Abstract
At the end of July beginning August 2014 an emergent bluetongue virus serotype 4 (BTV-4) was introduced in Albania and bluetongue disease (BTD) outbreak affected sheep, goats and cattle populations.
Materials and methods: In this study, there were include data collected from 210 cattle, 56 of them shown clinical sign and were investigate closely. This subpopulation was part of cattle that randomly were tested for presence of total IgG by employing a competitive ELISA (cELISA) method. ELISA positive samples were tested by serum neutralization (SN) assay for nine different bluetongue serotypes.
Results: Out of 210 individual bovine sera samples, 128 sera blood samples were positive in cELISA test for presence of total IgG against BTV. The most prevalent clinical signs in animal that developed BT disease were reduction of milk production, fever, lameness, conjunctivitis and appetite lost. The case fatality resulted 5.4%. The SN test confirmed the c-ELISA results revealing the presence of antibodies against BTV serotype 4 in 62.5% (80 samples) of cELISA positive samples and the titer varies from 1:10 to 1:640.
Conclusion: The epizootic outbreak of BT disease affected cattle apart sheep and goats and severe clinical disease was described. Majority of the clinically affected cattle were positive in cELISA and from nine serotypes known to be spread in Europe, only BTV serotype 4 was determine by serum neutralization test.
Key words
Bluetongue virus serotype 9, weak positive, sera neutralization, follow up
Introduction
Bluetongue is an infectious disease of ruminants, caused by Bluetongue virus (BTV), a member of the Reoviridae family, transmitted by certain insect species that serve as vectors [1-4]. In general cattle are susceptible to infection, however most infection cases have typical subclinical course. BT disease (BTD) due by classical 24 serotypes is a non-contagious viral disease. In the last years, next to the classical 24 serotypes which are transmitted by vectors, new serotypes, such as, BTV-25, BTV-26, BTV-27 [5-7] have been described. The last serotypes are characterized by some addition features, causing asymptomatic infections, being directly transmitted, at least BTV-26 [8] having long viraemia (BTV-250 [9] and using goats as reservoir hosts. Vector-independent transmission of BTV then can occur, although its significance is not fully known. Direct contact transmission of BTV-26, likely by aerosol, between livestock has been demonstrated [8]. In a study conducted in dromedaries in Mauritania slaughterhouse, BTV-26 neutralising antibodies were also found in a high number of animals confirming that horizontal transmission may have occurred in this case at the slaughterhouse, where animals are gathered together for several days before slaughtering. In one report, serotype 25 viral RNA was found for at least 2 years in individual animals, and the blood of some goats was infectious at 12-19 months [9]. Typical clinical signs of BTD occur when virulent BTV strains introduced into free areas by vectors or susceptible naive animals move to endemic areas [7]. Cattle are the main mammalian reservoir of BTV. Serologic testing can detect infection and cattle could be used as sentinel animal for BTV surveillance [10]. Identification of BTV serotype is important for vaccination programs, BTD control and for BTV epidemiology studies. Up to last decade, most reference laboratories used traditional identification methods such as: virus isolation, serum or virus neutralization tests (SNT or VNT) [11-13]. Today, there are available advanced molecular methods, which offer several advantages compare to classical methods, however classical method is valid, useful and is advised to use them in parallel with modern techniques.
The first case of BTD in Albania is recorded in 1998, when BTV-9 circulates in ruminant animals [14]. Starting by early second half 2014 an epizootic outbreak of BTD occur in several Balkan countries including Albania. All ruminants were affected; sheep, goats and cattle, however interestingly, a relatively large proportion of cattle shown clinical signs, even the case fatality was higher, compare to most published data. The mild winter and other ecological condition favorite growing of insect vector [15].
The aim of this study was to describe the clinical signs of BTD and to identify the serotype circulating in Albania during 2014.
Materials and methods
In this study, blood samples collected from 210 cattle without and with clinical signs in close proximity were analyzed. Between Octobers to November 2014, serum samples were collected from coccygeal vein by using 9 ml plane vacutainer. All the operations were humane according to the animal welfare and this study was approved on December 2013 by Department of Veterinary Public Health. The samples were left to clot and stored overnight at 4°C, centrifuged at 3000 rpm for 5 min and harvested sera were stored at -20°C until the serological tests were performed. In addition, there were collected data for all animals sampled, clinical signs and course of disease were investigated and recorded. The total antibodies in serum samples were detected using a competitive ELISA kit, produced by Istituto Zooprofilattico Sperimentale Teramo, Italy, according to the procedure described by the manufacturer. The results of the ELISA tests were expressed as the value of the sample (S) divided by value of the positive control serum (P) supplied in the ELISA kit, as determined by measurement of the optical density (OD450) with a “TECAN” ELISA plate reader[1]. The ELISA results were expressed as positive and negative. Serum neutralization test (SNT) was done at BT reference laboratory, Teramo, Italy.
The data were analyzed in Microsoft Excel using the add-in Data Analysis toolPak (Descriptive Statistics).
Clinical investigation
From 210 animals, only 56 cattle shown clinical signs. For each animal was applied a same protocol which consisted of the following: a clinical inspection was executed after a clinical suspicion of BT was reported to the veterinary authorities by a livestock farmer or veterinary practitioner. If a clinical suspect BT situation was encountered during a clinical inspection, all clinically sick animals were identified and sampled (serum). An infected area was defined as an epidemiological unit that have one or more animals tested positive in the cELISA
Serological test
Competitive ELISA
The samples were collected from 10 selected districts based on highest serological prevalence during previous survey, and or highest reports from Albanian Veterinary Services. All samples were analyzed in parallel in Albania and Italy, by cELISA in Albania and Italy, and positive samples were tested by serum neutralization test, at Bluetongue reference laboratory[2], Teramo, Italy.
At the Animal Health Laboratory, Albanian Faculty Veterinary Medicine, 210 bovine sera blood samples were tested for the presence of total BTV antibodies using c-ELISA manufactured by the IZSA&M in Teramo.
Serum neutralization test
In order to confirm the positive ELISA results all samples were sent at BT reference laboratory and both cELISA and SN test were performed on positive samples. Positive and negative controls for the SN were available and the microtitre neutralization method was used. Fifty µl of several sera dilutions, from 1:10 to 1:1280, were added to each test well of flat-bottomed microtiter plates and mixed with an equal volume of Office International des Epizooties standard reference BTV serotypes 1, 2, 4, 6, 8, 9, 14, 15 and 16 (100 TCID50). They were incubated at 37°C in 5% CO2. After 1 h incubation, approximately 104 Vero cells were added per well in a volume of 100µl of minimum essential medium (MEM) containing antibiotics and, after incubation for 4 to 6 days, the test was read using an inverted reference BTV serotypes.
Results
Of the 210 cattle tested, 61% (95% CI, 59.7–62.3%) were ELISA positive. Of the 128 ELISA, positive samples, 62.5% were positive based on SN test. There were confirmed only serotype 4 (BTV-4) and the titer varies from 1:10 to 1:640. The clinical results are presented on Table 1 and Figure 1-4. The ELISA results are presented in Table 1 -3 and Figure 5.
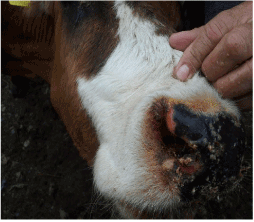
Figure 1. Typical lesions and crusts nasal mucosa
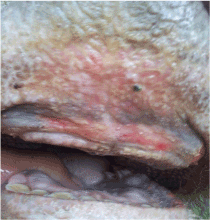
Figure 2. Erosions, ulceration of oral mucosa of cattle affected by BTV-4
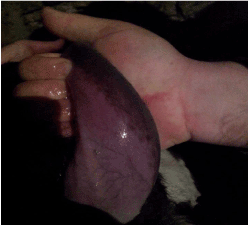
Figure 3. Hyperemic/purple coloration of tongue
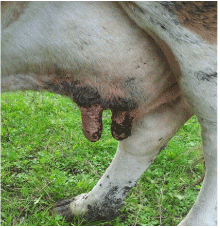
Figure 4. Purple coloration, lesions of teats
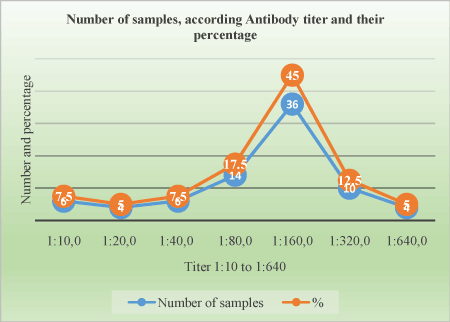
Figure 5. Titer distribution on positive animals
Table 1. Main clinical signs and their frequency in 56 cattle affected by BTV
Affected system |
Clinical manifestation |
Number of cases |
Percentage (%) |
General |
Fever |
55 |
98.2 |
Anorexia, weight loos, emaciation |
29 |
51.8 |
Drop of milk production |
56 |
100.0 |
Locomotor disorders |
Lameness |
39 |
69.6 |
Paresis |
1 |
1.8 |
Coronitis |
31 |
55.4 |
Mucosal disorders |
Lesions and nasal mucosa crusts |
6 |
10.7 |
Conjunctivitis |
42 |
75.0 |
Erosions/ulceration oral mucosa |
23 |
41.1 |
Hyperemic/purple coloration of tongue |
4 |
7.1 |
Respiratory disorders |
Dyspnea |
11 |
19.6 |
Cough |
8 |
14.3 |
Digestive disorders |
Anorexia |
51 |
91.1 |
Salivation |
33 |
58.9 |
Regurgitation |
3 |
5.4 |
Diarrhea |
3 |
5.4 |
| |
Abort |
0 |
0.0 |
Reproduction disorders |
Hyperemic/purple coloration, lesions of teats |
21 |
37.5 |
Table 2. Serological results based on cELISA and serum neutralization assay (performed at reference laboratory).
Test Method |
Results |
|
Positive (%) |
Negative |
Total |
cELISA |
128 (61.0) |
82 |
210 |
SN performed at reference laboratory (on 128 cELISA positive samples) |
80 (62.5) |
48 |
128 |
Table 3. Distribution of serological titers on 80 samples based on virus neutralization tests according their frequencies.
Titer |
Number of samples |
Percentage (%) |
1:10 |
6 |
7.5 |
1:20 |
4 |
5 |
1:40 |
6 |
7.5 |
1:80 |
14 |
17.5 |
1:160 |
36 |
45 |
1:320 |
10 |
17.5 |
1:640 |
4 |
5 |
Total |
80 |
100% |
Clinical investigation
Data on the frequency of clinical signs of affected animals are presented in Table 1. BTV-4 indicative clinical signs in affected animals were recorded in cattle. The most prominent clinical signs in BTV-4 affected cattle were: significant reduction of milk production, fever, anorexia, conjunctivitis, crusts/lesions of the oral mucosa, salivation, lameness, coronitis.
In addition, hyperemia, purple coloration and lesions of teats were recorded in certain proportion of animals. The less frequent clinical signs were paresis, regurgitation, diarrhea, and no abortions were reported. Four out of 56 animals that shown clinical disease died. The original figures (Figures 1, 2, 3 and 4) indicate some most prominent and visible clinical signs recorded during 2014 BTD outbreak.
The serological results based on cELISA and serum neutralization essay are presented in Table 2.
Serological results shown that the BTD was widespread. The cELISA test prevalence was 61%.
The Ab titer for positive samples ranges from 1:10 to 1:640. The table shows the proportion of samples according to antibody titer (Table 3).
Discussion
The awareness of veterinarians and farmers regarding clinical signs of BTD was likely to be low, particularly in the initial phase of the epizootic. As early as late June, beginning of August 2014, veterinary practitioners observed unusual clinical signs in a significant number of ruminant animals including cattle. The clinical manifestations and epidemiological situation in neighboring countries indicate presence of BTD which was diagnosed by serological methods and confirmed by laboratory advanced diagnostic tests. The interval between observation of the first clinical signs by the farmers and veterinary practitioners and an official notification to the veterinary authorities was relatively long. Promptly notification is very important, especially for highly contagious disease. BTD is one of the vesicular diseases and other vesicular disease must be included in differential diagnostic panel for cattle and sheep. Other diseases that should be included for differentiation in cattle include bovine viral diarrhea (BVD), infectious bovine rhinotracheitis (IBR), malignant catarrhal fever (MCF), photosensitization, and miscellaneous causes of bovine stomatitis [2,4]. Laboratory diagnosis of transboundary and notifiable animal diseases is compulsory as long as pathognomonic clinical signs almost don’t exist [13]. Farmers sometimes do not like to report early clinical signs of notifiable diseases; these facilitate further spread of infection. Training and encouraging both farmers and veterinarians to submit samples to the laboratory and reporting disease are key factors for controlling the outbreaks and stopping further spreading.
BTD in cattle, in gene2021 Copyright OAT. All rights reserv however a small proportion may show clinical cases, particularly during new serotypes incursion for the first year in naive population. In cattle, have been reported the occurrence and severity of the clinical disease, however they have always been much lower than in sheep during major outbreaks outside Africa, [2,3]. Cattle exposed to BTV under natural field conditions occasionally develop clinical signs of disease similar to those in BT-infected sheep, but in most instances the disease is unapparent [2]. The only exception is related to BTV-8 which affects cattle and they show clinical disease. In one study, only one out of four inoculated claves developed a mild conjunctivitis and another calve developed ulcers on the upper gum, but it was mild and the calf do not show salivation a detectable anorexia [16]. The majority of BTV-8 infected cattle within a herd were not clinically affected, but a few cattle within a herd, however, can show distinct clinical signs. Outbreaks with BTV-8 in cattle in the Netherlands in 2006 caused morbidity of 2.5%, with 0.22% mortality [16]. In this study, serological results shown a high serological prevalence based on cELISA (61%), only 39% of animals shown clinical signs, and more than 60% of infected individual did not shown any clinical signs, the case fatality was 7.14% (4 animal died). This could be explained by the fact that new serotype affects the naive population and no any immunity memory exists in population. The results of ELISA test performed in our laboratory and in Italy were the same. The sera prevalence of BTV infection, based on cELISA results, was 61%.
The serotype 4 was confirmed by SN test and the titer was significantly high. It ranges from 1:10 to 1:640, majority of samples have the titer 1:160 (45%). Some animals that were negative may do not seroconverted at the time they were bleed and test. Our study results are comparable to other studiesss [11,12,17]. Study of epidemiological “behavior” of BTV is important for controlling of BTD and reducing its negative impact in livestock. Applying active sero-surveillance of BTV in cattle is useful strategy for identifying of new serotypes of BTV and indicates proper preventive measures for impact minimalizing and controlling of potentially new epizootics.
Conclusion
In 2014, a new emergent serotype of bluetongue virus affected ruminant animals in Albania. Despite that BTD is a typical sheep disease, cattle were affected, and some of them shown severe clinical signs and fatal cases were recorded. The most prominent clinical signs were related with general clinical signs followed by locomotors and digestive tract and less affected was reproduction system. However, teat lesions of affected milking cow were present often. The case fatality rate in cattle was high, however it is within reported limits.
References
- Barratt-Boyes SM, MacLachlan NJ (1995) Pathogenesis of bluetongue virus infection of cattle. J Am Vet Med Assoc 206: 1322-1329. [Crossref]
- Radostits OM, Gay CC, Hinchcliff KW, Constable PD (2007) Bluetongue in Veterinary Medicine, Veterinary Medicine: A textbook of the diseases of cattle, horses, sheep, pigs and goats, Xth Edition,1299-1305.
- Quinn PJ, Markey BK, Leonard FC, Hartigan P, Fanning S, et al. (2011) Bluetongue species in Veterinary Microbiology and Microbial Disease Textbook, Second Edition, 640-642.
- Verwoerd DW, Erasmus BJ (2004) Bluetongue. In: Infectious Diseases of Livestock, 2nd Edition, JA Coetzer and RC Tustin, Eds, Oxford Press, Cape Town, 1201-1220.
- Hofmann MA, Renzullo S, Planzer J, Mader M, Chaignat V, et al. (2010) Detection of Toggenburg orbivirus by a segment 2–specific quantitative RT-PCR. Journal of Virological Methods 165: 325-329.
- Maan NS, Maan S, Belaganahalli MN, Ostlund EN, Johnson DJ, et al. (2012) Identification and differentiation of the twenty-six bluetongue virus serotypes by RT-PCR amplification of the serotype-specific genome segment 2. PLoS One 7: e32601. [Crossref]
- Zientara S, Sailleau C, Viarouge C, Höper D, Beer M, et al. (2014) Novel Bluetongue Virus in Goats, Corsica, France, 2014. Emerg Infect Dis 20: 2123-2125. [Crossref]
- Batten C, Darpel K, Henstock M, Fay P, Veronesi E, et al. (2014) Evidence for transmission of bluetongue virus serotype 26 through direct contact. PLoS One 9: e96049. [Crossref]
- Vögtlin A, Hofmann MA, Nenniger C, Renzullo S, Steinrigl A, et al. (2013) Long-term infection of goats with bluetongue virus serotype 25. Vet Microbiol 166: 165-173. [Crossref]
- Mayo CE, Mullens BA, Reisen WK, Osborne CJ, Gibbs EPJ, et al. (2014) Seasonal and Interseasonal Dynamics of Bluetongue Virus Infection of Dairy Cattle and Culicoides sonorensis Midges in Northern California – Implications for Virus Overwintering in Temperate Zones. PLoS ONE 9: e106975. [Crossref]
- Avci O, Yapici O, Bulut O, Kale M, Yavru S, et al. (2014) Detection of antibodies against Blue Tongue Virus in yaks (Bos Grunniens) in Issyk Kul, First Report. Journal of Animal and Plant Sciences 24: 1220-1223.
- Bulut O, Yavru S, Yapkiç O, Simsek A, Kale M, et al. (2006) Serological investigation of Bluetongue Virus infection by serum neutralization test and Elisa in sheep and goats. Bull Vet Inst Pulawy 50: 305-307.
- OIE (2016) Chapter 2.1.3. Bluetongue, In: Manual of Diagnostic Tests and Vaccines, Office International des Epizooties, Paris.
- Di Ventura M, Tittarelli M, Semproni G, Bonfini B, Savini G, et al. (2004) Serological surveillance of bluetongue virus in cattle, sheep and goats in Albania. Vet Ital 40: 101-104. [Crossref]
- Wilson A, Mellor P (2008) Bluetongue in Europe: vectors, epidemiology and climate change. Parasitol Res 103 Suppl 1: S69-77. [Crossref]
- Elbers AR, Backx A, Meroc E, Gerbier G, Staubach C (2008) Field observations during the bluetongue serotype 8 epidemic in 2006: I. Detection of first outbreaks and clinical signs in sheep and cattle in Belgium, France and the Netherlands. Prev Vet Med 87: 21-30. [Crossref]
- Sharma RN, Beckford S, Tiwari K, Vinet E, Thomas D, et al. (2016) Se- roprevalence of Bluetongue Virus Antibody in Ruminants from Grenada. Open Journal of Veterinary Medicine 6: 99-103.





