Comorbidity between cancer and psychiatric disorders including adjustment disorder, depressive disorders or angst can seriously influence the prognosis and the quality of life of patients. The identification of the psychological and biological profile of patients at risk for such comorbidity is not yet available. Classical candidate genes such as the BDNF, the 5-HTLPR and genes whose products are involved in inflammatory events have received some attention, but results are inconclusive. In the present review the association between cancer and psychiatric disorders is reviewed, a focus on the investigation of the Gene X Environment and the epigenetic control over the activation of the HPA axis is proposed as a tool to refine the definition of the biologic profile at risk for comorbidity between psychiatry and cancer.
Cancer is one of the most relevant causes of mortality and morbidity worldwide. It was the third cause of death in 1990 and the second one in 2013 [1,2]. The number of new cancer cases worldwide was 12 million in 2012, and deaths associated with cancer disease were 8.2 millions in the same period [3]. A year later, the number of new cancer cases rose till 14.9 millions and the number of patients death because of cancer did not decrease [2]. These numbers are expected to increase in the next decades, and stomach and colorectum are frequent sites of cancer in males and females in the developed countries, where the incidence rates of these diseases are significantly higher [2]. Breast cancer is the most common form of cancer for women in the western world, while 1-6 million incident cases of colon were recorded in 2013, associated with a number of deaths as high as 771000 [2]. Every effort made in the direction of a better treatment of cancer and a better prognosis is then to be highly prioritized. A consistent body of literature recently focused on the psychological reaction to a diagnosis of cancer and how this may influence the course of the treatment and the prognosis [4–9]. This may be in association with the fact that being diagnosed with cancer and the psychical symptoms of the disease concur to create a mental stress that may hamper the ability of patients to face their diagnosis and to comply to the medical prescriptions [10,11]. It is to be expected that a diagnosis of cancer and the following changes in patients life can prove to be overwhelming for a large number of patients. This may represent a critical point of cancer treatment, because patients diagnosed with cancer should retain the ability to react effectively to the diagnosis they have received and follow actively and energetically the medical indications they receive. Consistently, it is shown that the benefits of changing habits, and starting exercise or changing diet are strong enough to decrease the mortality associated with a diagnosis, for example, of colorectal cancer [12]. This statement proved to be true also for different kind of cancers [13-15]. Those are relevant interventions, because patients with cancer are particularly prone to depressive and anxiety disorders, with a prevalence of depressive disorders that is estimated to be as high as 25% [16] or higher [17].Patient with depressive symptoms developed after a diagnosis of cancer have cognitive disturbances (88%), sleeping disturbances (86%), and depressive mood (83%) [18]. These numbers are not without consequences. Stress per se has been reported to be a negative prognostic factor in patients with cancer [19]. It was estimated that patients that develop depressive symptoms during cancer show 26 % higher risk of mortality (39% for major depressive disorder) [20], prolonged hospitalization [21] and lower compliance to the treatment [22]. The reasons for the association between cancer and psychiatric disorder is to be found in the combined effect of a life-threatening diagnosis, the chronic pain, the extensive surgical interventions and the side effects of medical treatment [23,24]. Some patients appear to perform better than others after a diagnosis and treatment for a cancer disease, in a way that is dependent on biological, sociodemographic and psychological events [25]. In the present review the association between cancer and depression and anxiety related disorders is described, evidence from large epidemiological investigations is reported along with evidence of genetic association with a handful of relevant candidate genes. Figure 1 reports the flowchart and the key resulting points for the present contribution. The expected result of the present review is to help the identification of the sociodemographic and biologically related risk factors for the development of depression or angst after a diagnosis of cancer. Such achievement would pave the way to the implementation of strategies to prevent depression in the group of patients that are at risk. The effective implementations of such strategies would help abate the incidence of psychic sufferance following a diagnosis of cancer, with direct benefits the prognosis of patients and the quality of their lives [19]. A specific focus is set on the HPA axis (Figure 2) for its role in mediating response to stress and for its activity is plastic to early life experiences in a way that may be in causal relationship with the risk of cancer, as detailed in the following paragraphs.
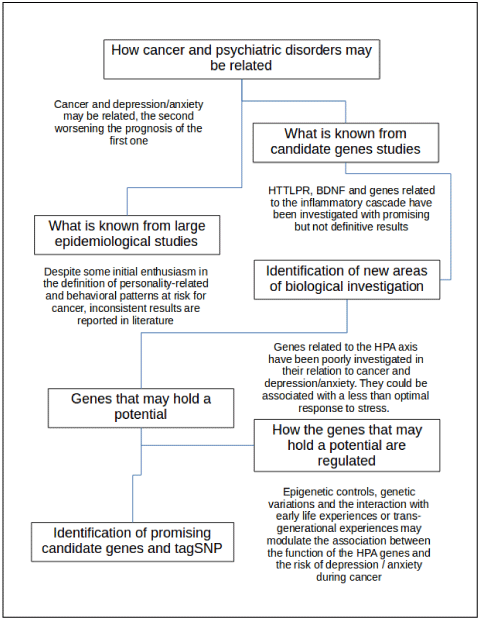
Figure 1. Flowchart for the present review. Critical points are reported
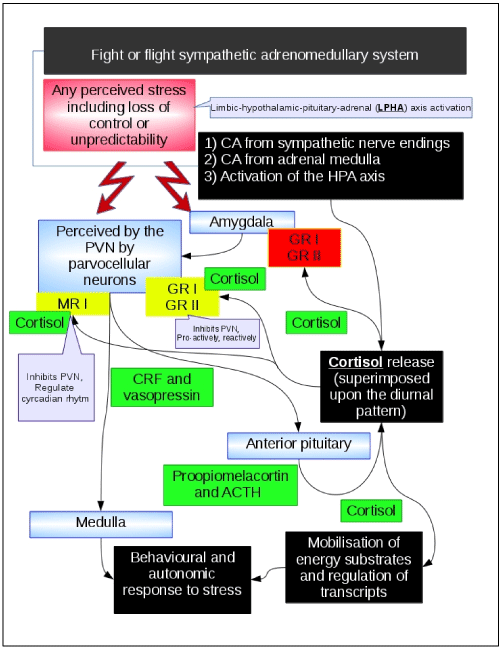
Figure 2. Limbic–hypothalamic–pituitary–adrenal axis
The limbic–hypothalamic–pituitary–adrenal (LHPA) axis is shown in the picture. Black boxes narratively describe the main events. The psychological related events that are processed by the cortex and limbic system in humans are described in the red box. Green boxes describe the neurological or endocrinological mediators. Yellow boxes describe receptors that mediate the negative feed-back of the system. Blue boxes describe the anatomical structures that are involved in the mechanism. Briefly, any perceived stress results in release of catecholamines (CA) and cortisol. Cortisol mediates its own negative feedback by interacting with mineralcorticoic receptors (MR) and corticoid receptors (CR). Cortisol is released independently from stressing events through a circadian rhythm (higher in the morning), and it is also released during stressful events. This latter release is superimposed over the diurnal release. MR receptors type I are thought to be associated with the negative feedback of the diurnal release of cortisol. CR type I receptors are high affinity receptors that are thought to mediate the negative feedback to diurnal cortisol release (pro-active negative feedback). CR type II are low affinity receptors that are thought to mediate the stressing events mediated acute release of cortisol (reactive negative feedback). Stressful events are encoded in the central nervous system (cortex and limbic system), this information is delivered to the hypothalamic paraventricular nucleus (PVN) where the corticotropin releasing factor (CRF) and vasopressin (VP) are released from nerve terminals in the median eminence into the hypophyseal-portal vascular system. At the level of the anterior pituitary, CRF stimulates the secretion of ACTH which is reversed in the systemic circulation to reach the adrenal gland cortex where cortisol is release to mediate its effects on energy metabolism that are necessary for the fight or flight reaction to stressing events. Of note, CRF also acts as a neurotransmitter in the central nervous system. CRF neurons are present in the cortex, limbic system and brain stem regions, CRF projections from the hypothalamus and the amygdala to the medullary noradrenergic nuclei may be relevant to the stress-mediated behaviour and autonomic reactions. It is interesting to note that the amygdala projects to the PVN and facilitates the release of CRF, but the the activation of the GR receptors expressed on the amygdala results into a increased signal from the amygdala to the PVN. This may be relevant in conditions of continued or repeated stress.
Pubmed, the Cochrane database and Google Scholar were searched using the following keywords in permutation: “gene”, “cancer”, “depres*”, “anxiety”, “risk”, “variation”, “SNP”, “methylation”, “epigenetic”, “acetylation”, “HPA axis”. References of the most relevant papers were manually searched in order to complete the bibliography research for the present contribution. AD and MC independently searched the literature and confronted the final results. Attention was dedicated in the inclusion of sociodemographic and psychological variables when describing the risk of having a depressive or angst episode after a diagnosis of cancer.
There are different kinds of cancers and their prevalence is different in the general population, as their distribution tends to variate through years. Among women the five most common cancer sites are breast (25.2%), colorectum (9.2%), lung (8.7%), cervix (7.9%) and stomach (4.8%). Among men the most common sites of cancer diagnosed are lung (16.7%), prostate (15.0%), colorectum (10.0%), stomach (8.5%) and liver (7.5%) [26]. A graphical representation of such differences as mirrored by the number of scientific articles published in specific cancer areas is reported in Figure 3. It is possible to assume that the strength of association between any specific kind of cancer and the risk of a psychiatric disorder varies among the different cancer types. This may be connected with the different prognoses and with the different impact of the proliferative diseases on body image and on the ability to maintain a normal life throughout the course of the disease. Moreover, it is a topic of debate whether the depressive status is a result of a poor biological condition which by itself holds the association with a poorer outcome, as some reports seem to suggest [27,28], or whether depression may play a role as an independent risk factor for a poorer prognosis in case of cancer. Other researchers have tried to address that association between a pre-cancer history of depression or personality traits (which may also have a biological basis [29-32]), and a diagnosis of cancer later in life. A selected number of large perspective such studies is reported in Table 1. Such an epidemiological connection may stress the possible overlap of a set of biological and psychological characteristics that are common, or rather, belong to a cause-effect paradigm, in pre-morbid personality or depression and cancer. If proven, this association would be of tremendous relevance, in that it could provide evidence for preventive strategies able to decrease the incidence of cancer diseases in the general population, by identifying subjects that shows a set of phenotypes (behavior or symptoms) that may underline a biologic sensitivity to proliferative diseases or may expose to risk behaviors that eventually end with increased risk for cancer. Nevertheless, results appear to be inconsistent as detailed in the following paragraph, and a biological common background that may be made accountable for a pre-morbid specific personality or psychiatric diagnosis and cancer later in life is still missing.
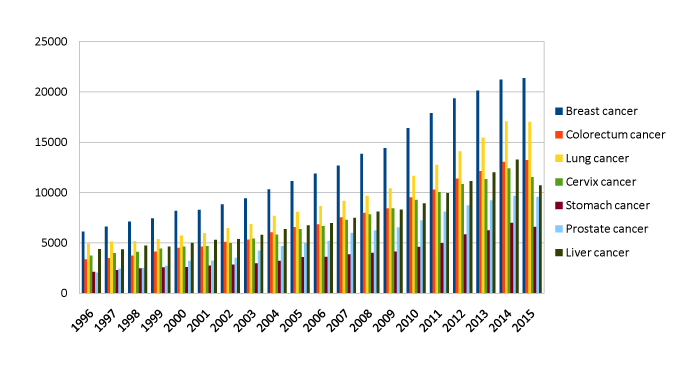
Figure 3. Number and distribution through time of publications about cancer in different districts
The picture was created using the following terms in the possible permutations pubmed: breast, colon, colorectum, colorectal, lung, cervix, cervical, stomach, cancer. Data of publication was set as a clustering variable in order to detect the distribution through time of the publications in specific areas. This was meant to obtain information about the current and past scientific interest in the different cancer diseases and have a brute quantity of the total number of articles published in a specific period about cancer.
Effort to determine an epidemiological association between a distinctive patterns of habits of nervous tension and the risk of develop cancer later in life have been numerous and ambitious in the final part of the last century. Thomas and colleagues enrolled 1337 medical graduates and assessed them from 1948 to 1964 through the habits of nervous tension (HNTQ), which also includes a measure of depression, anxiety, anger and loneliness [33]. The scale was designed under the assumption that these characteristics could be related to the psycho-physiological ability to react to their environments. The ability to cope with stress could be determinant in shaping the risk of develop a number of disorders later in life [34]. As a result of this large investigation, symptoms of anxiety and depression shown earlier in life were significantly associated with a higher risk of cancer. Moreover, the duration in time of depressive feelings was shown to be a strong predictor of later development of cancer in life in a sample including 1353 men and women of the age from 50 to 69 followed up for at period of 10 years [35,36]. A 20 year follow up study including 2107 individuals reported an association between depression and cancer, even though the strength of such association was not as high as expected [37]. Overall, these findings would link epidemiologically the presence of depressive feelings or reactions to events with the risk of cancer. Nevertheless, conflicting reports are also found (38–42), and it is difficult to drawn final conclusions on this association. A possible explanation for these discrepancies may be found in the different age of subjects involved in the studies, the different periods of observation and the number of patients included in the study. For example, studies that found significant association between the psychological variables or the pre-morbid psychiatric symptoms in the investigated samples were characterized by longer periods of follow up: 24 to 30 years for the positive association findings [35,36,43,44], 10 to 17 for the negative association findings [40–42]. An association of note was reported in one of the larger studies on this issue, conducted by Dalton and colleagues in 2002, showing that the correlation between a diagnosis of a psychiatric disorder and the risk of cancer was influenced time: the risk of receiving a diagnosis of cancer was higher when closer to the diagnosis of a psychiatric disorder [43]. This finding may be consistent with the hypothesis that response to stress is a key element to understand the risk of cancer later in life, and that there are “windows” of biologic reaction to stress during which one single individual is more or less sensitive to long-lasting changes in the physiological activation of the stress-related molecular cascades. In this light, the psychological characteristics or persistent depressive symptoms could be better understood in their relation to cancer when interpreted as a reaction to stress, or a crystallization of it, experienced earlier in life. Thus, the experiences suffered during the early years would be critical in understanding the reaction to stress to acute events that happen later in life. This concept is further explained in the following paragraphs. Another relevant piece of evidence that results from the large epidemiological investigations conducted in the past years on the association with premorbid personality traits or depression and anxiety and the later development of cancer, is that this association was reported as to be more evident for a number of specific cancers including the colon, rectum and bladder cancer [45]. The biological basis of such association is still to be elucidated, but the brain-gut synergy is a rapid evolving field of research interest in these years [46,47], and more could be unraveled to understand the association between psychic sufferance and somatic disorders.
The potential of genetic variations located in candidate genes to determine the risk for psychiatric disorders after a diagnosis or during the treatment of a cancer disease has been investigated in literature. Table 1 reports a selection of the main investigations conducted so far. The serotonin system, the BDNF related system and the inflammatory cascades have been extensively investigated. The rationale of such selection is mainly associated with the role of these genes in shaping the risk of a depressive disorder or an anxiety related disorder in the general population. Such investigations have been then conducted under the hypothesis that genes and variations that had been previously associated with depressive and anxiety disorders could be as well predictors of such episodes in patients with cancer or patients in treatment for cancer disorders. Such statement proved to be true in at least some investigations. Schillani and colleagues [48] reported that a variation in the promoter of the gene that codes for the serotonin transporter was associated with anxiety in a sample of 48 women with breast cancer. Of note, they also observed that the anxious preoccupation scores decreased with the time of follow up, this being more evident in the group of the L/L (long variation) carriers. This finding would suggest a faster and more efficacious psychological adaptation to the cancer and treatment of patients carrying the L variation when compared with other patients. If proven true by other independent examination, such finding could be instrumental in the identification of patients that would benefit from a psychological support during their illness, and for whom, such support should be prioritized. Nevertheless, the small number of patients included in the study does not allow conclusive statements about the given association and the ability to cope with a diagnosis of breast cancer and related treatment. Consistently with this, this finding could not be replicated in a sample of 94 patients with neck cancer (33 out of the sample were genotyped) in a previous analysis by Gilbert and colleagues [49], nor it was in a larger sample including 309 patients treated with mastectomy for breast cancer and genotyped for a number of serotoningergic variants [50]. It is of particular relevance that a set of genes implied in inflammation were reported to predict depression in a sample of women with breast cancer [51]. Consistently, the activation or the impairment of the immunological system has been claimed to be responsible for higher risk of proliferative disorders [52,53] and for psychiatric disorders as well through complex neurological events such as pruning [54]. The direct interaction between inflammation and depression in a sample of women treated with chemotherapy was analyzed by Torres and colleagues [55], this report showing that a high level of inflammation indexes (including the nuclear factor kappa B) was associated with persistent symptoms of depression after the treatment. The association between genetic variations located in genes that code for proteins involved in inflammatory events was confirmed by a large study conducted by Saad and colleagues [56] involving 335 patients with breast cancer. Patients who were homozygous for the rare allele in TNFA rs1799964 had an 87% decrease in the odds of belonging to the Subsyndromal class compared to the Resilient class. Due to the proven effects of depression towards the prognosis of cancer disease, this kind of study could help identify subjects that are particularly at risk of developing a depression during cancer treatment. Consistently with these results, variations rs1360780, rs9296158 and rs9470080 located in a gene that codes for a protein with a role in immunoregulation and basic cellular processes involving protein folding and trafficking, the FKBP5, was found to harbor variations associated with depression and anxiety in a sample of 130 newly diagnosed patients with gastric cancer [57]. The BDNF Val/Met variation has been the object of investigation in this field of research. The BDNF Val66Met (rs6265) polymorphism is a functional SNP of the BDNF gene. The Met allele of BDNF Val66Met may be related to a less adaptive genetic disposition toward adverse experience, as well it has been associated with a decreased expression of the BDNF and problems in learning [58]. This variation or the epigenetic regulation of BDNF were consistently [50,59,60] found to be associated with depression or anxiety in samples of patients with cancer , highlighting its potential role in predicting such psychological breakdown in patients when they receive a diagnosis of cancer or they are in treatment for it. Overall, the candidate gene approach could not provide definitive results in the identification of subjects that are particularly at risk of psychiatric comorbidity when diagnosed with a cancer illness. The reasons for such lack of results may rely on the small samples included in the analyses, that were not powered enough to detect a true genetic association, or the limited penetrance of single variations towards complex phenotypes as previously reviewed by our group [61-63]. Another reason may be that some potential candidate genes have not been exhaustively investigated. The HPA system is primary activated during stress response, this physiological role makes HPA a candidate for the biology of psychiatric comorbidity in cancer.
A perceived internal or external stress alongside with disease or infection or other physical traumas, leads to the activation of the limbic-hypothalamic-pituitary-adrenal (LPHA) axis (Figure 2), whose activation can be associated with a pathological response to stress or trauma at the moment the trauma is in place, or shape the response to traumas later in life. The possibility that an earlier trauma can influence how the LPHA system will later respond to stress during the adult life opens some new scenarios in the investigation of the biological determinants of risk of psychiatric diseases after a diagnosis of cancer. Some patients react better than others to a diagnosis of cancer, with positive effects on the prognosis of the disease, due to a better compliance to the treatment. The reason for such better performance could be found in a more or less efficient LPHA activation, which could be determined more by the exposure to previous stressing experiences in life than to the current psychological and sociodemographic current status. The most part of investigations published so far focused on the current sociodemographic and psychological characteristics of patients in order to infer a profile of patients that are at risk to develop depression or anxiety after a diagnosis of cancer. The reader will find a complete and interesting review on this topic here [64–66]. Nevertheless, a little effects of current psychological and sociodemographic status over psychological response to cancer has been consistently reported in literature [66,67], suggesting that the current status may not affect the psychological response to stress as much as other determinants. The analysis of early life stress factors could pave the way to the understanding of the geneXenvironment interactions in shaping resilience by the means of the epigenetic controls of the expression of key genes. Schuler and colleagues [68] suggested that a direct link may be found from the early life experiences and the risk of breast cancer, but to the best of our knowledge, there are no published investigations about the role of early life experiences and resilience to psychological distress after a diagnosis of cancer. On the contrary, there are consistent lines of evidence that show how the early life experiences may inferfere with the function of the LPHA, which determines response to stress and influence the risk of depression and anxiety [69-71]. It has been demonstrated that rats exposed to maternal separation in early periods of their life exhibit higher levels of ACTH (Adrenocorticotropic hormone), CFR (Corticotropin releasing factor) and VP (Vasopressin) compared to control animals (see for example [72,73]), but what is maybe more important as for the consequences in human health, it that it has been reported that are “windows” during which the same stressful condition may or may not induce permanent changes in the biological regulation of LPHA. Interestingly, parallel to the alterations in the LPHA, animals exposed to stress during early periods show a modified serotonin response to citalopram, a common drug used to treat depression and anxiety, which would suggest a direct biological association between stress during the early phases of life and depression or anxiety [74]. The GABAergic system has also been found to be profoundly modified by exposure to early stress in animal models [75], which is consistent with risk of developing anxiety related disorders in adult life for humans that are exposed to ACEs. Combining the evidence from retrospective studies in humans and perspective studies in animals, it is possible to assume safely that early life stressing events are statistically associated with an increased risk of mental disorders, and that the LPHA system may be the biological substrate for this statistical association, due to its ability to be influenced by early experiences and to develop accordingly in adult life. Consistently with this picture, there is evidence in humans that anxiety and depressive disorders are associated with increased levels of CRF [76]. It is then safe to assume that ACEs are able to shape resilience to stress later in life. Of note, the relationship between ACEs and mental disorders is more probabilistic than deterministic in nature, because resilience to stress is also determined by genetic influences. A number of studies have been conducted to test the hypothesis that genetic variations located in genes that are instrumental for the correct balance of the HPA axis could predict the biological stress response or the balance of the stress system. These were conducted under the evidence that an impaired or an imbalanced HPA axis represents a risk factor for further development of a psychiatric disorder [77–79] and up to the 70% of patients with major depressive disorder show a dysfunction of the HPA (80). Most part of the genetic investigations in this field focused on the CRH, its main receptor CRH1 and CHRBP (the CRH-binding protein). These proteins are thought to be crucial in the control of the HPA axis, other hormons such as oxytocin and vasopressine were proved to modulate it to a lesser extent [81–83]. Variations in these genes were able to control response to stress [84] and during depression [85]. Genetic association results for risk of depression and response to antidepressant treatment for the genes coding for CRH, CRH1 and CHRBP are reported in Table 2.
Table 2. Genetic association studies investigating CHR, CRH1 and CHRBP
Study |
Sample |
Genetic variations |
Outcome |
Results |
Variations tested in predicting risk of MDD during cancer |
Liu and colleagues, 2006 [126] |
MDD = 206
HCT = 195
ethnicity = Han Chinese |
CRHR1: rs1876828; rs242939; rs242941 |
Diagnosis of MDD |
Rs242939 G allele was more represented in MDD patients. Haplotype GGT at the investigated variations was twice as represented in MDD. |
NO |
Licinio and colleagues, [127] |
MDD = 80
Mexican-Americans treated with fluoxetine or desipramine for 8 weeks |
CRHR1: rs1876828; rs242939; rs242941 |
Response to treatment |
GAG haplotype was associated with better reponse in high anxiety MDD patients |
NO |
Smith and colleagues, 2016 [128] |
Over 106,000 subjects, non necessaily diagnosed with a psychiatric disorder |
GWAS analysis |
Neurotocism |
9 loci were significantly associated with neurotocism, one including the CRH1 |
NO |
Chen and colleagues, 2015 [129] |
502 rural Afrinca American families partecipating in the Strong African American families-teen, a randomized prevention trial |
CRHR1: rs720936; rs4792887; rs110402 // rs242924;
rs242940 //rs173365; rs242950 |
Depressive symptoms |
TG haplotype at the rs242924;
rs242940 variations had low levels of depressive symptoms.
GC haplotype at the variations rs173365; rs242950 reported fewer depressive symptoms. The interaction of family economic hardship and CRH1 variations predicted depressive symptoms. |
NO |
Ressler and colleagues, 2009 [130] |
1385 individuals of African American descent |
5-HTTLPR (long and short allele)
and CRHR1: rs7209436;, rs4792887; rs110402. |
Risk of depression |
No association with 5-HTTLPR alone. Significant association of rs110402 and haplotype TCA Protecitve effect of the TCA haplotype in careers of 5-HTTLPR S |
YES |
Brandely and colleagues, 2008 [131] |
422 Afro-American patients and and independent sample of 199 subjects, of which 74 with no MDD and 126 with MDD |
CRHR1: rs4076452; rs12942300; rs7209436;rs4792887;rs110402;rs242924;rs242940;rs173365;rs242950;rs242948 |
Risk of depression |
SNPs were associated wiuth MDD at nomila p levels when in combination with an history of child abuse |
NO |
Starr and colleagues, 2014 [132] |
Perspective study in an original sample of 815 youth at the age of 15, reassessed between age 22 and 25, 512 consented, 381 entered the study. |
5- HTTLPR
CRHR1 rs110402 |
Risk of depression |
Chronic stress, adverse life events and the A allele of the rs110402 and for the S allele at 5-HTTRLP were associated with the risk of depression |
NO |
Buttenschøn and colleagues, 2016 [133] |
408 MDD
289 HCT |
68 genetic variations in CRH, CRHR1, CRH2, FKBP5 and NC3C1 |
Risk of depression |
No significant association finding. Nominal significant associations were found at rs4309, rs4311, rs4329 and ACE I/D |
NO |
Wu and colleagues, 2012 [134] |
368 MDD
371 HTC;
A meta-analysis including 2476 MDD and 7744 HTC |
ACE I/D |
Risk of depression |
No significant association in the investigation study. Meta-analys showed a 18% increased risk for depression for careers of D/D variation |
NO |
Weber and colleagues, 2016 [135] |
239 PD
508 PD (replication sample)
508 HTC |
CRHR1 rs7225082; rs7209436;rs4458044; rs12936181; rs3785877; rs17689918; rs17689966; rs4792825 |
Risk of panic disorder |
Rs17689918 was associated with PD after correction for multiple testing |
NO |
Amstadter and colleagues, 2011 [136] |
Prospective study involving 103 pediatric injury patients |
CRHR1
rs4074461; rs12944712; rs4458044; rs12936181; rs242942; rs17763104; rs17690314; rs17763658 |
Risk of panic disorder |
Rs12944712 was associated with PTSD symptoms after Bonferroni correction |
NO |
White and colleagues, 2013 [137] |
Prospective study involving 626 individuals exposed to hurricane |
CRHR1
rs12938031;
rs7209436;
rs171441;
rs242924;
rs4792887;
rs2664008;
rs242936;
rs110402;
rs17689966;
rs242939;
rs16940686;
rs173365 |
Risk of panic disorder |
Rs12938031 and rs4792887 were associated with PTSD symptoms after Bonferroni correction |
NO |
Grabe and colleagues, 2010 [138] |
1638 individuals assessed for Childhood trauma and MDD |
CRHR1 TAT haplotype (rs7209436; rs110402; rs242924;) |
Risk of depression given genetic X E interaction |
TAT haplotype showed a significant interaction with childhood physical neglet, rs17689882 reached “gene-wide” signfificant association result |
NO |
Heim and colleagues, 2009 [139] |
1063 individuals assessed for Childhood trauma and MDD |
CRHR1 rs110402 |
Risk of depression given genetic X E interaction |
Protective effect of the rs110402 A-allele in men (N = 424) |
NO |
Wasserman and colleagues, 2009 [140] |
A sample of 672 suicide attempter offspring |
CRHR1
rs12936511 |
Risk for suicide |
T allele at the rs12936511 was significantly more transmitted in suicidal males |
NO |
DeYoung and colleagues, 2011 [141] |
339 maltreated children
275 HTC |
CRHR1
rs110402; rs242924; rs7209436 |
Association between maltreatment and neuroticism |
TAT haplotype was associated with higher levels of neuroticims |
NO |
Laucht and colleagues, 2013 [142] |
300 individuals longitudinally assessed for childhood trauma and depression at the age of 19, 22 and 23 |
CRHR1
rs7209436; rs110402; rs242924; and rs17689882 |
Association between maltreatment and depression |
TAT haplotype interacted with childhood trauma in driving risk for depression along with G/G at rs17689882 |
NO |
Kranzler and colleagues, 2011 [143] |
1,211 European-Americans (EAs) and 1,869 African-Americans (AAs) |
CRHR1 TAT haplotype (rs7209436; rs110402; rs242924;) |
Risk of depression given genetic X E interaction |
TAT haplotype had a significant interaction with adverse childhood life events and depression in African-American women |
NO |
Liu and colleagues, 2013 [144] |
256 MDD
272 HTC |
CRHR1
rs1876828;
rs242939;
rs242941 |
Risk of depression |
G allele of rs242939 was significantly over-represented in the MDD group
GGT haplotype was significantly over-represented in the MDD group |
NO |
Polanczyk and colleagues, 2009 [145] |
Follow up of two independent samples: 1116 followed up to the age of 40 ys and 1037 followed up to the age of 32 |
CRHR1 TAT haplotype (rs7209436; rs110402; rs242924;) |
Risk of depression given genetic X E interaction |
TAT haplotype had a significant interaction with adverse childhood life events and depression |
NO |
Hodges and colleagues, 2007 [146] |
120 multiplex PD pedigrees |
GRP
rs755719;
rs1517036;
rs1517035;
rs4108697;
rs1062557;
rs936431;
GRPR
rs4084832;
rs2353577;
rs4986945;
rs4986946;
rs3747411;
rs2353576;
CRHR1
rs242924;
rs242940;
rs173365;
rs1396862;
rs242944;
TACR1
rs754978;
rs4439987;
rs3755460;
rs741418;
rs2058860;
rs1477157. |
Risk of panic disorder |
Rs1517035, rs4108697 and rs4439987 were found to be associated with risk of PD in association with a story of childhood abuse. |
NO |
Yoshinobu Ishitobi and colleagues, 2013 [147] |
218 HTC |
CRHR1
rs4076452;
rs7209436;
rs110402;
rs242924;
rs242940;
rs173365
CRHR2
rs4722999;
rs3779250;
rs2267710;
rs1076292;
rs2284217;
rs2267716 |
Personality traits (NEO PI-R; TCI; STAI) |
No genetic association was found |
NO |
Soyka and colleagues, 2004 [148] |
170 alcohol dependent patients |
CRHR1
rs110402;
rs171440;
rs1396862;
rs878886 |
Personality traits (NEO PI-R; TCI; STAI) |
No genetic association was found |
NO |
Tochigi and colleagues, 2006 [149] |
243 HTC |
CRHR2
rs2267717 |
Personality traits (NEO PI-R; STAI) |
A allele at the rs2267717 was associated with Opennes |
NO |
Keck and colleagues, 2008 [150] |
186 PD
299 HTC
173 PD
495 anonymous blood donnors |
71 SNPs in CRH, CRHR1, CRHR2, AVP, AVPR1A, AVPR1B |
Risk of panic disorder |
The following variations were associated with PD in the combined sample: rs28632197 (AVPR1B), rs28607590 (AVPR1B), rs28575468 (AVPR1B), rs242937 (CRHR1), rs1396862 (CRHR1), rs878886 (CRHR1).
Rs878886 resisted correction for multiple testing |
NO |
Heitland and colleagues, 2016 [151] |
224 HTC |
5-HTTLPR
CRHR1 rs878886 |
Fear conditioned responses |
CRHR1 rs878886 G-allele carriers showed reduced acquisition of cue-specific fear-conditioned responses. An additional interaction effect of CRHR1 rs878886 and the triallelic 5-HTTLPR/rs25531 variant on cued fear acquisition |
NO |
Heitland and colleagues, 2013 [152] |
150 HTC |
5-HTTLPR
CRHR1 rs878886 |
Fear conditioned responses |
G allele at rs878886 was associated with no acquisition of fear response. When in combination with the short allele, it was associated with increased fear conditioned response |
NO |
Hsu and colleagues, 2012 [153] |
16 unmedicated MDD
83 HTC |
CRH
rs110402 |
Neuronal process of emotional stimuli |
A carriers showed decreased response in MDD in the hypothalamus, bilateral amygdala, and left nucleus accumbens |
NO |
Pgliaccio and colleagues, 2015 [154] |
306 HTC 9 – 14 years old |
CRHR1 rs4792887; rs110402; rs242941; rs242939; rs1876828
NR3C2
rs5522
NR3C1 rs41423247; rs10482605; rs10052957
FKBP5
rs1360780 |
Neuronal process of emotional stimuli |
The genetic profile scores
and life events experience interacted to predict connectivity with
the parahippocampal gyrus, caudate tail, MFG, and IFG |
NO |
Ching-Lopez and colleagues, 2015 [155] |
711 individuals from the local community |
91 candidate SNPs |
Risk for MDD |
Rs623580 in TPH1, rs9526236 upstream to HTR2A, rs17689966, rs173365, rs7209436, rs110402 and rs242924 in CRHR1 showed a marginal significance in association with MDD |
* |
Kohrt and colleagues, 2015 [156] |
682 individuals |
FKBP5
rs1360780; rs3800373; rs9296158; rs9470080 |
Cortisol levels and depressive symptoms |
Rs9296158 showed an association with depressive symptoms, which was stronger when considering interaction with the childhood trauma |
NO |
Woody and colleagues, 2016 [157] |
129 children with mother with MDD
126 children with mother without MDD |
CRHR1 TAT haplotype (rs7209436; rs110402; rs242924;) |
Association with brooding |
Children who were non carriers of the protective TAT haplotype showed more brooding |
NO |
Pagliaccio and colleagues, 2015 [158] |
107 children |
CRHR1 rs4792887; rs110402; rs242941; rs242939; rs1876828
NR3C2
rs5522
NR3C1 rs41423247; rs10482605; rs10052957
FKBP5
rs1360780 |
Neuronal process of emotional stimuli |
GeneXE predicted greater left amygdala responses to negative emotional stimuli. Genetic profile
scores interacted with sex and pubertal status |
NO |
Cicchetti and colleagues, 2014 [159] |
1096 children |
5-HTTLPR
BDNF
rs6265
rs925946
rs7103411
rs4923461
NET
rs168924
CRHR1
rs7209436
rs110402
rs242942 |
Internalizing symptoms in children exposed to trauma |
5-HTTLPR and rs7103411 interacted with gender and environmental variables prediction the degree of anxiety and depressive symptoms |
* |
Suzuki and colleagues, 2014 [160] |
300 HTC |
FKBP5
rs1360780; |
Personality traits (DAS-24) |
Rs1360780 G allele was associated with Achievement, Self-control and Dependency subscales |
NO |
Szczepankiewicz and colleagues, 2014 [161] |
528 BP
218 MDD
742 HTC |
FKBP5
rs1306780;
rs755658;
rs9470080;
rs4713916;
rs7748266;
rs9296158;
rs9394309;
rs9800373 |
Risk for affective disorder |
Rs136078, rs9470080, rs4713916, rs9296158 and rs9394309 were found to be associated with MDD but not BD |
NO |
Pagliaccio and colleagues, 2014 [162] |
120 pre-school children |
CRHR1 rs4792887; rs110402; rs242941; rs242939; rs1876828
NR3C2
rs5522
NR3C1 rs41423247; rs10482605; rs10052957
FKBP5
rs1360780 |
Neuronal process of emotional stimuli |
The interaction of genetic profile scores and early life stress predicted left hippocampal and left amygdala volumes |
NO |
Schatzberg and colleagues, 2014 [163] |
40 MDD (psychotic)
26 MDD (not psychotic)
29 HCT |
CRH
rs10098823
rs3176921
rs5030875
rs5030877
rs6999100
rs7350113
CRHR1
rs110402
rs12938031
rs16940674
rs171440
rs17689966
rs242924
rs242940
rs242948
rs4076452
rs4792887
rs4792888
rs7209436
rs3785877
rs4792825
rs4458044
rs12944712
rs17763104
rs2664008
rs17763658
rs242942
rs11657992
CHRH2
rs2240403
rs2267712
rs2267715
rs2267717
rs2270007
rs255100
rs4723003
rs7812133
rs255102
rs975537
rs2190242
rs2267716
rs2284216
rs2284217
rs4723000
rs12701020
rs17159371
rs929377
NRC1
rs6195
rs6198
rs33388
rs2918419
rs10052957
rs10482633
rs12521436
rs12655166
rs17209258
rs41423247
NRC2
rs5525
rs5530
rs1879829
rs2070951
rs2272089
rs3910052
rs4835488
rs6535578
rs7658048
rs7694064
rs10213471
rs17024360
rs17484245
rs2070950
rs5522
FKBP5
rs1360780
rs3800373 |
Risk of MDD |
Rs4076452 and rs4792825 were associated with depression |
NO |
Engineer and colleagues, 2013 [164] |
200 pregnant women |
NR3C1
BcII and ER22/23EK
rs1876828
rs242939
rs242941 |
Risk for depression during pregnancy |
BcII and rs242939 were found to be associated with MDD during pregancy |
NO |
Shinozaki and colleagues, 2011 [165] |
131 adult kidney transplant recipients |
5-HTTLPR
rs25531
Stin 2 VNTR
BDNF
rs4680
COMT
rs6265
CRHR1
rs110402
rs242924
rs7209436
rs4792887
FKBP5
rs1360780
rs3800373
rs9296158
rs9470080 |
Risk for depression |
360780, rs9296158, and
rs9470080 were associated with depressive symptoms |
NO |
Zimmermann and colleagues, 2011 [166] |
884 individuals in a prospective study |
FKBP5
rs3800373
rs9296158
rs1360780
rs9470080
rs4713916 |
First occurrence of a MDD episode |
All investigated variations were associated with trauma or severe trauma, but not MDD |
NO |
Lewis and colleagues, 2012 [167] |
271 mothers and children from the Predicting and Preventing Adolescent Depression Project
165 mothers and children from the CaStANET twin register |
CRHR1
rs7209439
rs242924
rs110404
FKBP5
rs1360780
rs4713916
rs3800373
NR3c1
rs41423247 |
Association of recurrence between mother and offspring depression |
No genetic association |
NO |
Appel and colleagues, 2011 [168] |
2157 individuals from the Study of Health in Pomerania |
FKBP5
rs1360780 |
Risk for depression |
Significant interaction between childhood trauma and the T allele of rs1360780 was detected towards depression |
NO |
Zobel and colleagues, 2010 [169] |
268 MDD
284 HTC |
FKBP5
rs3800373
rs755658
rs1360780
rs1334894
rs4713916 |
Risk for depression |
Rs3800373, rs1360780 and rs4713916 were associated with risk of depression |
NO |
Dong and colleagues, 2009 [170] |
246 MDD
272 HTC |
Multiple SNPs crossing 56280000bp exploring ABCB1, SLC6A2, SLC6A3, SLC6A4, CREB1 and NTRK2 |
Risk of depression |
CREB1 rs3730276 was associated with risk of depression among other SNPs in different genes |
NO |
Polanczyk and colleagues, 2009 [145] |
1116 women in the E-risk study follow up to age 40
1037 women and men in the Dunedin Study follow up to age 32 |
CRHR1 TAT haplotype (rs7209436; rs110402; rs242924;) |
Risk for depression |
The haplotype conferred resistance to depression to individuals exposed to violence in their childhood |
NO |
Willour and colleagues, 2009 [171] |
317 families with 554 bipolar offspring |
FKBP5
rs1043805; rs7757037; rs3798346; rs9296158; rs9380525; rs7763535; rs737054 |
Risk for bipolar disorder |
Rs4713902 and rs7757037 were associated with BD |
NO |
Dempster and colleagues, 2007 [172] |
328 families with affected probands with a diagnosis of early childhood depression |
AVPR1B
rs28536160
rs283703064
rs35369693
rs28632197
rs33985287
rs33933482 |
Risk for depression (early onset) |
Rs35369693 and rs33985287 showed association with depression |
NO |
Wasserman and colleagues, 2009 [140] |
542 trios with suicide attempter offspring |
CRH
rs1870393
re3176921
CRHR1
rs1396862
rs4792887 |
Risk for suicide |
Rs33985287 was associated with suicide risk |
NO |
Ben-Efraim and colleagues, 2013 [173] |
660 trios of offsprings who attempted suicide |
AVPR1B
rs35630000;
rs33911258;
rs33990840;
rs28529127;
rs33933482 |
Risk for suicide |
Rs33990840 associated with suicide behavior |
NO |
MDD: Major Depressive Disorder, HCT: Healthy Controls, PD: Panic Disorder, NEO-PI-R: Revised NEO Personality lnventory, TCI: Temperament and Character Inventory, STAI: State-Trait Anxiety Inventory, MFG: Middle Frontal Gyrus, IFG: Inferior Frontal Gyrus, DAS-24: Dysfunctional Attitude Scale, BP: Bipolar Disorder
As shown in Table 1, there are conflicting results as to how much the genetic personal makeup may influence response to stress when the genes that code for the molecular cascades that regulate stress response are analyzed. This may depend on many factors, including the statistical power of the studies, the different inclusion criteria and the different assessment of patients and their illness. Nevertheless, some relevant biological aspects of response to stress may have been not take into sufficient consideration in the previous genetic investigations. One of such relevant biological aspect is the epigenetic control over genes expression. Epigenetic controls which genes are expressed, and its investigation is thought to help in filling the “Missing Heredity” in psychiatric genetics [86,87]. Through the epigenetic control of genetic expression cells that share the same exact genetic background will differentiate in, for example, neurons rather than liver cells. The term epigenetic was first introduced in the fifties [88], and is the center of extensive research in these years. There are different epigenetic mechanisms known at the current time, methylation of DNA and histone methylation and acetylation have been extensively investigated, and other mechanisms such as the interaction with iRNA molecules represent new research horizons for better understand how the cell regulate its genetic expression profile. Not only this is relevant to understand how cells specialize in their function or how the control of cell proliferation and differentiation is lost, for example, in degeneration or proliferative diseases, but it is also relevant in understanding the biologic structure of memory formation and the adaptation of the molecular cascades to experiences or traumas in life, or in the life of our predecessors.
The DNA double chain can be directly regulated by epigenetics events, and so can some proteins called histones that bind the DNA in tight wraps. DNA methylation is a molecular reaction that consists on a molecular modification of the cytosine side-chain by adding a methyl (-CH3) group with a covalent bond. DNA methylation results in much cases in the silencing of the DNA sequence where it is present, even though cases of increased expression of methylated sequences are also reported [89,90]. This molecular event is facilitated when cytosines are immediately followed by a guanine base. Such a concatenation forms the so called “CpG” islands in the DNA, which represent areas of DNA regulation. The ability of the epigenetic control over gene expression to determine both long-lasting differentiation processes and short-medium term regulations of the cells' behavior is mirrored by the presence of two different class of enzymes that mediate the methylation of DNA. DNA methyltransferases are present in “maintenance” and “de novo” isoforms [91]. The first isoform methylates areas of the DNA in which the methylation of CpG islands is present but not complete, the second isoform can methylate areas of DNA that were not previously methylated. Not only the DNA can be methylated, but also histones, whose complexes for tight bounds with DNA preventing its expression. Methylation cann occur to histones along with acetylation, ubiquitination and phosphorylation, all these molecular events concurring the orchestration that regulate the DNA transcription [92,93]. These mechanisms were shown to be directly involved in the formation of new memories [94]. This evidence bridges the psychological experience of an event with the biological modifications that both represent a reaction to the experience and an adaptation to the environment [95]. Its role in anxiety and depressive response after a trauma is intuitive and the ability to understand and modify the biological events that trigger memories and reaction to them would pave the way to a better treatment of depressive disorders and anxiety disorders. It has been reported that impairing the histone system result in a decreased ability to store memories in animal models [96-98], and the use of drugs that interact with histones can alter the ability to form memories [94,99], a molecular event that may be of use in clinical practice, when the extinction of aversive or maladaptive memories is required. This treatment strategy is not clinically implemented at the time of writing, but evidence from animal studies appear promising [100,101]. As Table 1 shows, genetic results are stronger in assessing the risk of anxiety or other psychiatric disorders given a specific genetic background, when the early life traumatic events are taken into consideration. Notable, early life events seem to play a more relevant role than the actual cause of stress in determining the risk of having a psychiatric disorder as a consequence of acute or chronic stress. The biological explanation of this event may be found in reports that show how the early traumatic events have a deep impact in the epigenetic mechanisms that control gene expression [102-107]. Most interestingly, the epigenetic regulation that is a consequence of stress and may expose later in life to stress-related psychiatric disorders appears to be transgenerational in nature, so that not only early life experiences are associated with epigenetic modifications that increase the risk of disease later in life, but also the stressful experiences of the pregnant mothers can influence the further development of their offspring [108,109]. A finding that was recently proved true for complex behaviors [110], cognitive performance and prenatal cocaine exposure [111], but not psychiatric disorders per se [112]. Mathews and colleagues [113] reported that women (n=33) recently diagnoses with breast cancer showed an impaired immune system and a dyscontrol in the epigenetic regulation of histone acetylation and phosphorylation, which was in association with the perceived stress. Kim and colleagues reported on a significant association between an increased methylation rate of the BDNF and an increased risk of suicide in a sample of 279 patients with breast cancer [59], a finding that was confirmed in an independent research conducted by Kang and colleagues [114] and involving 309 patients with breast cancer. Consistently, Zhou and colleagues [115] reported a significant association between methylation rates in three different genomic sites and depressive scores in a sample of 73 women with breast cancer in stages from I to III.
Table 1. Genetic and psychological predictors of depressive disorders during cancer
Study |
Sample |
Kind of cancer |
Genetic predictors |
Direction of investigation |
Predicted outcome |
Genes associated with a better / worse outcome |
Genes associated with resilience, if present |
Ahles and colleagues, 2003 [118] |
Long term survivors of breast cancer (n=51) or lymphoma (n=29) |
Breast Cancer;
Lymphoma |
APOE ε4 |
Cancer + genetic background → psychiatric / neurological symptoms |
Neuropsychological tests; CES-D; STAI, FSI |
APOE variant associated with visual memory and spatial ability (APOE ε4 negative → better visual memory and spatia ability results) |
The distribution of the APOE alleles were similar between survivor and the general population |
Thomas and colleagues, 1979 – 1980 [44,119] |
1337 (90% men) follow up for a period of 31 years |
Any cancer disorder |
NA |
Personality (HNTQ) + genetic bakcground → cancer |
Development of a cancer disease |
Colon, Rectum and Bladder
cancer correlated with depression / neurotic patterns.
These are related to the Ras oncogene
family |
NA |
Shekelle and colleagues, 1981 [120] and Olderoy and colleagues, 1998 [121] |
2107 male laborers follow up of 19 years |
Any cancer disorder |
NA |
Personality (MMPI) → cancer |
Development of a cancer disease |
No association with Ras variations.
An association with depression was observed |
NA |
Grossarth-Maticek and colleagues, 1984 [35] and Pelosi and colleague, 1992 [36] |
1353 caucasian individuals. Follow up of 30 years |
Any cancer disorder |
NA |
Depressive symtoms → cancer |
Depressive symptoms prior to the diagnosis of cancer predicted such diagnosis |
Colon, Rectum and Bladder
cancer correlated with depression / neurotic patterns.
These are related to the Ras oncogene
family |
NA |
Kaplan and colleague, 1988 [40] |
6928 individuals, follow up of 17 years |
Any cancer disorder |
NA |
Depressive symptoms (HPLDI, BDI) → cancer |
No significant association between depression and a diagnosis of cancer later in life |
NA |
NA |
Hahn and colleague, 1988 [39] |
16600 women, 8932 completed the psychological assessment |
Breast cancer |
NA |
Personality (MMPI) → cancer |
No significant association between depression and a diagnosis of cancer later in life |
NA |
NA |
Zonderman and colleagues, 1989 [42] |
6913 individuals followd up for a period of 10 years |
Any cancer disorder |
NA |
Personality / depression (CES-D; GWB-D) → cancer |
No significant association between depression and a diagnosis of cancer later in life |
NA |
NA |
Linkins and colleague, 1990 [41] |
2264 individuals followed up for at period of 12 years |
Any cancer disorder |
NA |
Personality / depression (CES-D) → cancer |
No significant association between depression and a diagnosis of cancer later in life |
NA |
NA |
Friedman and colleagues, 1994 [38] |
923 individuals with depression or other psychiatric disorders
143574 individuals from the general population |
Any cancer disorder |
NA |
Psychiatric diagnosis → cancer |
No significant association between depression and a diagnosis of cancer later in life |
NA |
NA |
Pennix and colleagues, 1998 [122] |
1825 geriatric individuals, no follow up |
Any cancer disorder |
NA |
Personality / depression (CES-D) → cancer |
Depression correlated with cancer |
Colon, Rectum and Bladder
cancer correlated with depression / neurotic patterns.
These are related to the Ras oncogene
family |
NA |
Dattore and colleagues, 1980 [123] |
75 cancer and 125 non cancer patients |
Any cancer disorder |
NA |
Personality (MMPI) → cancer |
Premorbid depressive symtoms correlated with depression |
Colon, Rectum and Bladder
cancer correlated with depression / neurotic patterns.
These are related to the Ras oncogene
family |
NA |
Persky and colleagues, 1987 [37] |
2018 individuals |
Any cancer disorder |
NA |
Personality (MMPI; Cattel 16 Personality Factor Inverntory) → cancer |
Depressive symptoms but not personality treaits predicted cancer |
Colon, Rectum and Bladder
cancer correlated with depression / neurotic patterns.
These are related to the Ras oncogene
family |
NA |
Dalton and colleagues, 2002 [43] |
89491 individuals diagnosed with depression followd up for 24 years |
Any cancer disorder |
NA |
Psychiatric diagnosis → cancer |
Brain Cancer risk was higher after 1 year from diagnosis but association was not confirmed after the 1 year |
NA |
NA |
Eberhard and colleagues, 2010 [124] |
165 TGCC patients |
Testicular germ cancer |
Two polymorphic regions in the Androgen Receptor |
Cancer + genetic background → psychiatric / neurological symptoms |
Angst or Depression two or five years after treatment (HADS-A; HADS-D) |
No genetic association |
No genetic association |
Gilbert and colleagues, 2011 [49] |
94 patients with head and neck cancer (33 werer genotyped for 5-HTTLPR) |
Head and neck cancer |
5-HTTLPR |
Cancer + genetic background → psychiatric / neurological symptoms |
Depressive Disorder |
No genetic association |
No genetic association |
Kyung and colleagues, 2012 [125] |
186 patients with breast cancer
Nondepressed, n= 137
Depressed, n= 49 |
Breast cancer |
5-HTTLPR |
Cancer + genetic background → psychiatric / neurological symptoms |
Outcome 1 =
Association with development of depression
Outcome 2 = severity of depressive symptoms associated with depressed patients |
Outcome 1 =
No genetic association
Outcome 2 = patients with the short allele had significantly higher HAM-D scores |
No genetic association |
Kim and colleagues, 2012 [50] |
309 patients after mastectomy for breast cancer |
Breast cancer |
5-HTT,
5-HTR2a 1438A/G,
5-HTR2a 102T/C and
BDNF val66Met |
Cancer + genetic background → psychiatric / neurological symptoms |
Association between genes and depression after mastectomy for breast cancer |
No genetic association with variations of 5-HTT, 5-HTR2a 1438A/G and 5-HTR2a 102T/C genes
The BDNF Met/Met genotype was associated with prevalent and persistent depression |
No genetic association |
Kim and colleagues, 2013 [51] |
309 women after breast cancer surgery |
Breast cancer |
Pro-inflammatory and anti-inflammatory cytokines
6 variations located in pro: TNF-α-850C/T, TNF- α-308G/A, IL-1β-511C/T, IL-1β +3953C/T, IL-6-174G/C, IL-8-251T/A
2 anti variations located in: IL-4 +33T/C, IL-10-1082G/A |
Cancer + genetic background → psychiatric / neurological symptoms |
The alleles related to higher pro-inflammatory and/or lower anti-anflammatory cytokine production would associate with depression in a cohort with breast cancer |
The pro-inflammatory cytokines is associated with depression and provide potential genetic basis for depression in breast cancer patients. |
No genetic association |
Schillani and colleagues, 2012 [48] |
48 early breast cancer patients |
Breast cancer (mammary carcinoma) |
5-HTTLPR |
Cancer + genetic background → psychiatric / neurological symptoms |
Association with 5-HTTLPR allele variations and the mental adaptation to cancer diagnosis and treatment |
The 5-HTTLPR alleles associated with the breast cancer patients being in greater risk of mental suffering (anxiety and depression) |
No genetic association |
Koh and colleagues, 2014 [60] |
91 patients with newly diagnosed gastric cancer |
Gastric cancer |
BDNF Val66Met |
Cancer + genetic background → psychiatric / neurological symptoms |
Association with BDNF Val66Met polymorphism and coping response to stress |
The Met allele of BDNF Val66Met may be predictive of an anxious coping style |
No genetic association |
Kim and colleagues, 2015 [59] |
279 patients with breast cancer |
Breast cancer |
BDNF methylation rates |
Cancer + genetic background → psychiatric / neurological symptoms |
Association with BDNF and suicidal ideations |
Increased BDNF methylation was significantly associated with suicidal ideation and depression independent of potential covariates
BDNF gene methylation status may be a biological marker for suicidality |
No genetic association |
Kang and colleagues, 2012 [57] |
130 newly diagnosed advanced gastric cancer patients |
Gastric cancer |
Three SNPs harbored by FKBP5 rs1360780, rs9296158 and rs9470080 |
Cancer + genetic background → psychiatric / neurological symptoms |
Investigation of the influence of FKBP5 gene polymorphisms on distress and vulnerability to anxiety and depression |
All of the polymorphisms showed a significant predictors of anxiety and depression |
No genetic association |
Saad and colleagues, 2014 [56] |
335 patients with breast cancer |
Breast cancer |
Cytokine gene variations:
IFNGR1 rs9376268,
IL6 rs2069840 and TNFA rs1799964 |
Cancer + genetic background → psychiatric / neurological symptoms |
Association with cytokine gene variations and depressive symptoms |
Variations of cytokine genes may place patients at higher risk for development of Subsyndromal levels of depressive symptoms |
No genetic association |
Zhou and colleagues, 2015 [115] |
73 women with breast cancer in diffeerent stages |
Breast cancer |
Methylation rate a different locations in the genome |
Cancer + genetic background → psychiatric / neurological symptoms |
Methylation rate a different locations in the genome is correlated with Stress, anxiety and Depression |
Methylation rates at genes FAM101A and FOXJ1 were found to be associated with Depression (HADS), but not anxiety (HADA) or Stress (PSS) |
No genetic association |
CES-D: Center for Epidemiological Study - Depression, STAI: Spielberger State-Trait Anxiety Inventory, FSI: Fatigue Symptom Inventory, HNTQ: habits of nervous tension, MMPI: Minne-
sota Multiphasic Personality Inventory, HPLDI: Human Population Laboratory Depression Index, BDI: Beck Depression Inventory, GWB-D: General Well-being Schedule-Depression subscale, TGCC: testicular germ cell cancer, HADS-A: Hospital Anxiety and Depression Scale-Anxiety, HADS-D: Hospital Anxiety and Depression Scale-Depression, PSS: Perceived Stress Scale
Depression and anxiety may be complications during the treatment of the most form of cancers. A comorbidity with depressive symptoms or symptoms of anxiety results in a worse prognosis for patients with a diagnosis of cancer. The ability to identify subjects at risk of develop a psychiatric disorder later on in the treatment of a cancer would pave the way to the implementation of efficacious strategies to reduce comorbidity and increase the effectiveness of the current treatments for cancer. This topic has been the object of intensive research during the last decades, focusing on the social, psychological and biological characteristics of those patients that are more at risk for a psychiatric comorbidity after they receive a diagnosis of cancer or during the treatment of cancer. Such identification as so far frustrate researchers efforts and the current classifications used for prognostic and treatment related protocols in cancer disease (Box 1 and 2), do not implement psychiatric comorbidity or stress-related events. A younger age, being female, a lower income and treatment with surgery are significantly associated with more stress [33], education level proved to be associated with resistance to depressive symptoms [116] and social support was shown to play a relevant role also [117]. Psychological factors may also play a role as it was reported that neuritic traits in personality could increase the risk of a later development of a cancer. Nevertheless, the presence of conflicting results in literature does not allow final conclusions in this field either, as previously reported in the present contribution. The last decades of investigation have focused in the research for genetic variations that could predict depressive or anxiety related symptoms in cancer patients, and despite some of the more classical variations that have previously reported to be associated with such psychiatric disorders show a correlation with depressive and anxiety disorders in groups of patients that suffer from a cancer, negative results prevent final conclusions once again. Within this frame, a better understanding of the molecular events that mediate response to stress, with a particular attention of the timing of their activation and the presence of “windows” during which the stress response system can be deeply changed by the external experience during specific ages, may be the future horizon of research in this field. A number of genes have been selected in Table 3 by the implementation of the stress molecular cascades in Cytoskape. The program helped in defining a large molecular cascade reported in Figure 4, whose genetic and epigenetic characterization could be used as an object of investigation in the next researchers in the field of psychiatric liability in cancer patients.
Box 1. TNM classifications
What it is
TNM is a classification of a cancer disease by its dissemination in the body. It provides a description of the cancer disease and disease dissemination that is instrumental for prognosis and treatment.
When it is used
TNM is used to the classification of a number of cancer diseases, it is not specific for the CRC. It is mainly used in the clinical settings.
How it is used
T is the category that describes the first localization of the tumor
N is the category that describes the regional involvement of lymph nodes
M is the category the describes the presence of metastases
Published by
Box 2. Surveillance, Epidemiology, and End Results (SEER) classification
What it is
SEER is a classification of a cancer disease based on histological variables. Hundreds of different histological cancer classes are known. They are grouped in six categories including: 1) Carcinomas (from epithelial cells, malignant); 2) Sarcoma (originates in supportive and connective tissues); 3) Myeloma (originates in the plasma cells of bone marrows); 4) Leukemia (often associted with the overproduction of white blood cells); 5) Lymphoma (originates in the gland or nodes of the lymphatic system); 5) Mixed types.
When it is used
SEER was established in 1973 in response to the National Cancer Act of 1971, which mandated that the National Cancer Institute (NCI) collect, analyze, and disseminate all data useful in the prevention, diagnosis, and treatment of cancer. SEER is mainly used for statistical and research goals
How it is used
It is based on the histological characteristics of the cancer, it assembles and report annual estimates of cancer statistics that pertain to incidence, prevalence, and patient survival; monitor trends to identify important changes in cancer rates for population subgroups defined by geographic, demographic, and social characteristics; provide information on changes over time in stage of disease at diagnosis and types of therapy, as well as associated changes in cancer patient survival; carry out special studies that provide insight into trends in cancer rates, treatment patterns, and other relevant aspects of cancer control; and provide an infrastructure to support cancer research through its publicly available data.
Published by
Table 3. Selection of genes and tagging SNPs
symbol |
Ensembl |
chr |
start |
stop |
descritpion |
n_exons |
n_knonw_SNPs |
best_tagging |
previous evidence (pmid) |
NR3C1 |
ENSG00000113580 |
5q31.3 |
143254293 |
143459148 |
This gene encodes glucocorticoid receptor, which can function both as a transcription factor that binds to glucocorticoid response elements in the promoters of glucocorticoid responsive genes to activate their transcription, and as a regulator of other transcription factors. This receptor is typically found in the cytoplasm, but upon ligand binding, is transported into the nucleus. It is involved in inflammatory responses, cellular proliferation, and differentiation in target tissues. Mutations in this gene are associated with generalized glucocorticoid resistance. Alternative splicing of this gene results in transcript variants encoding either the same or different isoforms. Additional isoforms resulting from the use of alternate in-frame translation initiation sites have also been described, and shown to be functional, displaying diverse cytoplasm-to-nucleus trafficking patterns and distinct transcriptional activities (PMID:15866175). [provided by RefSeq, Feb 2011]</dd> |
16 |
318 |
rs312570,rs312571,rs7701729,rs7722075,rs11746024,rs1391251,rs17101265,rs2112197,rs2112198,rs312637, |
1557508(2),8297920(1),8373936(1),9380019(1),10520140(1),11033331(1),11274650(5),11383977(1),11593077(2),12242054(2),12511169(2),1262724,(1),12727135(3),14517580(1),14677081(1),15536494(1),15576061(1),15645065(1),15694112(1),15987954(1),16019586(4),16255837(1),16499076(1),16580345(1),16785275(1),17034955(1),17070667(2),18037007(1),18368033(1),18502552(1),19065455(1),19300389(1),19782477(1),19906241(1),19906242(1),20231081(1) |
AVP |
ENSG00000101200 |
20p13 |
3080909 |
3095165 |
This gene encodes a member of the vasopressin/oxytocin family and preproprotein that is proteolytically processed to generate multiple protein products. These products include the neuropeptide hormone arginine vasopressin, and two other peptides, neurophysin 2 and copeptin. Arginine vasopressin is a posterior pituitary hormone that is synthesized in the supraoptic nucleus and paraventricular nucleus of the hypothalamus. Along with its carrier protein, neurophysin 2, it is packaged into neurosecretory vesicles and transported axonally to the nerve endings in the neurohypophysis where it is either stored or secreted into the bloodstream. The precursor is thought to be activated while it is being transported along the axon to the posterior pituitary. Arginine vasopressin acts as a growth factor by enhancing pH regulation through acid-base transport systems. It has a direct antidiuretic action on the kidney, and also causes vasoconstriction of the peripheral vessels. This hormone can contract smooth muscle during parturition and lactation. It is also involved in cognition, tolerance, adaptation and complex sexual and maternal behaviour, as well as in the regulation of water excretion and cardiovascular functions. Mutations in this gene cause autosomal dominant neurohypophyseal diabetes insipidus (ADNDI). This gene is present in a gene cluster with the related gene oxytocin on chromosome 20. [provided by RefSeq, Nov 2015]</dd> |
4 |
72 |
rs6107250,rs6115805,rs6139019,rs2422859,rs3087802,rs3827132,rs6084276 |
|
BDNF |
ENSG00000176697 |
11p13 |
27644817 |
27732132 |
This gene encodes a member of the nerve growth factor family of proteins. Alternative splicing results in multiple transcript variants, at least one of which encodes a preproprotein that is proteolytically processed to generate the mature protein. Binding of this protein to its cognate receptor promotes neuronal survival in the adult brain. Expression of this gene is reduced in Alzheimer's, Parkinson's, and Huntington's disease patients. This gene may play a role in the regulation of the stress response and in the biology of mood disorders. [provided by RefSeq, Nov 2015]</dd> |
12 |
101 |
rs7931247,rs7934165,rs908867,rs962369,rs10835210,rs12273363,rs12288512,rs16917237,rs7103411,rs4923468 |
19898468 |
CRH |
ENSG00000147571 |
8q13 |
66176041 |
66178945 |
This gene encodes a member of the corticotropin-releasing factor family. The encoded preproprotein is proteolytically processed to generate the mature neuropeptide hormone. In response to stress, this hormone is secreted by the paraventricular nucleus (PVN) of the hypothalamus, binds to corticotropin releasing hormone receptors and stimulates the release of adrenocorticotropic hormone from the pituitary gland. Marked reduction in this protein has been observed in association with Alzheimer's disease. Autosomal recessive hypothalamic corticotropin deficiency has multiple and potentially fatal metabolic consequences including hypoglycemia and hepatitis. In addition to production in the hypothalamus, this protein is also synthesized in peripheral tissues, such as T lymphocytes, and is highly expressed in the placenta. In the placenta it is a marker that determines the length of gestation and the timing of parturition and delivery. A rapid increase in circulating levels of the hormone occurs at the onset of parturition, suggesting that, in addition to its metabolic functions, this protein may act as a trigger for parturition. [provided by RefSeq, Nov 2015]</dd> |
2 |
95 |
|
1361969(1), 8491091(2), 8643680(1), 8731457(2), 9854171(2), 10599479(2), 10867111(1), 10918705(1), 1128269,(1), 11337099(1), 12662131(1), 12769601(1), 12845406(1), 14563384(1), 14575894(1), 14698679(1), 15212620(1), 15214502(1), 15240401(1), 15278013(1), 15331578(1), 15509759(1), 15573019(1), 16594259(3), 16769145(1), 16870185(1), 16884458(1), 17044726(1), 17065965(1), 17287823(1), 18384079(1), 18412102(1), 18622363(1), 19520785(1), 19738931(1), 20010888(1) |
REST |
ENSG00000084093 |
4q12 |
56903680 |
56940039 |
This gene encodes a transcriptional repressor that represses neuronal genes in non-neuronal tissues. It is a member of the Kruppel-type zinc finger transcription factor family. It represses transcription by binding a DNA sequence element called the neuron-restrictive silencer element. The protein is also found in undifferentiated neuronal progenitor cells and it is thought that this repressor may act as a master negative regular of neurogenesis. Alternatively spliced transcript variants have been described [provided by RefSeq, Jul 2010]</dd> |
8 |
592 |
rs13148656,rs17086696,rs2899063,rs3796543,rs3796544,rs6554347,rs6554348,rs6812222,rs7693137,rs7696808 |
20071535 |
DRD1 |
ENSG00000184845 |
5q35.1 |
175440148 |
175444682 |
This gene encodes the D1 subtype of the dopamine receptor. The D1 subtype is the most abundant dopamine receptor in the central nervous system. This G-protein coupled receptor stimulates adenylyl cyclase and activates cyclic AMP-dependent protein kinases. D1 receptors regulate neuronal growth and development, mediate some behavioral responses, and modulate dopamine receptor D2-mediated events. Alternate transcription initiation sites result in two transcript variants of this gene. [provided by RefSeq, Jul 2008]</dd> |
2 |
198 |
|
19077056 |
DRD2 |
ENSG00000149295 |
11q23 |
113399741 |
113485131 |
This gene encodes the D2 subtype of the dopamine receptor. This G-protein coupled receptor inhibits adenylyl cyclase activity. A missense mutation in this gene causes myoclonus dystonia; other mutations have been associated with schizophrenia. Alternative splicing of this gene results in two transcript variants encoding different isoforms. A third variant has been described, but it has not been determined whether this form is normal or due to aberrant splicing. [provided by RefSeq, Jul 2008]</dd> |
|
238 |
rs3018331,rs2606726,rs2735201,rs1672713,rs12292595,rs10750043,rs1319730,rs7119141,rs7934726,rs11821039 |
8897112(1), 9513185(1), 19180583(1), 20122683(2), 9345554(1), 9513185(1), 10482381(1), 10512150(1), 10812530(1), 18251011(2), 18929622(1), 20067857(1) |
GRIN2B |
ENSG00000273079 |
12p12 |
13470636 |
14048701 |
N-methyl-D-aspartate (NMDA) receptors are a class of ionotropic glutamate receptors. NMDA receptor channel has been shown to be involved in long-term potentiation, an activity-dependent increase in the efficiency of synaptic transmission thought to underlie certain kinds of memory and learning. NMDA receptor channels are heteromers composed of three different subunits: NR1 (GRIN1), NR2 (GRIN2A, GRIN2B, GRIN2C, or GRIN2D) and NR3 (GRIN3A or GRIN3B). The NR2 subunit acts as the agonist binding site for glutamate. This receptor is the predominant excitatory neurotransmitter receptor in the mammalian brain. [provided by RefSeq, Jul 2008]</dd> |
16 |
638 |
rs1805509,rs1805526,rs3026164,rs3026175,rs3026186,rs4764006,rs7309380,rs7311774,rs7316060,rs7977989 |
19077056 |
GRIN2A |
ENSG00000183454 |
16p13.2 |
9688909 |
10247857 |
This gene encodes a member of the glutamate-gated ion channel protein family. The encoded protein is an N-methyl-D-aspartate (NMDA) receptor subunit. NMDA receptors are both ligand-gated and voltage-dependent, and are involved in long-term potentiation, an activity-dependent increase in the efficiency of synaptic transmission thought to underlie certain kinds of memory and learning. These receptors are permeable to calcium ions, and activation results in a calcium influx into post-synaptic cells, which results in the activation of several signaling cascades. Disruption of this gene is associated with focal epilepsy and speech disorder with or without mental retardation. Alternative splicing results in multiple transcript variants. [provided by RefSeq, May 2014]</dd> |
20 |
690 |
rs8048025,rs9941172,rs10744973,rs1448265,rs12708642,rs13330640,rs16966264,rs7200793,rs837686,rs837699 |
19077056 |
GRIN2C |
ENSG00000161509 |
17q25.1 |
74839096 |
74864437 |
This gene encodes a subunit of the N-methyl-D-aspartate (NMDA) receptor, which is a subtype of ionotropic glutamate receptor. NMDA receptors are found in the central nervous system, are permeable to cations and have an important role in physiological processes such as learning, memory, and synaptic development. The receptor is a tetramer of different subunits (typically heterodimer of subunit 1 with one or more of subunits 2A-D), forming a channel that is permeable to calcium, potassium, and sodium, and whose properties are determined by subunit composition. Alterations in the subunit composition of the receptor are associated with pathophysiological conditions such as Parkinson's disease, Alzheimer's disease, depression, and schizophrenia. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Jun 2013]</dd> |
16 |
619 |
rs4592695,rs4790015,rs7212305,rs7214890,rs7501499,rs8064769,rs8075657,rs8076595,rs907898,rs9898046 |
19077056 |
GRIN2D |
ENSG00000105464 |
19q13.33 |
48387365 |
48452445 |
N-methyl-D-aspartate (NMDA) receptors are a class of ionotropic glutamate receptors. NMDA channel has been shown to be involved in long-term potentiation, an activity-dependent increase in the efficiency of synaptic transmission thought to underlie certain kinds of memory and learning. NMDA receptor channels are heteromers composed of the key receptor subunit NMDAR1 (GRIN1) and 1 or more of the 4 NMDAR2 subunits: NMDAR2A (GRIN2A), NMDAR2B (GRIN2B), NMDAR2C (GRIN2C), and NMDAR2D (GRIN2D). [provided by RefSeq, Mar 2010]</dd> |
13 |
395 |
|
|
GRIN3A |
ENSG00000198785 |
9q31.1 |
101543967 |
101763963 |
This gene encodes a subunit of the N-methyl-D-aspartate (NMDA) receptors, which belong to the superfamily of glutamate-regulated ion channels, and function in physiological and pathological processes in the central nervous system. This subunit shows greater than 90% identity to the corresponding subunit in rat. Studies in the knockout mouse deficient in this subunit suggest that this gene may be involved in the development of synaptic elements by modulating NMDA receptor activity. [provided by RefSeq, Jul 2008]</dd> |
11 |
584 |
rs10819702,rs10819703,rs2416939,rs4743367,rs7024182,rs4743365,rs34148386,rs7020312,rs7850043,rs7865169 |
19077056 |
TRIM28 |
ENSG00000130726 |
19q13.4 |
58543531 |
58551651 |
The protein encoded by this gene mediates transcriptional control by interaction with the Kruppel-associated box repression domain found in many transcription factors. The protein localizes to the nucleus and is thought to associate with specific chromatin regions. The protein is a member of the tripartite motif family. This tripartite motif includes three zinc-binding domains, a RING, a B-box type 1 and a B-box type 2, and a coiled-coil region. [provided by RefSeq, Jul 2008]</dd> |
17 |
428 |
|
14519192; 19081377 |
DNMT1 |
ENSG00000130816 |
19p13.2 |
10124084 |
10204338 |
This gene encodes an enzyme that transfers methyl groups to cytosine nucleotides of genomic DNA. This protein is the major enzyme responsible for maintaining methylation patterns following DNA replication and shows a preference for hemi-methylated DNA. Methylation of DNA is an important component of mammalian epigenetic gene regulation. Aberrant methylation patterns are found in human tumors and associated with developmental abnormalities. Variation in this gene has been associated with cerebellar ataxia, deafness, and narcolepsy, and neuropathy, hereditary sensory, type IE. Alternative splicing results in multiple transcript variants. [provided by RefSeq, Jan 2016]</dd> |
42 |
703 |
rs721186,rs11672909,rs2116940,rs2288350,rs4804125,rs4804490,rs4804494,rs8111085,rs8112895,rs7247491 |
25065861 |
DNMT3a |
ENSG00000119772 |
2p23 |
25216516 |
25359033 |
CpG methylation is an epigenetic modification that is important for embryonic development, imprinting, and X-chromosome inactivation. Studies in mice have demonstrated that DNA methylation is required for mammalian development. This gene encodes a DNA methyltransferase that is thought to function in de novo methylation, rather than maintenance methylation. The protein localizes to the cytoplasm and nucleus and its expression is developmentally regulated. Alternative splicing results in multiple transcript variants encoding different isoforms. [provided by RefSeq, Jul 2008]</dd> |
33 |
609 |
rs7566506,rs11686210,rs7589318,rs6545976,rs6719226,rs3731634,rs17046887,rs17046931,rs28932473,rs3820935 |
25065861 |
DNMT3b |
ENSG00000088305 |
20q11.2 |
32755338 |
32816401 |
CpG methylation is an epigenetic modification that is important for embryonic development, imprinting, and X-chromosome inactivation. Studies in mice have demonstrated that DNA methylation is required for mammalian development. This gene encodes a DNA methyltransferase which is thought to function in de novo methylation, rather than maintenance methylation. The protein localizes primarily to the nucleus and its expression is developmentally regulated. Mutations in this gene cause the immunodeficiency-centromeric instability-facial anomalies (ICF) syndrome. Eight alternatively spliced transcript variants have been described. The full length sequences of variants 4 and 5 have not been determined. [provided by RefSeq, May 2011]</dd> |
24 |
475 |
rs6087625,rs6087626,rs6088590,rs6088594,rs6119516,rs6120708,rs6141509,rs910869,rs910871,rs959829 |
25065861 |
NFIX |
ENSG00000008441 |
19p13.3 |
12980299 |
13114251 |
The protein encoded by this gene is a transcription factor that binds the palindromic sequence 5'-TTGGCNNNNNGCCAA-3 in viral and cellular promoters. The encoded protein can also stimulate adenovirus replication in vitro. Three transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Aug 2012]</dd> |
13 |
143 |
rs7250834,rs12982296,rs10402645,rs11881337,rs10401962,rs10420597,rs2193519,rs34999793,rs7249150,rs7256436 |
25135974 |
EGR1 |
ENSG00000120738 |
5q31.1 |
138464918 |
138469887 |
The protein encoded by this gene belongs to the EGR family of C2H2-type zinc-finger proteins. It is a nuclear protein and functions as a transcriptional regulator. The products of target genes it activates are required for differentitation and mitogenesis. Studies suggest this is a cancer suppressor gene. [provided by RefSeq, Dec 2014]</dd> |
2 |
324 |
rs6596462,rs6861341 |
25135974 |
SLC6A4 |
ENSG00000108576 |
17q11.2 |
30188071 |
30242214 |
This gene encodes an integral membrane protein that transports the neurotransmitter serotonin from synaptic spaces into presynaptic neurons. The encoded protein terminates the action of serotonin and recycles it in a sodium-dependent manner. This protein is a target of psychomotor stimulants, such as amphetamines and cocaine, and is a member of the sodium:neurotransmitter symporter family. A repeat length polymorphism in the promoter of this gene has been shown to affect the rate of serotonin uptake and may play a role in sudden infant death syndrome, aggressive behavior in Alzheimer disease patients, and depression-susceptibility in people experiencing emotional trauma. [provided by RefSeq, Jul 2008]</dd> |
15 |
348 |
rs12601965,rs170923,rs17545903,rs234810,rs234815,rs234828,rs234832,rs4796011,rs8067780,rs877092 |
>100 |
HTR2A |
ENSG00000102468 |
13q14-q21 |
46821711 |
46906905 |
This gene encodes one of the receptors for serotonin, a neurotransmitter with many roles. Mutations in this gene are associated with susceptibility to schizophrenia and obsessive-compulsive disorder, and are also associated with response to the antidepressant citalopram in patients with major depressive disorder (MDD). MDD patients who also have a mutation in intron 2 of this gene show a significantly reduced response to citalopram as this antidepressant downregulates expression of this gene. Multiple transcript variants encoding different isoforms have been found for this gene. [provided by RefSeq, Sep 2009]</dd> |
4 |
215 |
rs7326674,rs9534606,rs17069936,rs4942625,rs4942634,rs17386667,rs1572872,rs9567851,rs17069953,rs17069957 |
>100 |
SLC6A3 |
ENSG00000142319 |
5p15.3 |
1384893 |
1453323 |
This gene encodes a dopamine transporter which is a member of the sodium- and chloride-dependent neurotransmitter transporter family. The 3' UTR of this gene contains a 40 bp tandem repeat, referred to as a variable number tandem repeat or VNTR, which can be present in 3 to 11 copies. Variation in the number of repeats is associated with idiopathic epilepsy, attention-deficit hyperactivity disorder, dependence on alcohol and cocaine, susceptibility to Parkinson disease and protection against nicotine dependence.[provided by RefSeq, Nov 2009]</dd> |
15 |
346 |
rs2963260,rs461193,rs11564772,rs2963262,rs6873098,rs7719363,rs37017,rs16877324,rs11133762,rs2306194 |
10435396(1), 11374331(1), 12185406(1), 15363105(1), 16644083(1), 17159542(1), 18295460(2), 19844206(1) |
DBH |
ENSG00000123454 |
9q34 |
133632915 |
133662790 |
The protein encoded by this gene is an oxidoreductase belonging to the copper type II, ascorbate-dependent monooxygenase family. It is present in the synaptic vesicles of postganglionic sympathetic neurons and converts dopamine to norepinephrine. It exists in both soluble and membrane-bound forms, depending on the absence or presence, respectively, of a signal peptide. [provided by RefSeq, Jul 2008]</dd> |
12 |
489 |
rs10793897,rs10901095,rs11243524,rs17148276,rs4740313,rs7851410,rs7861722,rs10901091,rs2031872,rs7869935 |
9707300(1), 11904129(1) |
COMT |
ENSG00000093010 |
22q11.21 |
19937504 |
19974209 |
Catechol-O-methyltransferase catalyzes the transfer of a methyl group from S-adenosylmethionine to catecholamines, including the neurotransmitters dopamine, epinephrine, and norepinephrine. This O-methylation results in one of the major degradative pathways of the catecholamine transmitters. In addition to its role in the metabolism of endogenous substances, COMT is important in the metabolism of catechol drugs used in the treatment of hypertension, asthma, and Parkinson disease. COMT is found in two forms in tissues, a soluble form (S-COMT) and a membrane-bound form (MB-COMT). The differences between S-COMT and MB-COMT reside within the N-termini. Several transcript variants are formed through the use of alternative translation initiation sites and promoters. [provided by RefSeq, Sep 2008]</dd> |
11 |
197 |
|
9754693(3), 15450911(1), 16232322(1), 16529167(1), 18046978(1), 18629431(1) |
CNR1 |
ENSG00000118432 |
6q14-q15 |
88135831 |
88170776 |
This gene encodes one of two cannabinoid receptors. The cannabinoids, principally delta-9-tetrahydrocannabinol and synthetic analogs, are psychoactive ingredients of marijuana. The cannabinoid receptors are members of the guanine-nucleotide-binding protein (G-protein) coupled receptor family, which inhibit adenylate cyclase activity in a dose-dependent, stereoselective and pertussis toxin-sensitive manner. The two receptors have been found to be involved in the cannabinoid-induced CNS effects (including alterations in mood and cognition) experienced by users of marijuana. Multiple transcript variants encoding two different protein isoforms have been described for this gene. [provided by RefSeq, May 2009]</dd> |
6 |
237 |
rs6454604,rs6907825,rs9450667,rs1203156,rs12661729,rs1884320,rs6454605,rs6454606,rs7744338,rs9359748 |
19839936(1) |
NPY |
ENSG00000122585 |
7p15.1 |
24283035 |
24293016 |
This gene encodes a neuropeptide that is widely expressed in the central nervous system and influences many physiological processes, including cortical excitability, stress response, food intake, circadian rhythms, and cardiovascular function. The neuropeptide functions through G protein-coupled receptors to inhibit adenylyl cyclase, activate mitogen-activated protein kinase (MAPK), regulate intracellular calcium levels, and activate potassium channels. A polymorphism in this gene resulting in a change of leucine 7 to proline in the signal peptide is associated with elevated cholesterol levels, higher alcohol consumption, and may be a risk factor for various metabolic and cardiovascular diseases. The protein also exhibits antimicrobial activity against bacteria and fungi. [provided by RefSeq, Oct 2014]</dd> |
4 |
51 |
rs1073317,rs12700524,rs16147,rs16148,rs16149,rs3905497 |
7561860(4), 8892391(2), 10208289(1), 14757324(1), 16713589(3), 16723308(1), 18281099(1), 19264362(1), 8097548(1), 11165308(1), 15078174(1), 19264362(1), |
MAO-B |
ENSG00000069535 |
Xp11.23 |
43749229 |
43899854 |
|
|
172 |
|
1581446(1), , 9179504(2), 12849931(2), , 19657584(1) |
MAO-A |
ENSG00000189221 |
Xp11.3 |
43641118 |
43760611 |
This gene is one of two neighboring gene family members that encode mitochondrial enzymes which catalyze the oxidative deamination of amines, such as dopamine, norepinephrine, and serotonin. Mutation of this gene results in Brunner syndrome. This gene has also been associated with a variety of other psychiatric disorders, including antisocial behavior. Alternatively spliced transcript variants encoding multiple isoforms have been observed. [provided by RefSeq, Jul 2012]</dd> |
16 |
143 |
|
10335676(1), 11204346(1), 12595913(1), 15450911(3), 16529167(1), 18046978(1), 12555227(2), 15450911(1), 1498903(1), 7635005(1), 7717091(1), 8313400(2), 8923574(1), 9020990(1), 9179504(3), 9807650(1), 10564737(4), 11121185(1), 12502014(1), 12555227(2), 15486489(2), 15564894(1), 15956990(1), 17088501(1), 17417064(3), 17884271(4),,18337637(4), , 8544183(2), 19224413(2), 19382113(2), 19657584(1), 19859025(1), 19996035(1),20010318(3), 20078943(2), 20175604(1) |
GABRA2 |
ENSG00000151834 |
4p12 |
46221573 |
46412012 |
|
2 |
153 |
rs10029664,rs10470769,rs10938448,rs11725004,rs3922601,rs4073266,rs4264808,rs4395477,rs4401483,rs4645250 |
19842164, 19842164, 24022508 |
CNR1 |
ENSG00000118432 |
6q14-q15 |
88135831 |
88170776 |
This gene encodes one of two cannabinoid receptors. The cannabinoids, principally delta-9-tetrahydrocannabinol and synthetic analogs, are psychoactive ingredients of marijuana. The cannabinoid receptors are members of the guanine-nucleotide-binding protein (G-protein) coupled receptor family, which inhibit adenylate cyclase activity in a dose-dependent, stereoselective and pertussis toxin-sensitive manner. The two receptors have been found to be involved in the cannabinoid-induced CNS effects (including alterations in mood and cognition) experienced by users of marijuana. Multiple transcript variants encoding two different protein isoforms have been described for this gene. [provided by RefSeq, May 2009]</dd> |
6 |
237 |
Rs1049353,
rs806376 ,rs806369 ,rs12205430 ,rs806381 |
/ |
CNRIP1 |
ENSG00000119865 |
2p14 |
68278788 |
68325432 |
This gene encodes a protein that interacts with the C-terminal tail of cannabinoid receptor 1. Two transcript variants encoding different isoforms have been described for this gene. [provided by RefSeq, Jul 2013]</dd> |
6 |
76 |
Rs806376 ,rs806367 ,rs6454673 ,rs4707436 ,rs12205430 |
/ |
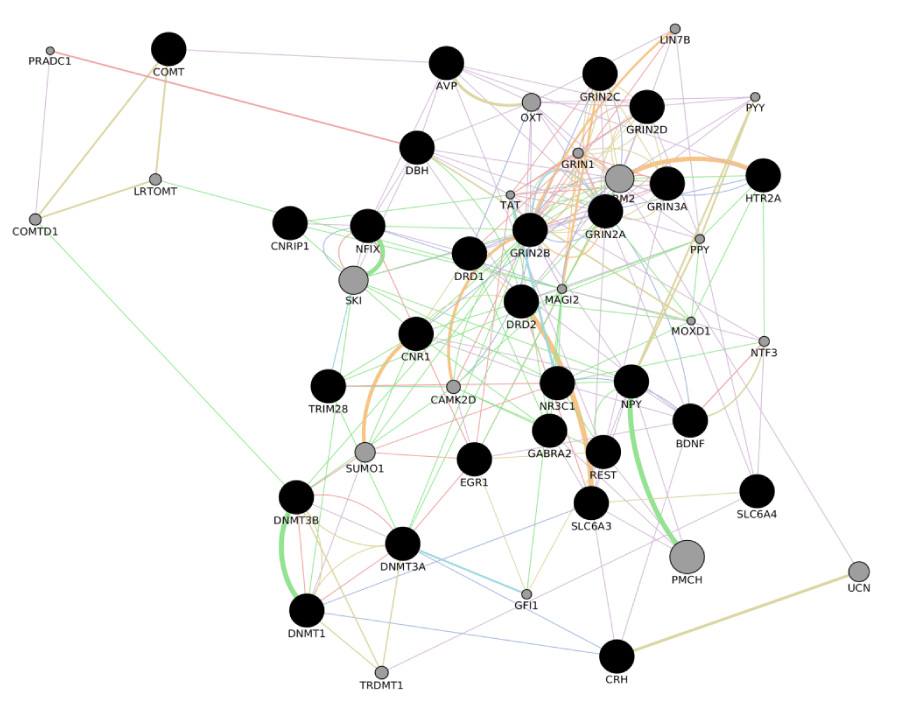
Figure 4. The molecular pathway proposed to further investigations
The characteristics of the genes are detailed in Table 3. The molecular pathway was extrapolated from the Cytoskape and genemania plugin database after entering the genes found to be associated with stress and cancer. The distance from the single genes is a function of the strength of the molecular interaction of their products. The arrows symbolize the direction of the molecular chain flow between the represented genes' products. Brown lines indicate physical connections, green lines indicate genetic connections, violet lines indicate co-expression, blu lines indicated co-localization. The following GO annotations were found to be significantly overrepresented in the molecular pathway in the picture: Behavior (Q-value = 3.8e-09, coverage = 12/228), response to ethanol (Q-value = 2.8e-06, coverage = 5/16), single-organism behavior (Q-value = 1.6e-05, coverage = 7/93), learning and memory (Q-value = 2.4e-05, coverage = 6/57) and cognition (Q-value = 6.7e-05, coverage = 6/70).
- GBD 2013 Mortality and Causes of Death Collaborators (2015) Global, regional, and national age-sex specific all-cause and cause-specific mortality for 240 causes of death, 1990-2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet 385: 117-171. [Crossref]
- Global Burden of Disease Cancer Collaboration, Fitzmaurice C, Dicker D, Pain A, Hamavid H, et al. (2015) The Global Burden of Cancer 2013. JAMA Oncol 1: 505-527. [crossref]
- Bernard W, Christopher P (2014) World Cancer Report 2014. International Agency for Research on Cancer.
- Slatore CG, Sullivan DR, Pappas M, Humphrey LL (2014) Patient-centered outcomes among lung cancer screening recipients with computed tomography: a systematic review. J Thorac Oncol 9: 927-934. [crossref]
- Salani R, Andersen BL (2012) Gynecologic care for breast cancer survivors: assisting in the transition to wellness. Am J Obstet Gynecol 206: 390-397. [crossref]
- Lydiatt WM, Moran J, Burke WJ (2009) A review of depression in the head and neck cancer patient. Clin Adv Hematol Oncol 7: 397-403. [crossref]
- Bishop SC, Basch S, Futterweit W (2009) Polycystic ovary syndrome, depression, and affective disorders. Endocr Pract 15: 475-482. [crossref]
- Haman KL (2008) Psychologic distress and head and neck cancer: part 1--review of the literature. J Support Oncol 6: 155-163. [crossref]
- Gibson CA, Lichtenthal W, Berg A, Breitbart W (2006) Psychologic issues in palliative care. Anesthesiol Clin 24: 61-80, viii. [crossref]
- Demark-Wahnefried W, Aziz NM, Rowland JH, Pinto BM (2005) Riding the crest of the teachable moment: promoting long-term health after the diagnosis of cancer. J Clin Oncol 23: 5814-5830. [crossref]
- Lawson PJ, Flocke SA (2009) Teachable moments for health behavior change: a concept analysis. Patient Educ Couns 76: 25-30. [crossref]
- Van Blarigan EL, Meyerhardt JA (2015) Role of physical activity and diet after colorectal cancer diagnosis. J Clin Oncol 33: 1825-1834. [crossref]
- Eyigor S, Kanyilmaz S (2014) Exercise in patients coping with breast cancer: An overview. World J Clin Oncol 5: 406-411. [crossref]
- Gardner JR, Livingston PM, Fraser SF (2014) Effects of exercise on treatment-related adverse effects for patients with prostate cancer receiving androgen-deprivation therapy: a systematic review. J Clin Oncol32: 335-346.
- Cormie P, Nowak AK, Chambers SK, Galvão DA, Newton RU (2015) The potential role of exercise in neuro-oncology. Front Oncol 5: 85. [crossref]
- Mitchell AJ, Chan M, Bhatti H, Halton M, Grassi L, et al. (2011) Prevalence of depression, anxiety, and adjustment disorder in oncological, haematological, and palliative-care settings: a meta-analysis of 94 interview-based studies. Lancet Oncol 12: 160-174. [crossref]
- Akechi T, Nakano T, Okamura H, Ueda S, Akizuki N, et al. (2001) Psychiatric disorders in cancer patients: descriptive analysis of 1721 psychiatric referrals at two Japanese cancer center hospitals. Jpn J Clin Oncol 31: 188-194. [crossref]
- Hund B, Reuter K, Härter M, Brähler E, Faller H, Keller M, et al. (2016) Stressors, symptom profile, and predictors of adjustment disorder in cancer patients. Results from an epidemiological study with the composite international diagnostic interview, adaptation for oncology (CIDI-O). Depress Anxiety 33: 153-161. [Crossref]
- Kim-Fuchs C, Le CP, Pimentel MA, Shackleford D, Ferrari D, et al. (2014) Chronic stress accelerates pancreatic cancer growth and invasion: a critical role for beta-adrenergic signaling in the pancreatic microenvironment. Brain Behav Immun 40: 40-47. [Crossref]
- Satin JR, Linden W, Phillips MJ (2009) Depression as a predictor of disease progression and mortality in cancer patients: a meta-analysis. Cancer 115: 5349-5361. [crossref]
- Prieto JM, Blanch J, Atala J, Carreras E, Rovira M, et al. (2002) Psychiatric morbidity and impact on hospital length of stay among hematologic cancer patients receiving stem-cell transplantation. J Clin Oncol 20: 1907-1917. [crossref]
- Colleoni M, Mandala M, Peruzzotti G, Robertson C, Bredart A, et al. (2000) Depression and degree of acceptance of adjuvant cytotoxic drugs. Lancet 356: 1326-1327. [crossref]
- Onitilo AA, Nietert PJ, Egede LE (2006) Effect of depression on all-cause mortality in adults with cancer and differential effects by cancer site. Gen Hosp Psychiatry 28: 396-402. [crossref]
- Irwin MR (2013) Depression and insomnia in cancer: prevalence, risk factors, and effects on cancer outcomes. Curr Psychiatry Rep 15: 404. [crossref]
- Shelton RC (2007) The molecular neurobiology of depression. Psychiatr Clin North Am 30: 1-11. [crossref]
- WHO (2008): The Global Burden of Disease 2004 Update. Geneva.
- Nakaya N, Goto K, Saito-Nakaya K, Inagaki M, Otani T, et al. (2006) The lung cancer database project at the National Cancer Center, Japan: study design, corresponding rate and profiles of cohort. Jpn J Clin Oncol 36: 280-284. [crossref]
- Nakaya N, Saito-Nakaya K, Akechi T, Kuriyama S, Inagaki M, et al. (2008) Negative psychological aspects and survival in lung cancer patients. Psychooncology 17: 466-473. [crossref]
- Amad A, Ramoz N, Thomas P, Jardri R, Gorwood P (2014) Genetics of borderline personality disorder: systematic review and proposal of an integrative model. Neurosci Biobehav Rev 40: 6-19. [crossref]
- Cummings JL (1998) Frontal-subcortical circuits and human behavior. J Psychosom Res 44: 627-628. [crossref]
- Cummings MA (2015) The neurobiology of psychopathy: recent developments and new directions in research and treatment. CNS Spectr 20: 200-206. [crossref]
- Iofrida C, Palumbo S, Pellegrini S (2014) Molecular genetics and antisocial behavior: where do we stand? Exp Biol Med (Maywood) 239: 1514-1523. [crossref]
- Thomas BC, Waller A, Malhi RL, Fung T, Carlson LE, et al. (2014) A longitudinal analysis of symptom clusters in cancer patients and their sociodemographic predictors. J Pain Symptom Manage. 47: 566-578. [crossref]
- Arneaud SL, Douglas PM (2016) The Stress Response Paradox: Fighting Degeneration at the Cost of Cancer. FEBS J. [crossref]
- Grossarth-Maticek R, Frentzel-Beyme R, Becker N (1984) Cancer risks associated with life events and conflict solution. Cancer Detect Prev 7: 201-209. [crossref]
- Pelosi AJ, Appleby L (1992) Psychological influences on cancer and ischaemic heart disease. BMJ 304: 1295-1298. [crossref]
- Persky VW, Kempthorne-Rawson J, Shekelle RB (1987) Personality and risk of cancer: 20-year follow-up of the Western Electric Study. Psychosom Med 49: 435-449. [crossref]
- Friedman GD (1994) Psychiatrically-diagnosed depression and subsequent cancer. Cancer Epidemiol Biomarkers Prev 3: 11-13. [crossref]
- Hahn RC, Petitti DB (1988) Minnesota Multiphasic Personality Inventory-rated depression and the incidence of breast cancer. Cancer 61: 845-848. [crossref]
- Kaplan GA, Reynolds P (1988) Depression and cancer mortality and morbidity: prospective evidence from the Alameda County study. J Behav Med 11: 1-13. [crossref]
- Linkins RW, Comstock GW (1990) Depressed mood and development of cancer. Am J Epidemiol 132: 962-972. [crossref]
- Zonderman AB, Costa PT Jr, McCrae RR (1989) Depression as a risk for cancer morbidity and mortality in a nationally representative sample. JAMA 262: 1191-1195. [crossref]
- Dalton SO, Mellemkjaer L, Olsen JH, Mortensen PB, Johansen C (2002) Depression and cancer risk: a register-based study of patients hospitalized with affective disorders, Denmark, 1969-1993. Am J Epidemiol 155: 1088-1095. [crossref]
- Thomas CB, McCabe OL (1980) Precursors of premature disease and death: habits of nervous tension. Johns Hopkins Med J 147: 137-145. [crossref]
- Brewer JK (2008) Behavioral genetics of the depression/cancer correlation: a look at the Ras oncogene family and the “cerebral diabetes paradigm.” J Mol Neurosci MN 35: 307-322. [crossref]
- Filaretova L, Bagaeva T (2016) The Realization of the Brain-Gut Interactions with Corticotropin-Releasing Factor and Glucocorticoids. Curr Neuropharmacol. [crossref]
- Kelly JR, Clarke G, Cryan JF, Dinan TG (2016) Brain-gut-microbiota axis: challenges for translation in psychiatry. Ann Epidemiol 26: 366-372. [crossref]
- Schillani G, Era D, Cristante T, Mustacchi G, Richiardi M, et al. (2012) 5-HTTLPR polymorphism and anxious preoccupation in early breast cancer patients. Radiol Oncol 46: 321-327. [crossref]
- Gilbert J, Haman KL, Dietrich MS, Blakely RD, Shelton RC, et al. (2012) Depression in patients with head and neck cancer and a functional genetic polymorphism of the serotonin transporter gene. Head Neck 34: 359-364. [crossref]
- Kim JM, Kim SW, Stewart R, Kim SY, Shin IS, et al. (2012) Serotonergic and BDNF genes associated with depression 1 week and 1 year after mastectomy for breast cancer. Psychosom Med 74: 8-15. [crossref]
- Kim JM, Stewart R, Kim SY, Kang HJ, Jang JE, et al. (2013) A one year longitudinal study of cytokine genes and depression in breast cancer. J Affect Disord 148: 57-65. [crossref]
- Axelrad JE, Lichtiger S, Yajnik V (2016) Inflammatory bowel disease and cancer: The role of inflammation, immunosuppression, and cancer treatment. World J Gastroenterol 22: 4794-4801. [crossref]
- Dmitrieva OS, Shilovskiy IP, Khaitov MR, Grivennikov SI (2016) Interleukins 1 and 6 as Main Mediators of Inflammation and Cancer. Biochemistry (Mosc) 81: 80-90. [crossref]
- Cocchi E, Drago A, Serretti A (2016) Hippocampal Pruning as a New Theory of Schizophrenia Etiopathogenesis. Mol Neurobiol 53: 2065-2081. [crossref]
- Torres MA, Pace TW, Liu T, Felger JC, Mister D, et al. (2013) Predictors of depression in breast cancer patients treated with radiation: role of prior chemotherapy and nuclear factor kappa B. Cancer 119: 1951-1959. [crossref]
- Saad S, Dunn LB, Koetters T, Dhruva A, Langford DJ, et al. (2014) Cytokine gene variations associated with subsyndromal depressive symptoms in patients with breast cancer. Eur J Oncol Nurs 18: 397-404. [crossref]
- Kang JI, Chung HC, Jeung HC, Kim SJ, An SK, et al. (2012) FKBP5 polymorphisms as vulnerability to anxiety and depression in patients with advanced gastric cancer: a controlled and prospective study. Psychoneuroendocrinology 37: 1569-1576. [crossref]
- Dincheva I, Glatt CE, Lee FS (2012) Impact of the BDNF Val66Met polymorphism on cognition: implications for behavioral genetics. Neuroscientist 18: 439-451. [crossref]
- Kim JM, Kang HJ, Kim SY, Kim SW, Shin IS, et al. (2015) BDNF promoter methylation associated with suicidal ideation in patients with breast cancer. Int J Psychiatry Med 49: 75-94. [crossref]
- Koh MJ, Jeung HC, Namkoong K, Chung HC, Kang JI (2014) Influence of the BDNF Val66Met polymorphism on coping response to stress in patients with advanced gastric cancer. J Psychosom Res 77: 76-80. [crossref]
- Drago A, Serretti A (2009) Focus on HTR2C: A possible suggestion for genetic studies of complex disorders. Am J Med Genet Part B Neuropsychiatr Genet 150B: 601-637. [Crossref]
- [crossref] Drago A, Alboni S, Brunello N, De Ronchi D, Serretti A (2010) HTR1B as a risk profile maker in psychiatric disorders: a review through motivation and memory. Eur J Clin Pharmacol 66: 5-27.
- Serretti A, Drago A, De Ronchi D (2007) HTR2A gene variants and psychiatric disorders: a review of current literature and selection of SNPs for future studies. Curr Med Chem 14: 205-2069. [Crossref]
- De Vries AM, de Roten Y, Meystre C, Passchier J, Despland JN, et al. (2014) Clinician characteristics, communication, and patient outcome in oncology: a systematic review. Psychooncology 23: 375-381. [crossref]
- Robinson S, Kissane DW, Brooker J, Burney S (2015) A systematic review of the demoralization syndrome in individuals with progressive disease and cancer: a decade of research. J Pain Symptom Manage 49: 595-610. [crossref]
- Sales PM, Carvalho AF, McIntyre RS, Pavlidis N, Hyphantis TN (2014) Psychosocial predictors of health outcomes in colorectal cancer: a comprehensive review. Cancer Treat Rev 40: 800-809. [crossref]
- Nakaya N (2014) Effect of psychosocial factors on cancer risk and survival. J Epidemiol 24: 1-6. [crossref]
- Schuler LA, Auger AP (2010) Psychosocially influenced cancer: diverse early-life stress experiences and links to breast cancer. Cancer Prev Res (Phila) 3: 1365-1370. [crossref]
- Cameron OG (2006) Anxious-depressive comorbidity: effects on HPA axis and CNS noradrenergic functions. Essent Psychopharmacol 7: 24-34. [crossref]
- Faravelli C, Lo Sauro C, Lelli L, Pietrini F, Lazzeretti L, et al. (2012) The role of life events and HPA axis in anxiety disorders: a review. Curr Pharm Des 18: 5663-5674. [crossref]
- Faravelli C, Lo Sauro C, Godini L, Lelli L, Benni L, Pietrini F, et al. (2012) Childhood stressful events, HPA axis and anxiety disorders. World J Psychiatry 2: 13-25. [Crossref]
- Dent GW, Okimoto DK, Smith MA, Levine S (2000) Stress-induced alterations in corticotropin-releasing hormone and vasopressin gene expression in the paraventricular nucleus during ontogeny. Neuroendocrinology 71: 333-342. [Crossref]
- Fahlke C, Lorenz JG, Long J, Champoux M, Suomi SJ, et al. (2000) Rearing experiences and stress-induced plasma cortisol as early risk factors for excessive alcohol consumption in nonhuman primates. Alcohol Clin Exp Res 24: 644-650. [Crossref]
- Arborelius L, Linnér L, Wallsten C, Ahlenius S, Svensson TH (2000) Partial 5-HT1A receptor agonist properties of (-)pindolol in combination with citalopram on serotonergic dorsal raphe cell firing in vivo. Psychopharmacology (Berl) 151: 77-84. [crossref]
- Caldji C, Francis D, Sharma S, Plotsky PM, Meaney MJ (2000) The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology 22: 219-229. [Crossref]
- Wong ML, Kling MA, Munson PJ, Listwak S, Licinio J, et al. (2000) Pronounced and sustained central hypernoradrenergic function in major depression with melancholic features: relation to hypercortisolism and corticotropin-releasing hormone. Proc Natl Acad Sci U S A 97: 325-330. [Crossref]
- Rao U, Hammen C, Ortiz LR, Chen LA, Poland RE (2008) Effects of early and recent adverse experiences on adrenal response to psychosocial stress in depressed adolescents. Biol Psychiatry 64: 521-526. [crossref]
- Risbrough VB, Stein MB (2006) Role of corticotropin releasing factor in anxiety disorders: a translational research perspective. Horm Behav 50: 550-561. [crossref]
- Shea A, Walsh C, Macmillan H, Steiner M (2005) Child maltreatment and HPA axis dysregulation: relationship to major depressive disorder and post traumatic stress disorder in females. Psychoneuroendocrinology 30: 162-178. [Crossref]
- Holsboer F (2000) The corticosteroid receptor hypothesis of depression. Neuropsychopharmacology 23: 477-501. [crossref]
- Schmidt MV, Enthoven L, van der Mark M, Levine S, de Kloet ER, et al. (2003) The postnatal development of the hypothalamic-pituitary-adrenal axis in the mouse. Int J Dev Neurosci 21: 125-132. [crossref]
- Schmidt MV, Deussing JM, Oitzl MS, Ohl F, Levine S, et al. (2006) Differential disinhibition of the neonatal hypothalamic- pituitary-adrenal axis in brain-specific CRH receptor 1-knockout mice. Eur J Neurosci 24: 2291-2298. [Crossref]
- Venihaki M, Majzoub J (2002) Lessons from CRH knockout mice. Neuropeptides 36: 96-102. [crossref]
- Mahon PB, Zandi PP, Potash JB, Nestadt G, Wand GS (2013) Genetic association of FKBP5 and CRHR1 with cortisol response to acute psychosocial stress in healthy adults. Psychopharmacology (Berl) 227: 231-241. [crossref]
- Menke A, Klengel T, Rubel J, Brückl T, Pfister H, et al. (2013) Genetic variation in FKBP5 associated with the extent of stress hormone dysregulation in major depression. Genes Brain Behav 12: 289-296. [crossref]
- Crow TJ (2011) 'The missing genes: what happened to the heritability of psychiatric disorders?'. Mol Psychiatry 16: 362-364. [crossref]
- Danchin É, Charmantier A, Champagne FA, Mesoudi A, Pujol B, et al. (2011) Beyond DNA: integrating inclusive inheritance into an extended theory of evolution. Nat Rev Genet 12: 475-486. [crossref]
- Waddington C (1957) The strategy of the genes. A discussion of some aspects of theoretical biology. New York, MacMillan.
- Chahrour M, Jung SY, Shaw C, Zhou X, Wong ST, et al. (2008) MeCP2, a key contributor to neurological disease, activates and represses transcription. Science 320: 1224-1229. [crossref]
- Cohen S, Zhou Z, Greenberg ME (2008) Medicine. Activating a repressor. Science 320: 1172-1173. [Crossref]
- Pradhan S, Esteve PO (2003) Mammalian DNA (cytosine-5) methyltransferases and their expression. Clin Immunol 109: 6-16. [crossref]
- Gurard-Levin ZA, Almouzni G (2014) Histone modifications and a choice of variant: a language that helps the genome express itself. F1000Prime Rep 6: 76. [crossref]
- Strahl BD, Allis CD (2000) The language of covalent histone modifications. Nature 403: 41-45. [crossref]
- Levenson JM, O'Riordan KJ, Brown KD, Trinh MA, Molfese DL, et al. (2004) Regulation of histone acetylation during memory formation in the hippocampus. J Biol Chem 279: 40545-40559. [crossref]
- Zhang TY, Meaney MJ (2010) Epigenetics and the environmental regulation of the genome and its function. Annu Rev Psychol 61: 439-466, C1-3. [crossref]
- Alarcón JM, Maller2021 Copyright OAT. All rights reserv, et al. (2004) Chromatin acetylation, memory, and LTP are impaired in CBP+/- mice: a model for the cognitive deficit in Rubinstein-Taybi syndrome and its amelioration. Neuron 42: 947-959. [Crossref]
- Bourtchouladze R, Lidge R, Catapano R, Stanley J, Gossweiler S, et al. (2003) A mouse model of Rubinstein-Taybi syndrome: defective long-term memory is ameliorated by inhibitors of phosphodiesterase 4. Proc Natl Acad Sci U S A 100: 10518-10522. [crossref]
- Korzus E, Rosenfeld MG, Mayford M (2004) CBP histone acetyltransferase activity is a critical component of memory consolidation. Neuron 42: 961-972. [crossref]
- Yeh SH, Lin CH, Gean PW (2004) Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Mol Pharmacol 65: 1286-1292. [crossref]
- Bredy TW, Barad M (2008) The histone deacetylase inhibitor valproic acid enhances acquisition, extinction, and reconsolidation of conditioned fear. Learn Mem Cold Spring Harb N 15: 39-45. [crossref]
- Lattal KM, Barrett RM, Wood MA (2007) Systemic or intrahippocampal delivery of histone deacetylase inhibitors facilitates fear extinction. Behav Neurosci 121: 1125-1131. [crossref]
- Klengel T, Binder EB (2015) Epigenetics of Stress-Related Psychiatric Disorders and Gene × Environment Interactions. Neuron 86: 1343-1357. [crossref]
- McGowan PO (2013) Epigenomic Mechanisms of Early Adversity and HPA Dysfunction: Considerations for PTSD Research. Front Psychiatry 4: 110. [crossref]
- McGowan PO, Sasaki A, D'Alessio AC, Dymov S, Labonté B, et al. (2009) Epigenetic regulation of the glucocorticoid receptor in human brain associates with childhood abuse. Nat Neurosci 12: 342-348. [crossref]
- Mehta D, Klengel T, Conneely KN, Smith AK, Altmann A, et al. (2013) Childhood maltreatment is associated with distinct genomic and epigenetic profiles in posttraumatic stress disorder. Proc Natl Acad Sci U S A 110: 8302-8307. [crossref]
- Radley JJ, Kabbaj M, Jacobson L, Heydendael W, Yehuda R, et al. (2011) Stress risk factors and stress-related pathology: neuroplasticity, epigenetics and endophenotypes. Stress 14: 481-497. [crossref]
- Yehuda R, Koenen KC, Galea S, Flory JD (2011) The role of genes in defining a molecular biology of PTSD. Dis Markers 30: 67-76. [crossref]
- Oberlander TF, Weinberg J, Papsdorf M, Grunau R, Misri S, et al. (2008) Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 3: 97-106. [Crossref]
- Painter RC, Osmond C, Gluckman P, Hanson M, Phillips DI, et al. (2008) Transgenerational effects of prenatal exposure to the Dutch famine on neonatal adiposity and health in later life. BJOG 115: 1243-1249. [crossref]
- Mitchell E, Klein SL, Argyropoulos KV, Sharma A, Chan RB, et al. (2016) Behavioural traits propagate across generations via segregated iterative-somatic and gametic epigenetic mechanisms. Nat Commun 7: 11492. [Crossref]
- Zhao Q, Hou J, Chen B, Shao X, Zhu R, et al. (2015) Prenatal cocaine exposure impairs cognitive function of progeny via insulin growth factor II epigenetic regulation. Neurobiol Dis 82: 54-65. [Crossref]
- Schroeder JW, Smith AK, Brennan PA, Conneely KN, Kilaru V, et al. (2012) DNA methylation in neonates born to women receiving psychiatric care. Epigenetics 7: 409-414. [crossref]
- Mathews HL, Konley T, Kosik KL, Krukowski K, Eddy J, et al. (2011) Epigenetic patterns associated with the immune dysregulation that accompanies psychosocial distress. Brain Behav Immun 25: 830-839. [Crossref]
- Kang HJ, Kim JM, Kim SY, Kim SW, Shin IS, et al. (2015) A Longitudinal Study of BDNF Promoter Methylation and Depression in Breast Cancer. Psychiatry Investig 12: 523-531. [crossref]
- Zhou Q, Jackson-Cook C, Lyon D, Perera R, Archer KJ (2015) Identifying molecular features associated with psychoneurological symptoms in women with breast cancer using multivariate mixed models. Cancer Inform 14: 139-145. [Crossref]
- Stommel M, Kurtz ME, Kurtz JC, Given CW, Given BA (2004) A longitudinal analysis of the course of depressive symptomatology in geriatric patients with cancer of the breast, colon, lung, or prostate. Health Psychol 23: 564-573. [Crossref]
- Singer S, Krauss O, Keszte J, Siegl G, Papsdorf K, et al. (2012) Predictors of emotional distress in patients with head and neck cancer. Head Neck 34: 180-187. [crossref]
- Ahles TA, Saykin AJ, Noll WW, Furstenberg CT, Guerin S, et al. (2003) The relationship of APOE genotype to neuropsychological performance in long-term cancer survivors treated with standard dose chemotherapy. Psychooncology 12: 612-619. [crossref]
- Thomas CB, Duszynski KR, Shaffer JW (1979) Family attitudes reported in youth as potential predictors of cancer. Psychosom Med 41: 287-302. [crossref]
- Shekelle RB, Raynor WJ Jr, Ostfeld AM, Garron DC, Bieliauskas LA, et al. (1981) Psychological depression and 17-year risk of death from cancer. Psychosom Med 43: 117-125. [crossref]
- Olderøy G, Daehlin L, Ogreid D (1998) Low-frequency mutation of Ha-ras and Ki-ras oncogenes in transitional cell carcinoma of the bladder. Anticancer Res 18: 2675-2678. [crossref]
- Penninx BW, Guralnik JM, Pahor M, Ferrucci L, Cerhan JR, et al. (1998) Chronically depressed mood and cancer risk in older persons. J Natl Cancer Inst 90: 1888-1893. [crossref]
- Dattore PJ, Shontz FC, Coyne L (1980) Premorbid personality differentiation of cancer and noncancer groups: a test of the hypothesis of cancer proneness. J Consult Clin Psychol 48: 388-394. [crossref]
- Eberhard J, Ståhl O, Cohn-Cedermark G, Cavallin-Ståhl E, Giwercman Y, et al. (2010) Emotional disorders in testicular cancer survivors in relation to hypogonadism, androgen receptor polymorphism and treatment modality. J Affect Disord 122: 260-266. [Crossref]
- Kim KR, Chung HC, Lee E, Kim SJ, Namkoong K (2012) Body image, sexual function and depression in Korean patients with breast cancer: modification by 5-HTT polymorphism. Support Care Cancer 20: 2177-2182. [crossref]
- Liu Z, Zhu F, Wang G, Xiao Z, Wang H, et al. (2006) Association of corticotropin-releasing hormone receptor1 gene SNP and haplotype with major depression. Neurosci Lett 404: 358-362. [crossref]
- Licinio J, O’Kirwan F, Irizarry K, Merriman B, Thakur S, et al. (2004) Association of a corticotropin-releasing hormone receptor 1 haplotype and antidepressant treatment response in Mexican-Americans. Mol Psychiatry 9: 1075-1082. [crossref]
- Smith DJ, Escott-Price V, Davies G, Bailey ME, Colodro-Conde L, et al. (2016) Genome-wide analysis of over 106 000 individuals identifies 9 neuroticism-associated loci. Mol Psychiatry 21: 749-757. [crossref]
- Chen Y, Brody GH (2015) Family Economic Hardship, Corticotropin-Releasing Hormone Receptor Polymorphisms, and Depressive Symptoms in Rural African-American Youths. J Adolesc Health 57: 235-240. [Crossref]
- Ressler KJ, Bradley B, Mercer KB, Deveau TC, Smith AK, et al. (2010) Polymorphisms in CRHR1 and the serotonin transporter loci: gene x gene x environment interactions on depressive symptoms. Am J Med Genet Part B Neuropsychiatr Genet 153B: 812-824. [Crossref]
- Bradley RG, Binder EB, Epstein MP, Tang Y, Nair HP, et al. (2008) Influence of child abuse on adult depression: moderation by the corticotropin-releasing hormone receptor gene. Arch Gen Psychiatry 65: 190-200. [crossref]
- Starr LR, Hammen C, Conway CC, Raposa E, Brennan PA (2014) Sensitizing effect of early adversity on depressive reactions to later proximal stress: Moderation by polymorphisms in serotonin transporter and corticotropin releasing hormone receptor genes in a 20-year longitudinal study. Dev Psychopathol. 26: 1241-1254. [Crossref]
- Buttenschøn HN, Krogh J, Nielsen MN, Kaerlev L, Nordentoft M, et al. (2016) Association analyses of depression and genes in the hypothalamus-pituitary-adrenal axis. Acta Neuropsychiatr. [crossref]
- Wu Y, Wang X, Shen X, Tan Z, Yuan Y (2012) The I/D polymorphism of angiotensin-converting enzyme gene in major depressive disorder and therapeutic outcome: a case-control study and meta-analysis. J Affect Disord 136: 971-978. [crossref]
- Weber H, Richter J, Straube B, Lueken U, Domschke K, et al. (2016) Allelic variation in CRHR1 predisposes to panic disorder: evidence for biased fear processing. Mol Psychiatry 21: 813-822. [crossref]
- Amstadter AB, Nugent NR, Yang BZ, Miller A, Siburian R, et al. (2011) Corticotrophin-releasing hormone type 1 receptor gene (CRHR1) variants predict posttraumatic stress disorder onset and course in pediatric injury patients. Dis Markers 30: 89-99. [Crossref]
- White S, Acierno R, Ruggiero KJ, Koenen KC, Kilpatrick DG, et al. (2013) Association of CRHR1 variants and posttraumatic stress symptoms in hurricane exposed adults. J Anxiety Disord 27: 678-683. [crossref]
- Grabe HJ, Schwahn C, Appel K, Mahler J, Schulz A, et al. (2010): Childhood maltreatment, the corticotropin-releasing hormone receptor gene and adult depression in the general population. Am J Med Genet B Neuropsychiatr Genet 153B: 1483-1493. [Crossref]
- Heim C, Bradley B, Mletzko TC, Deveau TC, Musselman DL, et al. (2009) Effect of Childhood Trauma on Adult Depression and Neuroendocrine Function: Sex-Specific Moderation by CRH Receptor 1 Gene. Front Behav Neurosci 3: 41. [crossref]
- Wasserman D, Wasserman J, Rozanov V, Sokolowski M (2009) Depression in suicidal males: genetic risk variants in the CRHR1 gene. Genes Brain Behav 8: 72-79. [Crossref]
- DeYoung CG, Cicchetti D, Rogosch FA (2011) Moderation of the association between childhood maltreatment and neuroticism by the corticotropin-releasing hormone receptor 1 gene. J Child Psychol Psychiatry 52: 898-906. [Crossref]
- Laucht M, Treutlein J, Blomeyer D, Buchmann AF, Schmidt MH, et al. (2013) Interactive effects of corticotropin-releasing hormone receptor 1 gene and childhood adversity on depressive symptoms in young adults: findings from a longitudinal study. Eur Neuropsychopharmacol 23: 358-367. [Crossref]
- Kranzler HR, Feinn R, Nelson EC, Covault J, Anton RF, et al. (2011) A CRHR1 haplotype moderates the effect of adverse childhood experiences on lifetime risk of major depressive episode in African-American women. Am J Med Genet Part B Neuropsychiatr Genet 156B: 960-968. [Crossref]
- Liu Z, Liu W, Yao L, Yang C, Xiao L, et al. (2013) Negative life events and corticotropin-releasing-hormone receptor1 gene in recurrent major depressive disorder. Sci Rep 3: 1548. [crossref]
- Polanczyk G, Caspi A, Williams B, Price TS, Danese A, et al. (2009): Protective effect of CRHR1 gene variants on the development of adult depression following childhood maltreatment: replication and extension. Arch Gen Psychiatry 66: 978-985. [Crossref]
- Hodges LM, Weissman MM, Haghighi F, Costa R, Bravo O, et al. (2009) Association and linkage analysis of candidate genes GRP, GRPR, CRHR1, and TACR1 in panic disorder. Am J Med Genet BNeuropsychiatr Genet 150B: 65-73. [crossref]
- Ishitobi Y, Nakayama S, Kanehisa M, Higuma H, Maruyama Y, Okamoto S, et al. (2013) Association between corticotropin-releasing hormone receptor 1 and 2 (CRHR1 and CRHR2) gene polymorphisms and personality traits. Psychiatr Genet 23: 255-257. [crossref]
- Soyka M, Preuss UW, Koller G, Zill P, Hesselbrock V, Bondy B (2004) No association of CRH1 receptor polymorphism haplotypes, harm avoidance and other personality dimensions in alcohol dependence: results from the Munich gene bank project for alcoholism. Addict Biol 9: 73-79. [crossref]
- Tochigi M, Kato C, Otowa T, Hibino H, Marui T, Ohtani T, et al. (2006) Association between corticotropin-releasing hormone receptor 2 (CRHR2) gene polymorphism and personality traits. Psychiatry Clin Neurosci 60: 524-526. [crossref]
- Keck ME, Kern N, Erhardt A, Unschuld PG, Ising M, et al. (2008) Combined effects of exonic polymorphisms in CRHR1 and AVPR1B genes in a case/control study for panic disorder. Am J Med Genet B Neuropsychiatr Genet 147B: 1196-1204. [crossref]
- Heitland I, Groenink L, van Gool JM, Domschke K, Reif A, et al. (2016) Human fear acquisition deficits in relation to genetic variants of the corticotropin-releasing hormone receptor 1 and the serotonin transporter--revisited. Genes Brain Behav 15: 209-220. [crossref]
- Heitland I, Groenink L, Bijlsma EY, Oosting RS, Baas JMP (2013) Human fear acquisition deficits in relation to genetic variants of the corticotropin releasing hormone receptor 1 and the serotonin transporter. PloS One 8: e63772. [crossref]
- Hsu DT, Mickey BJ, Langenecker SA, Heitzeg MM, Love TM, et al. (2012) Variation in the corticotropin-releasing hormone receptor 1 (CRHR1) gene influences fMRI signal responses during emotional stimulus processing. J Neurosci32: 3253-3260. [crossref]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, et al. (2015) Amygdala functional connectivity, HPA axis genetic variation, and life stress in children and relations to anxiety and emotion regulation. J Abnorm Psychol 124: 817-833. [crossref]
- Ching-López A, Cervilla J, Rivera M, Molina E, McKenney K, et al. (2015) Epidemiological support for genetic variability at hypothalamic-pituitary-adrenal axis and serotonergic system as risk factors for major depression. Neuropsychiatr Dis Treat 11: 2743-2754. [crossref]
- Kohrt BA, Worthman CM, Ressler KJ, Mercer KB, Upadhaya N, et al. (2015) Cross-cultural gene- environment interactions in depression, post-traumatic stress disorder, and the cortisol awakening response: FKBP5 polymorphisms and childhood trauma in South Asia. Int Rev Psychiatry 27: 180-196. [crossref]
- Woody ML, Kudinova AY, McGeary JE, Knopik VS, Palmer RH, et al. (2016) Influence of maternal depression on children's brooding rumination: Moderation by CRHR1 TAT haplotype. Cogn Emot 30: 302-314. [crossref]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, et al. (2015) HPA axis genetic variation, pubertal status, and sex interact to predict amygdala and hippocampus responses to negative emotional faces in school-age children. NeuroImage 109: 1-11. [crossref]
- Cicchetti D, Rogosch FA (2014) Genetic moderation of child maltreatment effects on depression and internalizing symptoms by serotonin transporter linked polymorphic region (5-HTTLPR), brain-derived neurotrophic factor (BDNF), norepinephrine transporter (NET), and corticotropin releasing hormone receptor 1 (CRHR1) genes in African American children. Dev Psychopathol 26: 1219-1239. [crossref]
- Suzuki A, Matsumoto Y, Sadahiro R, Enokido M, Goto K, et al. (2014) Relationship of the FKBP5 C/T polymorphism with dysfunctional attitudes predisposing to depression. Compr Psychiatry 55: 1422-1425. [crossref]
- Szczepankiewicz A, LeszczyÅ ska-Rodziewicz A, Pawlak J, Narozna B, Rajewska-Rager A, et al. (2014) FKBP5 polymorphism is associated with major depression but not with bipolar disorder. J Affect Disord 164: 33-37. [crossref]
- Pagliaccio D, Luby JL, Bogdan R, Agrawal A, Gaffrey MS, et al. (2014) Stress-system genes and life stress predict cortisol levels and amygdala and hippocampal volumes in children. Neuropsychopharmacology 39: 1245-1253. [crossref]
- Schatzberg AF, Keller J, Tennakoon L, Lembke A, Williams G, et al. (2014) HPA axis genetic variation, cortisol and psychosis in major depression. Mol Psychiatry 19: 220-227. [crossref]
- Engineer N, Darwin L, Nishigandh D, Ngianga-Bakwin K, Smith SC, et al. (2013) Association of glucocorticoid and type 1 corticotropin-releasing hormone receptors gene variants and risk for depression during pregnancy and post-partum. J Psychiatr Res 47: 1166-1173. [crossref]
- Shinozaki G, Jowsey S, Amer H, Biernacka J, Colby C, et al. (2011) Relationship between FKBP5 polymorphisms and depression symptoms among kidney transplant recipients. Depress Anxiety 28: 1111-1118. [crossref]
- Zimmermann P, Brückl T, Nocon A, Pfister H, Binder EB, et al. (2011) Interaction of FKBP5 gene variants and adverse life events in predicting depression onset: results from a 10-year prospective community study. Am J Psychiatry 168: 1107-1116. [crossref]
- Lewis G, Collishaw S, Harold G, Rice F, Thapar A (2012) Maternal depression and child and adolescent depression symptoms: an exploratory test for moderation by CRHR1, FKBP5 and NR3C1 gene variants. Behav Genet 42: 121-132. [crossref]
- Appel K, Schwahn C, Mahler J, Schulz A, Spitzer C, et al. (2011) Moderation of adult depression by a polymorphism in the FKBP5 gene and childhood physical abuse in the general population. Neuropsychopharmacology 36: 1982-1991. [crossref]
- Zobel A, Schuhmacher A, Jessen F, Höfels S, von Widdern O, et al. (2010) DNA sequence variants of the FKBP5 gene are associated with unipolar depression. Int J Neuropsychopharmacol 13: 649-660. [crossref]
- Dong C, Wong ML, Licinio J (2009) Sequence variations of ABCB1, SLC6A2, SLC6A3, SLC6A4, CREB1, CRHR1 and NTRK2: association with major depression and antidepressant response in Mexican-Americans. Mol Psychiatry 14: 1105-1118. [crossref]
- Willour VL, Chen H, Toolan J, Belmonte P, Cutler DJ, et al. (2009) Family-based association of FKBP5 in bipolar disorder. Mol Psychiatry 14: 261-268. [crossref]
- Dempster EL, Burcescu I, Wigg K, Kiss E, Baji I, et al. (2007) Evidence of an association between the vasopressin V1b receptor gene (AVPR1B) and childhood-onset mood disorders. Arch GenPsychiatry 64: 1189-1195. [crossref]
- Ben-Efraim YJ, Wasserman D, Wasserman J, Sokolowski M (2013) Family-based study of AVPR1B association and interaction with stressful life events on depression and anxiety in suicide attempts. Neuropsychopharmacology 38: 1504-1511. [crossref]




