Abstract
The aim of the present study is to provide a review of main histopathological changes of the carotid body with potential forensic interest. Developmental changes in the carotid body have been reported in Sudden Infant Death Syndrome. SIDS victims frequently show alterations in respiratory regulation which may partly be ascribed to peripheral arterial chemoreceptors. Histopathological findings regarding cellular populations, connective components and inflammatory infiltrates have also been observed in opiate-related deaths. Better awareness about the structure of the carotid body and possible histopathological changes may be useful also for histopathological investigations in other cases of forensic relevance.
Introduction
The carotid body is the main arterial chemoreceptor directly involved in the control of respiratory and cardiocirculatory functions. It is located at the carotid bifurcation and consists of lobules separated by connective tissues, each one is organized in clusters of two different populations of cells (Figure 1). The first one is represented by type I (or chief) cells, in turn classified into light, dark and pyknotic cells. They are the real chemoreceptors which store and release neurotransmitters and neuromodulators, which are contained in dense-cored granules (Figure 1C-D). The second type is made by type II (or sustentacular) cells, which are considered supportive cells [1-3]. The carotid body is highly susceptible to reductions in pO2 pressure and pH and to increases in pCO2 pressure, in response of which rises the frequency and the volume of ventilation [4-6]. The sensory innervation of the carotid body is given by the carotid sinus nerve, a branch of the glossopharyngeal nerve. Moreover, the carotid body receives post-ganglionic sympathetic nerve fibers from the superior cervical ganglion, mainly acting on the microvascularization, and some parasympathetic fibers [6,7].
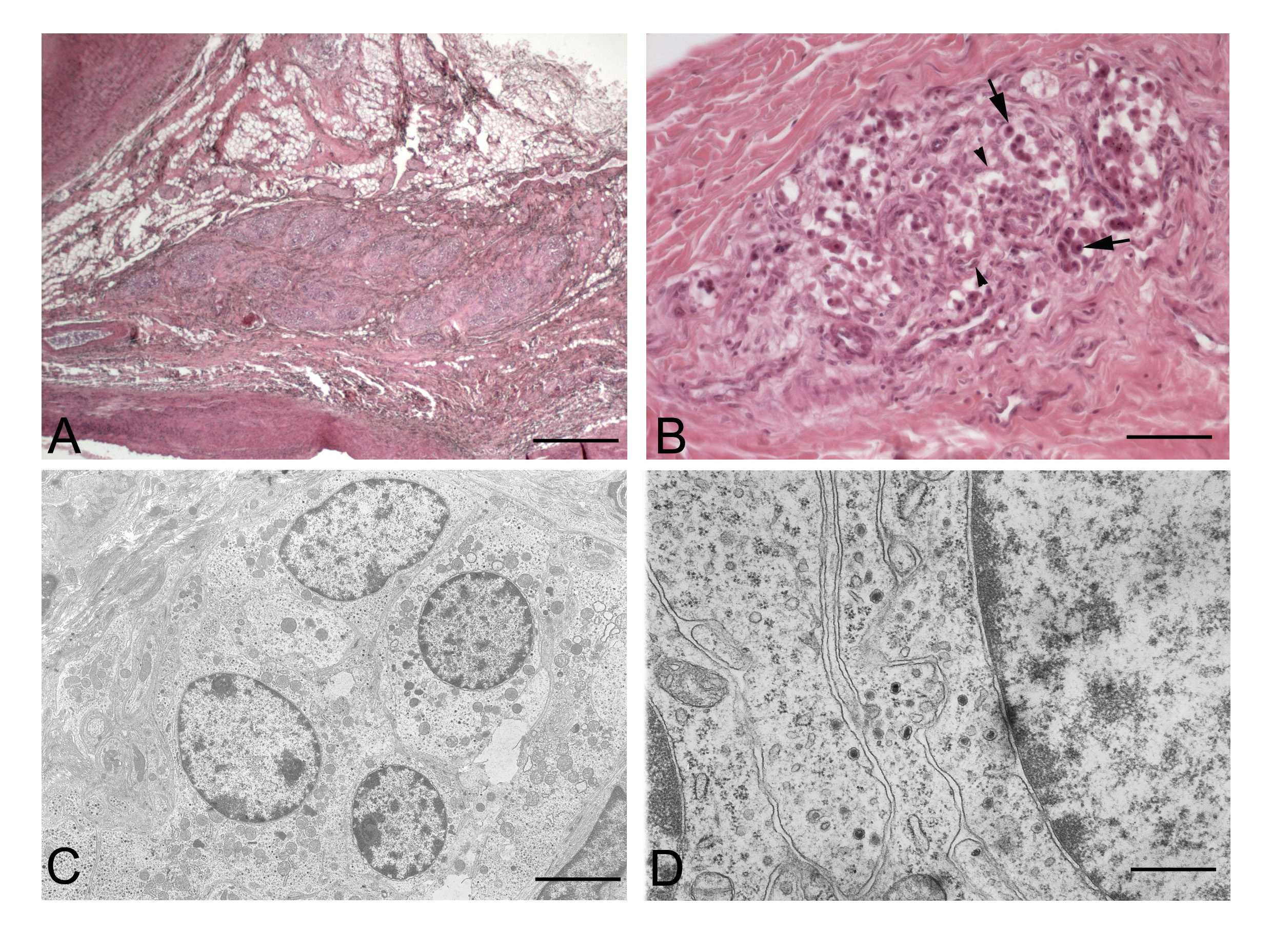
Figure 1. Normal structure of the carotid body. A: Carotid body of an adult human subject. B: Higher magnification of a lobule, showing the coexistence of roundish type I cells (arrows) and elongated type II cells (arrowheads). C-D: Electron micrographs of carotid body of 2-weeks-old rat showing a cluster of type I cells (C) and many dense-cored granules in the cytoplasms of adjacent type I cells (D). (Scale bars: A: 1 mm; B: 75 mm; C: 2.5 mm; D: 1 mm).
The carotid body may undergo structural and functional modifications in response to a series of environmental stimuli, some of which may show forensic implications. The carotid body, for instance, may be affected by chronic hypoxia, a condition which may involve humans living at high altitudes and therefore exposed to low atmospheric air pressure. The adaptive response mainly produces glomic hypertrophy due to increased number of type I cells. In particular, it has recently been highlighted that, as a consequence of a chronic hypoxic stimulus, type II cells may differentiate into precursor neural cells (also expressing nestin) which then give rise to mature glomus cells [8,9]. In this sense, type II cells are considered the stem cells of the carotid body [10].
The structure and function of the carotid body has also been reported to change along postnatal development and ageing. In particular, during the postnatal period, it has been demonstrated an increase of the total volume of the carotid body, accompanied by a progressive increment in vascularization [4,11]. Furthermore, ultrastructural studies revealed increased numbers of dense-core granules of type I cells and type I-type II cells synapses. The innervation of the carotid body has also been reported to develop in the postnatal period, due to an increase in afferent nerve endings and a decrease in the efferent ones [11].
The carotid body in sudden infant death syndrome
In contrast, defects in carotid body development or maturation have been associated with several neonatal respiratory deficiencies, such as sudden infant death or congenital central hypoventilation syndrome.
Hypotheses of alteration of reflexes triggered by peripheral arterial chemoreceptors have also been reported for Sudden Infant Death Syndrome (SIDS), a condition which is frequently put in differential diagnosis with homicide or accidental death [4,5,12]. It has been evidenced, for instance, that the period of postnatal maturation of the carotid body seems to correspond with the age range in which the risk of SIDS is highest [13,14]. Moreover, a series of cytochemical findings have been reported in the carotid bodies of SIDS victims [4,5,15].
The Sudden infant death syndrome (SIDS) is the sudden unexpected death of infants under 1 year of age, which remains unexplained after a thorough investigation, including performance of a complete autopsy and review of the circumstances of death and the clinical history [16]. Since the carotid body plays a pivotal role in the control of cardiorespiratory functions, prolonged sleep apnea, excessive periodic breathing and reduced hyperventilatory activity under hypoxic conditions (conditions increasing the risk of SIDS) could be ascribable to defective carotid body development. In literature, animal experimental studies have reported that carotid body denervation in the first postnatal period may cause the alteration of rhythmic ventilation and possibly unexpected deaths [17].
From a morphological point of view, conflicting observations have been reported about possible increases or decreases in the volume of the carotid body of SIDS victims [18,19]. Higher percentages of sustentacular and progenitor cells have been reported by other authors [20-22]. Besides, a prominent reduction or absence of dense-cored granules has been highlighted with ultrastructure analyses [21]. As it regards the content of various neurotransmitters, some reports showed conflicting results. Perrin et al. (1984) [23] reported ten- and three-fold higher concentrations of dopamine and noradrenaline, respectively, in SIDS carotid bodies but these findings were not confirmed by another research group [24].
Histopathology of carotid body in opiate-related deaths
Ageing is another situation in which the carotid body undergoes a series of structural and functional changes. These alterations include increase in interlobular and intralobular connective tissue and in type II cells, together with presence of inflammatory infiltrates [25,26]. Similar modifications of the carotid body structure have been described also in some clinical pathologies. For example, histological analyses demonstrated a consistent enlargement of the glomic lobules due to high proliferation of type II cells resulting in an increase in total volume of the carotid body in subjects affected by cardiac hypertrophy. These hyperplastic effect has been also observed in other pathologies like bronchial asthma, chronic bronchitis and emphysema [27].
In a forensic context, particular attention has been put by our group to the histopathological changes of the carotid body in opiate addiction [6,7,25,26]. Morphometric analyses have demonstrated an increase in the total volume of the carotid body in opiate-related deaths with respect to age-matched controls. Moreover, structural changes similar to age-related changes have also been seen, i.e., increases in interlobular and intralobular connective tissue, together with increased number of type II cells. These changes have been ascribed to heroin-dependent progressive arteriosclerosis of glomic arteries [24]. The above morphometric analyses also revealed histopathologic alterations specific of opiate-addiction and not present in ageing, i.e., decreased percentage of light cells. Apart from increased content in connective tissue, the disposition and complexity of the connective components have also been recently evaluated with reference to novel image analyses based on analysis of dispersion (Morisita’s index), gray level co-occurrence matrix (entropy, angular second moment, variance, correlation) and fractal (fractal dimension, lacunarity) parameters. Significant changes were found in all the above morphometric parameters with respect to age-matched controls, indicating higher complexity and irregularity of the connective tissue disposition. It was also intriguing that carotid bodies of opiate-related deaths showed higher fractal dimension and lower lacunarity also with respect to aged cases, confirming a branching of the connective components even more irregular than in aging [6].
Apart from the above histopathological changes in the parenchymal and connective components of the carotid body, we also demonstrated in opiate-addiction an higher incidence of chronic carotid glomitis, a pathological condition defined by the presence of lympho-monocyte aggregates throughout the carotid body. Such alterations have been previously reported only in aged persons and furtherly support the hypothesis of degenerative mechanisms in the carotid body of opiate addicted. In particular, in heroin addict subjects, chronic carotid glomitis could arise as inflammatory response against infective agents, heroin itself and other drugs, resulting in modification of the excitability of the glomus cells as well as their survival, proliferation and differentiation [5,7]. Inflammatory infiltrates resulted to be preferentially T cells (CD3+), in particular CD8-positive T suppressor/cytotoxic cells even if T helper lymphocytes, Natural killer cells and macrophages have also been detected [7] (Figure 2).
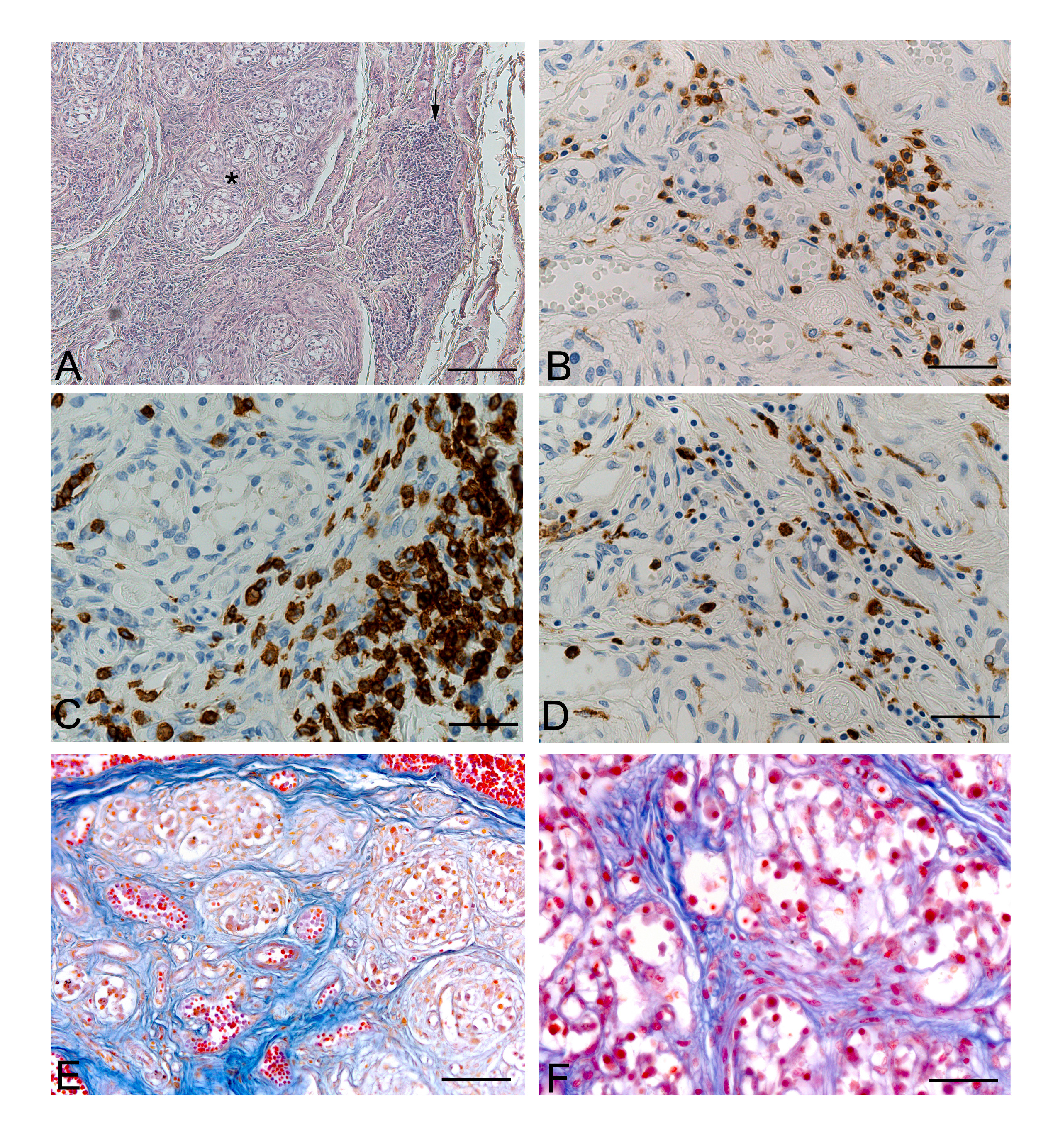
Figure 2. Carotid bodies of opiate-related deaths. A: Chronic carotid glomitis, characterised by an inflammatory aggregate (arrow) partially infiltrating a glomic lobule (star) (H.E.). B-D: Carotid body sections immunohistochemically stained with anti-CD45 (B), -CD8 (C), and -CD68 (D), showing inflammatory aggregates mainly composed of CD8-positive cytotoxic lymphocytes with macrophagic component. E-F: Increased interlobular and intralobular connective components of carotid bodies of opiate-related deaths. (Scale bars: A: 150 mm; B-D, F: 37.5 mm; E: 75 mm).
References
- Verna A (1979) Ulstrastructure of the carotid body in the mammals. Int Rev Cytol 60: 271-330. [Crossref]
2021 Copyright OAT. All rights reserv
- Pallot DJ, Al Neamy KW, Blakeman N (1986) Quantitative studies of rat carotid body type I cells. Acta Anat (Basel) 126: 187-192. [Crossref]
- Pallot DJ (1987) The mammalian carotid body. Adv Anat Embryol Cell Biol 102: 1-91. [Crossref]
- Porzionato A, Macchi V, Parenti A, Matturri L, De Caro R (2008a) Peripheral chemoreceptors: postnatal development and cytochemical findings in Sudden Infant Death Syndrome. Histol Histopathol 23: 351-65.
- Porzionato A, Macchi V, Parenti A, De Caro R (2008) Trophic factors in the carotid body. Int Rev Cell Mol Biol 269: 1-58. [Crossref]
- Guidolin D, Porzionato A, Tortorella C, Macchi V, De Caro R (2014) Fractal analysis of the structural complexity of the connective tissue in human carotid bodies. Front Physiol 5: 432. [Crossref]
- Porzionato A, Macchi V, Parenti A, De Caro R (2009) Chronic carotid glomitis in heroin addiction. Histol Histopathol 24: 707-715. [Crossref]
- Pardal R, Ortega-Sáenz P, Durán R, López-Barneo J (2007) Glia-like stem cells sustain physiologic neurogenesis in the adult mammalian carotid body. Cell 131: 364-77.
- Platero-Luengo A, González-Granero S, Durán R, Díaz-Castro B, Piruat JI, et al. (2014) An O2-sensitive glomus cell-stem cell synapse induces carotid body growth in chronic hypoxia. Cell Jan 16: 156: 291-303.
- López-Barneo J, Macías D (2016) Carotid body oxygen sensing and adaptation to hypoxia. Pflugers Arch 468: 59-70. [Crossref]
- De Caro R, Macchi V, Sfriso MM, Porzionato A (2013) Structural and neurochemical changes in the maturation of the carotid body. Respir Physiol Neurobiol 185: 9-19. [Crossref]
- Zara S, Porzionato A, De Colli M, Macchi V, Cataldi A, et al. (2013) Human carotid body neuroglobin, vascular endothelial growth factor and inducible nitric oxide synthase expression in heroin addiction. Histol Histopathol 28: 903-11.
- Quinn R (2005) Comparing rat's to human's age: how old is my rat in people years? Nutrition 21: 775-777. [Crossref]
- Harris MB (2012) Rat homologues to the human post-neonatal period: models for vulnerability to the sudden infant death syndrome. Pediatr Pulmonol 47: 729-730. [Crossref]
- Porzionato A, Macchi V, Stecco C, De Caro R (2013) The carotid body in Sudden Infant Death Syndrome. Respir Physiol Neurobiol 185: 194-201. [Crossref]
- Krous HF, Beckwith JB, Byard RW, Rognum TO, Bajanowski T, et al. (2004) Sudden infant death syndrome and unclassified sudden infant deaths: a definitional and diagnostic approach. Pediatrics 114: 234-8.
- Bureau MA, Lamarche J, Foulon P, Dalle D (1985) The ventilatory response to hypoxia in the newborn lamb after carotid body denervation. Respir Physiol 60: 109-119. [Crossref]
- Naeye RL, Fisher R, Ryser M, Whalen P (1976) Carotid body in the sudden infant death syndrome. Science 191: 567-569. [Crossref]
- Cole S, Lindenberg LB, Galioto FM Jr, Howe PE, DeGraff AC Jr, et al. (1979) Ultrastructural abnormalities of the carotid body in sudden infant death syndrome. Pediatrics 63: 13-7.
- Heath D, Khan Q, Smith P (1990) Histopathology of the carotid bodies in neonates and infants. Histopathology 17: 511-519. [Crossref]
- Cutz E, Ma TK, Perrin DG, Moore AM, Becker LE (1997) Peripheral chemoreceptors in congenital central hypoventilation syndrome. Am J Respir Crit Care Med 155: 358-363. [Crossref]
- Pávai Z, Töro K, Keller E, Jung J (2005) Morphometric investigation of carotid body in sudden infant death syndrome. Rom J Morphol Embryol 46: 93-97. [Crossref]
- Perrin DG, Cutz E, Becker LE, Bryan AC, Madapallimatum A, Sole MJ (1984) Sudden infant death syndrome: increased carotid-body dopamine and noradrenaline content. Lancet 2: 535-537.
- Lack EE, Perez-Atayde AR, Young JB (1986) Carotid bodies in sudden infant death syndrome: a combined light microscopic, ultrastructural, and biochemical study. Pediatr Pathol 6: 335-350.
- Porzionato A, Macchi V, Guidolin D, Parenti A, Ferrara SD, et al. (2005) Histopathology of carotid body in heroin addiction. Possible chemosensitive impairment. Histopathology 46: 296-306. [Crossref]
- Porzionato A, Macchi V, De Caro R (2009) Sudden infant death syndrome. N Engl J Med 361: 2580. [Crossref]
- Sivridis E, Pavlidis P, Fiska A, Pitsiava D, Giatromanolaki A (2011) Myocardial hypertrophy induces carotid body hyperplasia. J Forensic Sci 56 Suppl 1: S90-94. [Crossref]


