ng/L) was found [22]. Giannitsis [13] reported an overall 99P cut-point of 13.5 ng/L for 616 Germans with a mean age of 44+13.4 y while that in men was 14.5 and women 10.0. In a multi-center trans-Atlantic study (n=533 by questionnaire, mean age 37 y), Saenger [14] cited the following 99P URL of 14 (overall), 15.5 (male) and 9.0 (female) ng/L that was quite close to the publication from Germany. The Saenger study formed the data set cited in the hs-TnT product insert. However, this hs-TnT reference limits differ from that quoted in the new hs-TnT product insert (Elecsys Troponin T Gen 5 Stat package insert, March 2017) recently launched in the US. The 99P URL were higher in 1301 American subjects aged 21-89 y - overall 19, men 22 and women 14 ng/L respectively.
We also noted age-related differences in serum hs-TnT in both sexes, but more pronounced in men. Troponin elevations with age, contributed in part by age-related co-morbidities, have been emphasized [23–25]. Sandoval [9] and Collinson [26] underscore the vital role of population selection and pre-screening on the 99P decision limit. However, the data supporting the need to pre-qualify reference subjects with more tests (biochemical, imaging and ECG) is far from clear. In the Collinson study, questionnaires are a vital first step in volunteer recruitment. Thereafter the influence of additional testing (BP, ECG, NTproBNP, echocardiography, and estimated GFR) on the 99P URL for hs-TnT versus questionnaire alone is underwhelming – 21.5-23.8 versus 23.2 ng/L in men and 12.8-13.9 versus 13.6 in women. Besides the study is underpowered with less than 340 subjects. Thus, laboratories will need to verify the transferability of the 99 percentile URL to their intended populations. When they establish their own reference limits, institutions need to carefully choose whether additional tests are needed for the reference population recruits. Moreover, as noted previously [27] almost all younger women (20-39) have undetectable hs-TnT. Thus, guidance is needed from learned societies and experts regarding the advisability of including them as part of reference population studies. Besides, this group also has a low prevalence of acute coronary syndrome.
The current reference range study comprises a sufficiently large number of subjects (>500 per gender) encompassing equal numbers of men and women evenly distributed in their age groups. This should provide a solid basis for hsTnT cut-points in clinical decision-making. This study is unique in that it is the first and only large-scale hs-TnT reference range study in an Asian population. The sample size is adequately powered and secure sex-dependent reference limits for serum hs-TnT has been established. Our study population comprises mainly local residents (Chinese, Malay, and Indian origin) and some foreigners (from the Philippines, China, Malaysia, India, and Indonesia). Our data may not be applicable to other ethnic groups in Asia and studies in these populations are needed. Reference to recent literature in this regard [10] will be helpful.
We acknowledge some limitations in our study. Participants were not pre-screened with electrocardiograms, cardiac imaging or other serum biomarkers such as creatinine or NT-proBNP as they are costly. Subjects > 65 y were not included as it is difficult to recruit subjects without medical co-morbidities. While applying lower hs-TnT cut-offs in subjects over 65 y may misclassify risk, this may be mitigated by serial troponin testing [7]. We have not assessed the diagnostic efficiency of this hs-TnT assay in clinical situations such as chest pain, chronic kidney disease, diabetes or community screening. However, this assay has been widely studied with favorable performance in several clinical studies – diagnosis of acute myocardial infarction [28], early rule-in rule-out strategies for myocardial infarction [29], myocardial infarction after percutaneous coronary intervention [30], and heart failure [31].
In conclusion, the Roche hs-TnT assay exhibits hs performance (supporting a 99 percentile URL with a CV < 10% and > 50% detectable values especially in a large number of healthy male subjects). It qualifies for the proposed high-sensitivity troponin designation in the current literature. Gender differences in hs-TnT values are seen. Thus sex-specific URLs should be used just as is done for CK, CK-MB, natriuretic peptides, uric acid, creatinine, and blood cell counts. Our findings allow us to report a robust overall and gender-based reference ranges for hs-TnT in our multi-ethnic Asian cohort. The multi-center collaboration in this effort strengthens its wider utility nationally since it covers a broader spectrum of laboratories and patients. Age-related differences in hs-TnT were also found. Laboratories need to verify or establish their own hs-TnT reference values. Reference interval studies should be sufficiently large to accurately reflect the effects of gender and age. The impact of using gender- or age-specific cTn limits for the management of ACS is emerging and merits further studies. The use of group-specific URLs can only improve the diagnostic specificity of hs-TnT in the evaluation of ACS.
References
- Mozaffarian D (2017) Global Scourge of Cardiovascular Disease: Time for Health Care Systems Reform and Precision Population Health. J Am Coll Cardiol 70: 26-28. [Crossref]
- Fuster V (2014) Global burden of cardiovascular disease: time to implement feasible strategies and to monitor results. J Am Coll Cardiol 64: 520-522. [Crossref]
- Tan JWC, Lam CSP, Kasim SS, Aw TC, Abanilla JM, et al. (2017) Asia-Pacific consensus statement on the optimal use of high-sensitivity troponin assays in acute coronary syndrome diagnosis: focus on hs-TnI. Heart Asia 9: 81-87. [Crossref]
- Morrow DA, Cannon CP, Jesse RL, Newby LK, Ravkilde J, et al. (2007) National Academy of Clinical Biochemistry practice guidelines: clinical characteristics and utilization of biomarkers in acute coronary syndromes. Clin Chem 53: 552-574. [Crossref]
- Apple FS, Collinson PO (2012) Analytical characteristics of high-sensitivity cardiac troponin assays. Clin Chem 58: 54-61. [Crossref]
- Thygesen K, Alpert JS, Jaffe AS, Simooons ML, Chaitman BR, et al. (2012) Third universal definition of myocardial infarction. J Am Coll Cardiol 60: 1581-1598. [Crossref]
- Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B, et al. (2012) How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 33: 2252-2257. [Crossref]
- Apple FS, Jaffe AS, Collinson P, Mockel M, Ordonez-Llanos J, et al. (2015) IFCC educational materials on selected analytical and clinical applications of high-sensitivity cardiac troponin assays. Clin Biochem 48: 201-203. [Crossref]
- Sandoval Y, Apple FS (2014) The global need to define normality: the 99th percentile value of cardiac troponin. Clin Chem 60: 455-62. [Crossref]
- Apple FS, Sandoval Y, Jaffe A, Ordonez-Llanos J (2017) Cardiac troponin assays: guide to understanding analytical characteristics and their impact on clinical care. Clin Chem 63: 73-81. [Crossref]
- Hickman PE, Badrick T, Wilson SR, McGill D (2007) Reporting of cardiac troponin - problems with the 99th population percentile. Clin Chim Acta 381: 182-183. [Crossref]
- Aw TC, Phua SK, Tan SP (2013) Measurement of cardiac troponin I in serum with a new high-sensitivity assay in a large multi-ethnic Asian cohort and the impact of gender. Clin Chim Acta 422: 26-28. [Crossref]
- Giannitsis E, Kurz K, Hallermayer K, Jarausch J, Jaffe AS, et al. (2010) Analytical validation of a high-sensitivity cardiac troponin T assay. Clin Chem 56: 254-261. [Crossref]
- Sanger AK, Beyrau R, Braun S, Cooray R, Dolci A, et al. (2011) Multicenter analytical evaluation of a high-sensitivity troponin T assay. Clin Chim Acta 412: 748-754. [Crossref]
- National Committee for Clinical Laboratory Standards (2004) Protocols for determination of limits of detection and limits of quantitation; approved guideline. NCCLS document EP17-A. NCCLS, Wayne, PA: NCCLS.
- National Committee for Clinical Laboratory Standards (2004) Evaluation of precision performance of quantitative measurement methods; approved guideline – second edition. NCCLS document EP5-A2. NCCLS, Wayne, PA: NCCLS.
- Sadler WA (2008) Imprecision profiling. Clin Biochem Rev 29: S33-S36. [Crossref]
- Clinical Laboratory Standards Institute (2013) User evaluation of between-reagent lot variation; approved guideline. CLSI document EP26-A. CLSI, Wayne, PA: CLSI.
- Clinical Laboratory Standards Institute (2008) Establishing and verifying reference intervals in the Clinical Laboratory; approved guideline - third edition. CLSI document C28-A3c. CLSI, Wayne, PA: CLSI; 2008.
- Aw TC, Pua SK, Yew L, Lim WR, Tan SP (2010) Performance of a new rapid automated high-sensitive Troponin T (hsTnT) immunoassay. Clin Chem 56: Abstract C-88, A133-134.
- Apple FS, Ler R, Murakami MM (2012) Determination of 19 cardiac troponin I and T assay 99th percentile values from a common presumably healthy population. Clin Chem 58: 1574-1581. [Crossref]
- Gaggin HK, Dang PV, Do LD, deFilippi CR, Christenson RH, et al (2014) Reference interval evaluation of high-sensitivity troponin T and N-terminal B-type natriuretic peptide in Vietnam and the US: The North South East West trial. Clin Chem 60: 758-764. [Crossref]
- Gore MO, Seliger SL, Defilippi CR, Nambi V, Christenson RH, et al. (2014) Age- and sex-dependent upper reference limits for the high-sensitivity cardiac troponin T assay. J Am Coll Cardiol 63: 1441-1448. [Crossref]
- Franzini M, Lorenzoni V, Masotti S, Prontera C, Chiappino D, et al. (2015) The calculation of the cardiac troponin T 99th percentile of the reference population is affected by age, gender, and population selection: a multicenter study in Italy. Clin Chim Acta 438:376-381. [Crossref]
- Collinson PO, Heung YM, Gaze D, Boa F, Senior R, et al. (2012) Influence of population selection on the 99th percentile reference value for cardiac troponin assays. Clin Chem 58: 219-225. [Crossref]
- Normann J, Mueller M, Biener M, Vafaie M, Katus HA et al. (2012) Effect of older age on diagnostic and prognostic performance of high-sensitivity troponin T in patients presenting to an emergency department. Am Heart J 164: 698-705. [Crossref]
- Aw TC, Phua SK (2014) Troponin concentrations in young women measured by high-sensitivity troponins T and I. Clin Chem 60: Abstract B-338, S228-S229.
- Zhelev Z, Hyde C, Youngman E, Rogers M, Fleming S, et al. (2015) Diagnostic accuracy of single baseline measurement of Elecsys troponin T high-sensitive assay for diagnosis of acute myocardial infarction in emergency department: systematic review and meta-analysis. BMJ 350:h15 doi: 10,1136/bmj.h15.
- Cullen LA, Mills NL, Mahler S, Body R (2017) Early Rule-Out and Rule-In Strategies for Myocardial Infarction. Clin Chem 63: 129-139. [Crossref]
- Tricoci P (2017) Consensus or Controversy: Evolution of Criteria for Myocardial Infarction After Percutaneous Coronary Intervention. Clin Chem 63: 82-90. [Crossref]
- Seliger SL, Hong SN, Christenson RH, Kronmal R, Daniels LB, et al. (2017) High-sensitive cardiac troponin T as an early biochemical signature for clinical and subclinical heart failure: MESA (Multi-ethnic study of atherosclerosis). Circulation 135:1494-1505. [Crossref]

