The aim of this research was to develop antithrombotic polyelectrolyte multilayer nanocoatings that can decrease platelet adhesion to surfaces of blood contacting implants and devices and prevent thrombus formation. It has been shown that developed in this study sulfated derivatives of highly pure fungal chitosan fabricated under controlled conditions can be more effective in the decrease of platelet adhesion compared with heparin when they are used as polyanions in polyelectrolyte multilayer coatings. The application of novel coatings based on sulfated fungal chitosan in stents and other blood contacting devices can essentially improve blood compatibility and safety of the use of implants and decrease the probability of medical complications. The stiffness of nanocoatings depends on their hydration degree and plays important role in prevention of protein adsorption and platelet adhesion.
polyelectrolyte multilayers, platelet adhesion, sulfated chitosan, hydration, stiffness
Blood contacting surfaces may activate platelets and result in serious medical problems, such as thrombosis. Although a lot of biomaterials are used in the coatings for medical devices and implants, the need still exists for better polymer systems to regulate biological processes at the blood-material interface and to prevent thrombosis. Rational design of nanostructured multilayer coatings can direct cellular processes at interfaces [1]. The potential of chitosan in prevention and treatment of age-related diseases has been recently reviewed [2]. The effect of chitosan and its derivatives on biochemical and biophysical processes depends on their molecular weight and structure. Actually, the term “chitosan” corresponds to a group of polymers with different properties and applications due to varying molecular weights and degrees of deacetylation. The raw material for production of chitosan can also affect the properties and areas of applications. Extraction of chitosan from fungal sources has a number of advantages over the extraction of chitosan from crustacean sources, because demineralization stage can be eliminated, raw material is free from heavy metal contamination, and also free from seasonal variations of chemical composition [3]. The standardized procedures for selection of a raw material and controlled production of fungal chitosan in a bioreactor allow producing stable material with tailor-made molecular parameters, free from seasonal variations that are common for crustacean chitosans, for targeted biomedical applications. The degree of deacetylation and molecular weight of the fungal chitosan are more stable than the same properties of crustacean chitosans. Chitosan produced from fungal mycelia has been reported to have lower molecular weight compared to the molecular weight of chitosan obtained from crustacean sources, and higher deacetylation degree [4].
Platelets adhesion plays an important role in thrombus formation. In this study, chitosan/chitosan sulfate multilayer coatings were designed to prevent platelet adhesion. It has been found that fungal sulfated chitosan is more effective in the decrease of platelet adhesion in comparison with heparin and surface hydration plays an important role in the prevention of platelet adhesion.
KiOmedine-CSU® is an ultra-pure chitosan of non-animal origin, produced from white mushrooms (Agaricus bisporus). KiOmedine-CSU® was used in sulfating process to obtain chitosan sulfates with different sulfation degrees. The scheme of sulfating process is presented in Figure 1.
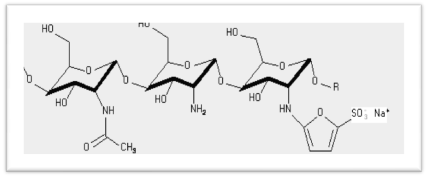
Figure 1. Scheme of fungal chitosan sulfation
- Sulfation with 5-formylfuran-2-sulfonic
acid (FFSA)
- Dispersion of CsU into HAC 1N
- Addition of FFSA
- Stirring overnight at 25°C
- Reduction
- Addition of sodium cyanoborohydride NABH3CN
- Stirring during 36 hours at 25°C
Three bilayers of chitosan/chitosan sulfate CS60 (1), chitosan/ chitosan sulfate CS80 (2), chitosan sulfate CS60/chitosan (3) and chitosan/heparin (4) were alternately deposited on cobalt-chromium (CoCr) discs using layer-by-layer assembly technique. The degree of sulfation of chitosan sulfate CS60 was 44%, and degree of sulfation of chitosan sulfate CS80 was 71%. The multilayers were fabricated by alternately and successively immersing the CoCr discs in the heparin or sulfated chitosan as polyanion and chitosan as polycation solution, followed by 10 minutes of adsorption and subsequently rinsing two times with de-ionized water for 1 minute every cycle. The cycle can be repeated n times to obtain a coating with desired number of nanolayers.
Platelet adhesion was studied using the procedure described previously [5]. Briefly, fresh venous blood was collected from healthy human volunteers, free from medications known to interfere with platelet function in concordance with the guidelines of the ethical committee of the P. Stradins Clinical University Hospital. Vacutainers (Vacutest KIMA, Italy) containing anticoagulant (K2EDTA 5.4mg) were used for collection of blood. Prior to testing polymer coated discs were sterilized by UV – irradiation (wavelength 253.7 nm) for 30 min. Each polymer coated disc was immersed in 1 ml of blood and incubated at 37 ± 2°C, 5% CO2 for 24 hours. The control was 1 ml blood sample. Cell counter (Beckman Coulter, Coulter LH 750, and USA) was used to determine platelet count in blood samples before and after incubation with the polymer coated disc. Experiment was carried out in triplicates, and a mean value was calculated.
The extent of platelet adhesion relative to the whole human blood control was calculated as follows:
Platelet adhesion, % = (n0 – nt)/n0 x 100 %
Where n0 and nt are the platelet counts in whole blood before and after contact, respectively.
Zwitterionic polymers are well known as nonfouling materials because they have both a positively and a negatively charged groups within the same side chain segment and so they have overall charge neutrality [6]. The hydration layer around the charged groups in these polymers prevents nonspecific protein adsorption followed by platelet adhesion, platelet aggregation, and thrombus formation [7,8].
In polyelectrolyte multilayers (PEMs) it is also possible to design surface with overall charge neutrality. Charges on polyanions and polycations balance each other due to extensive interpenetration within polyelectrolyte multilayers [9]. Recently the method of sum frequency generation (SFG) vibrational spectroscopy demonstrated the effect of sodium chloride (NaCl), present in the polyelectrolyte solutions, on interfacial structures in PEMs. During aging process in metastable PEMs interpenetration of polyanion, polycation and NaCl due to mutual diffusion results in charge compensation [10]. Mechanisms of charge compensation in PEMs have been discussed in a recent review [11].
It has been shown using X-ray photoelectron spectroscopy and fluorescence staining of collagen that in multilayer coatings of titanium surfaces positively charged collagen interpenetrates the negatively charged heparin layer [12]. This coating improved biocompatibility of titanium in contact with blood.
Though the sulfation degree and negative electrical charge of CS80 is higher than sulfation degree and negative charge of CS60, the platelet adhesion on coating with CS60 outermost layer is lower, Figure 2, probably because the stiffness of CS60 is also lower. It is well known at present that surfaces with lower stiffness have lower protein adsorption and, as a consequence, lower platelet adhesion [13,14].
It has been recently shown [15] that stronger electrostatic interactions between oppositely charged polyelectrolytes in multilayer coatings results in reduced hydration and increase of stiffness. It has been also reported that platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Increasing substrate stiffness leads to increased platelet adhesion and spreading [13].
The stiffness of environment in which cells are located could be closely related with changes in hydrated water content. Strong surface hydration has been proposed as a significant condition in the formation of nonfouling surfaces [16]. So it can be suggested that due to higher charge and stronger interaction with adjacent polycationic layer leading to reduced hydration and increased stiffness the CS80 is less effective compared to CS60 in prevention of platelet adhesion.
The role of polymer coating hydration and binding energy of water molecules with chitosan in the prevention of platelet adhesion has been recently also reported for chitosan coatings [5].
The water molecules with different mobility were detected in polymers using nuclear magnetic resonance (NMR) and differential thermal analysis [17] indicating different binding energy of water molecules to functional groups of macromolecules. Strongly bound, weakly bound and free water molecules have been detected in the NMR spectrum by a broadened and shifted line and a shift of the endothermic peak was observed on the thermal analysis diagrams.
Schwarz and Schonhoff [18,19] observed water immobilization due to adsorption of a polycation layer on polyelectrolyte multilayers. A much larger water immobilization of the 5th layer as compared with the first layer was also observed.
Increase of water binding energy can be associated with the decrease of protein adsorption and platelet adhesion [5,20,21]. So the polyatomic chitosan in the outermost layer can also resist platelet adhesion due to the presence of tightly bound hydration water (Figure 2).
McCormick et al. [22] found that polymer dynamics in the PEMs depends on the layer number and water content. Relaxation and line width measurements by NMR have shown that the adsorbed water has lower water molecules mobility in the films than in the analogous polyelectrolyte complexes. The diffuse interpenetrating model of the different layers and a partitioning of the water to the surface layer have been proposed by these investigators.
So the data from literature and our results show that PEMs even with polycationic chitosan in the outermost layer can decrease platelet adhesion. These results support the idea of interpenetrated layers.
Aggarwal et al. [23] studied multilayer coatings of polycationic chitosan paired with polyanionic cellulose sulfates with high and intermediate sulfation degree or heparin prepared using layer-by-layer technique. They indicated formation of stiffer layers when cellulose sulfate with high sulfation degree was used when compared to the use of heparin and cellulose sulfates with intermediate sulfation degree layers. Moreover, the use of cellulose sulfates leads into formation of more fuzzy intermingled multilayers compared with the using heparin as polyanion.
Figure 2 shows that even if polycationic chitosan is located in the outermost layer, the lower platelet adhesion was observed compared with coating with heparin in outermost layer. Chitosan is a weak polycation and heparin is a strong polyanion. It has been shown that the structure of the chitosan/heparin thin film was highly interpenetrated without clear boundaries between each layer [24].
Heparin is used widely in antithrombogenic applications, but it is commonly recognized that it is not right for every application because heparin usually is fabricated from animal products and can cause allergic reactions in patients that are sensitive to reagents derived from pig and bovine intestine, so it is important to avoid the potential risk for contamination. Chitosan derivatives manufactured by Kytozyme have plant origin and so novel chitosan sulfates can have potential to replace heparin in coatings for patients that have allergies to animal-based reagents.
Our results, Figure 2, demonstrate platelet adhesion do not depend essentially on the charge (positive or negative) of outermost layer. So interpenetration of chitosan (CS) and sulfated chitosan (CSS) layers can be suggested in CS/CSS multilayer nanocoatings. It has been also recently reported that cells could sense the viscoelasticity of the underlying layers at the nanometer level [25].

Figure 2. Platelet adhesion on chitosan/chitosan sulfate CS60 (1), chitosan/chitosan sulfate CS80 (2), chitosan sulfate CS60/chitosan (3) and chitosan/heparin (4) 3-layer coatings on CoCr discs. Outermost layers in italic are emphasized.
2021 Copyright OAT. All rights reserv
The adsorption of fucoidan and chitosan sulfate on poly (ethylene terephthalate) (PET) film surface was studied using the quartz crystal microbalance technique with a dissipation unit. It was reported that chitosan/fucoidan multilayer films were more compressed and thinner compared to chitosan/chitosan sulfate films. The formation of a loose and thick adsorbed film by sulfated chitosan was observed [26]. It was suggested that chitosan/fucoidan and chitosan/chitosan sulfate covered films reduced human serum albumin (HSA) adsorption due to steric repulsion [27]. So we can suggest that loose and thick coatings are necessary to prevent platelet adhesion.
Researchers at Southwest Jiaotong University, Chengdu, China reported [28] that heparin binding surface inhibited human umbilical artery smooth muscle cell adhesion and proliferation. But enhanced cell adhesion, proliferation and migration, and the release of nitric oxide were observed for human umbilical vein endothelial cells. The heparinized surface promoted in vivo re-endothelialization and inhibited thrombosis formation. Yao and co-authors demonstrated that heparin promoted the growth of human umbilical vein endothelial cells, but inhibited the proliferation of vascular smooth muscle cells [29]. Matthew and coworkers [30] reported that chitosan supported proliferation of both vascular cell types, but retarded smooth muscle cell growth to a greater extent than endothelial growth.
Sulfated chitosan can be used in biomedical applications as alternative to heparin.
Fungal chitosan can be used as a polycationic layer in combination with a polyanionic layer of sulfated fungal chitosan in polyelectrolyte multilayer coatings. Stronger electrostatic interactions between oppositely charged polyelectrolytes in multilayer coatings results in reduced hydration and increase of stiffness. Both these processes can increase adsorption of proteins and platelet adhesion. The hydration water plays an important role in biological interfacial interactions of implant coatings with blood and related molecular mechanisms of interaction of water molecules with biopolymer macromolecules have to be addressed by means of further research. Lower stiffness of outermost layer due to the use of sulfated fungal chitosan can lead to the decrease of platelet adhesion. Further studies investigating how platelets interact with the mechanical microenvironment have potential to improve essentially the current state of knowledge about biophysical and biochemical processes at blood/implant interfaces. The platelet adhesion on polyelectrolyte multilayers containing sulfated fungal chitosan is lower than on polyelectrolyte multilayers containing heparin.
Interpenetration of adjacent layers can lead to the formation of fuzzy structure of highly hydrated polyelectrolyte multilayers. In such a case even a coating with a cationic outermost layer can prevent platelet adhesion. The polyelectrolyte multilayer nanocoatings based on fungal chitosan and sulfated fungal chitosan with improved safety and biocompatibility might have versatile potential applications in blood contacting devices. Our results show that sulfated chitosan derived from ultrapure fungal chitosan can be designed to have superior properties compared to heparin when used in multilayer coatings of blood contacting implants and devices.
This work was supported by EU FP7 project "PREvention of late Stent Thrombosis by an Interdisciplinary Global European effort" (acronym PRESTIGE). Grant Number: 260309.
- Mendelsohn JD, Yang SY, Hiller J, Hochbaum AI and Rubner MF (2003) Rational design of cytophilic and cytophobic polyelectrolyte multilayer thin films. Biomacromolecules 4: 96-106. [Crossref]
- Kerch G (2015). The potential of chitosan and its derivatives in prevention and treatment of age-related diseases. Mar Drugs 13: 2158-2182. [Crossref]
- Nwe N and Stevens, W. F. (2002). Production of fungal chitosan by solid substrate fermentation followed by enzymatic extraction. Biotechnol.Lett. 24: 131-134.
- Kaur S and Dhillon GS (2014). The versatile biopolymer chitosan: potential sources, evaluation of extraction methods and applications. Crit Rev Microbiol 40: 155-175. [Crossref]
- Kerch G., Zicans J., Meri R.M., Stunda-Ramata A and Jakobsons E. (2015). The use of thermal analysis in assessing the effect of bound water content and substrate rigidity on prevention of platelet adhesion. J. Therm. Anal. Calorim. 120: 533-539.
- Sin M.C., Chen S.H and Chang, Y. (2014). Hemocompatibility of zwitterionic interfaces and membranes. Polym. J. 46: 436-443.
- Chen S, Li L, Zhao C and Zheng J. (2010). Surface hydration: principles and applications toward low-fouling/nonfouling biomaterials. Polymer 51: 5283-5293.
- Wang J-J, Wu M-B, Xiang T, Wang R, Sun S-D, et al. (2015). Antifouling and blood-compatible poly (ether sulfone) membranes modified by zwitterionic copolymers via in situ crosslinked copolymerization. J. Appl. Polym. Sci.132: 41585.
- Schlenoff J.B., Ly H., Li M. (1998). Charge and mass balance in polyelectrolyte multilayers. J. Am. Chem. Soc. 120: 7626-7634.
- Ge, A., Matsusaki, M., Qiao, L., Akashi, M., Ye, S. (2016). Salt effects on surface structures of polyelectrolyte multilayers (PEMs) investigated by vibrational sum frequency generation (SFG) spectroscopy. Langmuir 32: 3803-3810.
- Volodkin D and von Klitzing R. (2014). Competing mechanisms in polyelectrolyte multilayer formation and swelling: polycation–polyanion pairing vs. polyelectrolyte–ion pairing. Curr. Opin. Colloid. In., 19: 25-31.
- Chen J., Chen C., Chen Z., Chen J., Li Q., et al. (2010). Collagen/heparin coating on titanium surface Improves the biocompatibility of titanium applied as a blood-contacting biomaterial. J. Biomed. Mater. Res. A 95: 341-349. [Crossref]
- Qiu, Y., Brown, A.C., Myers, D.R., Sakurai, Y., Mannino, R.G., et al. 2014. Platelet mechanosensing of substrate stiffness during clot formation mediates adhesion, spreading, and activation. Proc. Natl. Acad. Sci. U.S.A. 111: 14430-14435. [Crossref]
- Liu D, Guo J and Zhang J.H. (2016). Chain mobility and film softness mediated protein antifouling at the solid–liquid interface. J. Mater. Chem. B. 4: 6134-6142.
- Teixeira R, Reis RL and Pashkuleva I (2016). Influence of the sulfation degree of glycosaminoglycans on their multilayer assembly with poly-l-lysine. Colloids Surf B Biointerfaces 145: 567-575. [Crossref]
- Leng C, Hung HC, Sun S, Wang D, Li Y, et al. (2015) Probing the Surface Hydration of Nonfouling Zwitterionic and PEG Materials in Contact with Proteins. ACS Appl Mater Interfaces 7: 16881-16888. [Crossref]
- Matis I.G, Kerch G.M and Aniskevich A.N. (1990). Investigation of the state of water sorbed by an organic polymer by high-resolution1H nuclear magnetic resonance and differential thermal analysis. Mech. Compos. Mater. 26: 259-262.
- Schwarz B and Schönhoff M.A. (2002). 1H NMR relaxation study of hydration water in polyelectrolyte mono and multilayers adsorbed to colloidal particles. Colloids Surf. A: Physicochem. Eng. Asp. 198: 293-304.
- Schwarz B and Schönhoff M. 2002. Surface potential driven swelling of polyelectrolyte multilayers. Langmuir 18: 2964-2966.
- Zheng J., Li L., Tsao H.K., Sheng Y.J., Chen S., et al. 2005. Strong repulsive forces between protein and oligo (ethylene glycol) self-assembled monolayers: A molecular simulation study. Biophys. J. 89: 158-166.
- Sheikh S, Blaszykowski C, Nolan R, Thompson D and Thompson M. (2015). On the hydration of subnanometric antifouling organosilane adlayers: A molecular dynamics simulation. J. Colloids Interf. Sci. 437: 197-204. [Crossref]
- McCormick M, Smith R.N, Graf R, Barrett C.J, Reven L, et al. (2003). NMR studies of the effect of adsorbed water on polyelectrolyte multilayer films in the solid state. Macromolecules 36: 3616-3625. [Crossref]
- Aggarwal N., Altgärde N., Svedhem S., Zhang K., Fischer S., et al. 2014. Study on multilayer structures prepared from heparin and semi-synthetic cellulose sulfates as polyanions and their influence on cellular response. Colloids Surf. B: Biointerfaces 116: 93-103. [Crossref]
- Lundin M, Blomberg E and Tilton RD (2010) Polymer dynamics in layer-by-layer assemblies of chitosan and heparin. Langmuir 26: 3242-3251. [Crossref]
- Azuma T, Teramura Y and Takai M (2016). Cellular response to non-contacting nanoscale sublayer: cells sense several nanometer mechanical properties. ACS Appl. Mater. Interfaces 8: 10710-10716.
- Indest T, Laine J, Johansson LS, Stana-Kleinschek K, Strnad S, et al. (2009). Adsorption of fucoidan and chitosan sulfate on chitosan modified PET films monitored by QCM-D. Biomacromolecules 10: 630-637. [Crossref]
- Indest T, Laine J, Kleinschek K.S and Zemljic L.F. 2010. Adsorption of human serum albumin (HSA) on modified PET films monitored by QCM-D, XPS and AFM. Colloids Surf. A: Physicochem. Eng. Asp. 360: 210-219.
- Yang Z, Tu Q, Wang J and Huang N (2012) The role of heparin binding surfaces in the direction of endothelial and smooth muscle cell fate and re-endothelialization. Biomaterials 33: 6615-6625. [Crossref]
- Yao Y, Wang J, Cui Y, Xu R., Wang Z, et al. (2014) Effect of sustained heparin release from PCL/chitosan hybrid small-diameter vascular grafts on anti-thrombogenic property and endothelialization. Acta Biomaterials. 10: 2739-2749. [Crossref]
- Chupa JM, Foster AM, Sumner SR, Madihally SV and Matthew HW (2000). Vascular cell responses to polysaccharide materials: in vitro and in vivo evaluations. Biomaterials 21: 2315-2322. [Crossref]


