Choriocarcinoma of the ovary is a rare and highly malignant germ cell tumor. There are three ways in which an ovarian choriocarcinoma can arise: as a primary gestational choriocarcinoma that results from an ectopic ovarian pregnancy, as a metastatic choriocarcinoma that arises from a gestational choriocarcinoma, and as a germ cell tumor with differentiation into trophoblastic structures. Ovarian choriocarcinomas are therefore classified as gestational or nongestational. Recently, DNA polymorphism analysis has allowed investigators to determine the etiology of choriocarcinoma (gestational versus nongestational). Herein, in this scoping review, we detail the classification of, and clinical aspects of, pure ovarian choriocarcinoma.
Choriocarcinoma is a very rare and highly malignant germ cell tumor that accounts for <1% of all malignant germ cell tumors [1]. There are three ways in which an ovarian choriocarcinoma can arise: as a primary gestational choriocarcinoma that results from an ectopic ovarian pregnancy, as a metastatic choriocarcinoma that arises from a gestational choriocarcinoma from another primary site in the female genital tract, and as a germ cell tumor with differentiation into trophoblastic structures [2].
Choriocarcinoma is classified as gestational or nongestational. Gestational choriocarcinoma is a form of gestational trophoblastic disease, which arises from a partial mole, a complete mole, or a normal pregnancy. Gestational choriocarcinoma is estimated to occur in about 2 to 7 pregnancies per 100,000 in the United States [3]. Nongestational choriocarcinoma does not arise from a pregnancy event and is an extremely rare occurrence. The incidence of primary ovarian nongestational choriocarcinoma is estimated to be 1 in 369,000,000 [4]. Differentiating gestational and nongestational choriocarcinoma can be difficult, as their clinical presentation and pathology can be identical. Traditionally, a definitive diagnosis of nongestational choriocarcinoma has been restricted to prepubescent females in whom the possibility of a pregnancy event can be eliminated with certainty.
Choriocarcinoma of the ovary can be pure choriocarcinoma or, more commonly, mixed with other germ cell components [1]. When examined histologically, if other germ cell components are present mixed with choriocarcinoma, a diagnosis of a nongestational origin can be made. However, if no other germ cell component is present, differentiating a nongestational from gestational origin is impossible using histologic means alone.
Recently, however, DNA polymorphism analysis has allowed investigators to determine the etiology of choriocarcinoma via analysis of the patient’s DNA, her partner’s DNA, and the DNA of the choriocarcinoma. By examining the DNA composition of the tumor and comparing it to the maternal and paternal DNA, the etiology of the choriocarcinoma can be determined. If the DNA of the tumor exactly matches the maternal DNA, the tumor is of non-gestational origin. However, if the tumor contains any alleles matching paternal DNA, the tumor is of gestational origin [5].
In 1982, Jacobs et al. published a comprehensive literature review on pure ovarian choriocarcinoma [6]. The study classified all published cases of pure ovarian choriocarcinoma at the time into three categories: gestational ovarian choriocarcinoma, pure nongestational ovarian choriocarcinoma, and choriocarcinoma of uncertain etiology. The pure nongestational ovarian choriocarcinoma category was assigned to all pure ovarian choriocarcinoma cases occurring in prepubertal females, and the uncertain etiology was assigned to all pure ovarian choriocarcinoma cases in postpubertal women who were said to be sexually abstinent or virginal. Since the publication of this review, several other literature reviews on this topic have been published; however, limitations exist, including lack of definitive categorization strategies. As a result, little is known about the incidence, clinical course, most effective treatment regimen, as well as outcomes for pure ovarian nongestational choriocarcinoma. The purpose of this scoping review is to provide a strict definition of pure ovarian choriocarcinoma that allows appropriate classification of nongestational and gestational origin in order to better understand this disease process.
The search strategy was developed in collaboration with a librarian at Penn State Hershey College of Medicine. We searched PubMed for articles published in English using the following search string: ((("choriocarcinoma"[MeSH Major Topic] OR choriocarcinoma [Title/Abstract]) AND (nongestational[Title/Abstract] OR non-gestational[Title/Abstract])) AND ((ovary[Title/Abstract] OR ovarian[Title/Abstract]) OR gonad[Title/Abstract])) AND english[Language] which resulted in 66 titles. Additionally, we searched Web of Science using All Databases with the following search string: (ts=(choriocarcinoma AND (nongestational OR non-gestational)) OR ti=(choriocarcinoma AND (nongestational OR non-gestational))) AND LANGUAGE: (English), which yielded 57 titles for a sum total of 123 titles. The final search in PubMed and Web of Science was performed on January 6, 2016. Duplicates (n=43) were excluded.
We included case reports and retrospective chart reviews. Review articles and book chapters were excluded (n=7). Review articles and those articles included in the analysis were cross-referenced to identify additional studies missed in initial database search (n=6). Included articles were limited to those dealing with pure non-gestational choriocarcinoma of the ovary.
Criteria used to define a non-gestational origin of the tumor were cases occurring in prepubertal girls or a cases establishing a diagnosis of non-gestational origin via DNA analysis. All other cases were excluded from the results and discussion. Some literature described young G0 females without mention of their history of sexual activity. In these cases, if the patient was <20 years of age, these cases were included (unless nongestational origin was confirmed upon DNA analysis). Because Jacobs et al published an exhaustive literature review on pure choriocarcinoma of the ovary of cases occurring prior to 1981; we included only studies published after 1980. Those articles not meeting this criteria (n=49) were excluded, see Table 4. Additionally, nine articles were inaccessible to us despite multiple requests for full-text articles, and so these were excluded [6-14]. The total number of articles included in this review is 21.
Table 4. Excluded citations
Reason for Exclusion |
Title |
Authors |
Gestational choriocarcinoma |
Primary choriocarcinoma of the ovary. Report of two cases |
Gangadharan VP, Mathew BS, Kumar KS, Chitrathara K |
Intra-operative cytodiagnosis of primary ovarian choriocarcinoma with ki67 immunoexpression |
Kar A, Kar T, Mahapatra S, Dehuri P |
Pure ovarian choriocarcinoma: a report of two cases |
Mood NI, Samadi N, Rahimi-Moghaddam P, Sarmadi S, Eftekhar Z, Yarandi F |
Ovarian choriocarcinoma arising from partial mole as evidenced by deoxyribonucleic acid microsatellite analysis |
Namba A, Nakagawa S, Nakamura N, Takazawa Y, Kugu K, Tsutsumi O, Taketani Y |
Pure choriocarcinoma of ovary diagnosed by fine needle aspiration cytology |
Naniwadekar MR, Desai SR, Kshirsagar NS, Angarkar NN, Dombale VD, Jagtap S |
Not ovarian choriocarcinoma |
Genotyping Diagnosis of Nongestational Choriocarcinom Involving Fallopian Tube and Ligament: A Case Study |
Buza N, Rutherford T, Hui P |
Extraovarian nongestational choriocarcinoma in a postmenopausal woman |
Dilek S, Pata O, Tok E, Polat A |
Endometrial carcinoma in elderly women |
Hoffman K, Nekhlyudov L, Deligdisch L |
Primary non-gestational choriocarcinoma of the uterine cervix: a case report |
Maesta L, Michelin OC, Traiman P, Hokama P, Rudge MVC |
Fallopian tube choriocarcinoma presenting as ovarian tumour: a case report |
Mundkur A, Rai L, Hebbar S, Guruvare S, Adiga P |
Concurrent ovarian-type primary peritoneal adenocarcinoma and peritoneal choriocarcinoma. A case report and review of the literature |
Pentheroudakis G, White J, Davis J, Brown I, Vasey P
|
Primary omental gestational choriocarcinoma ascertained by deoxyribonucleic acid polymorphism analysis |
Sakumoto K, Nagai Y, Inamine M, Kanazawa K |
Testicular choriocarcinoma metastatic to the skin: an additional case and literature review |
Tinkle LL, Graham BS, Spillane TJ, Barr RJ |
Primary renal artery choriocarcinoma causing secondary renovascular hypertension |
Usta TA, Karacan T, Ozyurek E, Naki MM, Omeroglu SN, Demirkiran F |
Pure nongestational uterine choriocarcinoma in a postmenopausal Chinese woman confirmed with short tandem repeat analysis |
Wang YM, Yang YF, Teng F, Zhang HY, Xue FX |
Primary choriocarcinoma of the vulva |
Weiss S, Amit A, Schwartz MR, Kaplan AL |
Not pure choriocarcinoma |
Ovarian nongestational choriocarcinoma mixed with various epithelial malignancies in association with endometriosis |
Hirabayashi K, Yasuda M, Osamura RY, Hirasawa T, Murakami M |
Serous carcinoma of the endometrium with choriocarcinomatous differentiation: A case report and review of the literature indicate the existence of 2 prognostically relevant tumor types |
Horn LC, Hanel C, Bartholdt E, Dietel J |
Malignant mixed ovarian germ cell tumor with embryonal component |
Moniaga NC, Randall LM |
Nongestational choriocarcinoma arising from a primary ovarian tumour |
Oladipo A, Mathew J, Oriolowo A, Lindsay I, Fisher R, Secki M, Yiannakis D |
Nongestational choriocarcinoma of the ovary—a case report |
Pai MR, Naik R |
Postmenopausal patient |
Choriocarcinoma of the ovary in a postmenopausal woman |
Babu MK, Kini U |
A case of non-gestational choriocarcinoma arising in the ovary of a postmenopausal woman |
Park SH, Park A, Kim JY, Kwon JH, Koh SB |
Patient ≥20 years old |
Primary pure ovarian choriocarcinoma mimicking ectopic pregnancy: a report of fulminant progression |
Balat O, Kutlar I, Ozkur A, Bakir K, Aksoy F, Ugur MG |
Primary ovarian choriocarcinoma mimicking ectopic pregnancy managed with laparoscopy--a case report |
Chen YX, Xu J, Lv WG, Xie X |
Pure nongestational choriocarcinoma of the ovary: a case report |
Choi YJ, Chun KY, Kim YW, Ro Dy |
Pure nongestational choriocarcinoma of ovary |
Corakci A, Ozeren S, Ozkan S, Gurbus Y, Ustun H, Yucesoy I |
Pure primary non-gestational ovarian choriocarcinoma: a diagnostic dilemma |
Gon S, Majumdar B, Barui G, Karmakar R, Bhattacharya A |
Management of non-gestational ovarian choriocarcinoma: laparoscopy can be essential. Report of two cases |
Gremeau AS, Bourdel N, Kondo W, Jardon K, Canis M |
Primary pure choriocarcinoma of the ovary |
Grover V, Grover RK, Usha R, Logani KB |
Leydig cell tumor, mature teratoma, and nongestational choriocarcinoma in a single ovary |
Jain T, VanKessel K, Reed S, Paley P |
Pure choriocarcinoma of the ovary: a case report |
Lv L, Yang K, Wu H, Lou J, Peng Z |
Ovarian choriocarcinoma: a difficult diagnosis of an unusual tumor and a review of the hook effect |
Wheeler CA, Davis S, Degefu S, Thorneycroft IH, O’Quinn AG |
Developing retroperitoneal anaplastic carcinoma with choriocarcinoma focus after ovarian nongestational choriocarcinoma: a case report |
Nikolic B, Ljubic A, Terzic M, Arandjelovic A, Babic S, Vucic M |
Primary pure choriocarcinoma of the ovary in reproductive ages: a case report |
Simsek T, Trak B, Tunc M, Karaveli S, Uner M, Sonmez C |
Primary ovarian nongestational choriocarcinoma. Report of a case in a young woman of childbearing age |
Vogler C, Schmidt WA, Edwards CL |
Case labeled as nongestational choriocarcinoma, but no further information of clinical context provided |
Primary chemotherapy and the role of second-look laparotomy in non-dysgerminomatous malignancies of the ovary
|
Pippitt CH Jr, Cain JM, Hakes TB, Pierce VK, Lewis JL Jr |
Patient <20 years old but with history of sexual activity |
Pure choriocarcinoma of the ovary in Silver-Russell Syndrome |
Haruma T, Ogawa C, Nishida T, Kusumoto T, Nakamura K, Seki N, Katayama T, Hiramatsu Y |
Animal study |
Immunohistological Description of Nongestastional Ovarian Choriocarcinoma in Two Female Mice with Conditional Loss of Trp53 Driven by the Tie2 Promoter |
Castiglioni V, Ghahremani MF, Goosens S, Maglie MD, Ardizzone M, Haigh JJ, Radelli E |
Non-gestational malignant placental site trophoblastic tumor of the ovary in a 4-year-old rhesus monkey |
Marbaix E, Defrere S, Duc KH, Lousse JC, Dehoux JP |
Cell line study/bench research/no human case study |
Limitation of differential expression of HLA-A,B,C antigens on choriocarcinoma cell lines by messenger RNA for HLA heavy chain but not by beta 2-microglobulin |
Kawata M, Sizer K, Sekiya S, Parnes JR, Herzenberg LA |
Epidermal growth-factor receptors in human corpora-lutea during the menstrual cycle and pregnancy |
Khandawood FS, Ayyagari RR, Dawood MY |
Localization of the cellular expression of inhibin in trophoblastic tissue |
McCluggage WG, Ashe P, McBride H, Maxwell P, Sloan JM |
Establishment and properties of a human choriocarcinoma cell line of ovarian origin |
Sekiya S, Kaiho T, Shirotake S, Iwasawa H, Inaba N, Kawata M, Higaki K, Ishige H, Takamizawa H, Minamihisamatsu M, Kuwata T |
Usefulness of intraoperative imprint cytology in ovarian germ cell tumors |
Abe A, Sugiyama Y, Furuta R, Matoda M, Takeshima N |
|
The impact of molecular genetic diagnosis on the management of women with hCG-producing malignancies |
Fisher RA, Savage PM, MacDermott C, Hook J, Sebire NJ, Lindsay I, Secki MJ |
Published prior to 1980 |
Nongestational choriocarcinoma of ovary—report of a case |
Dehaan QC |
Primary non-gestational choriocarcinoma of the ovary. Report of a case |
Panayotou PP |
Author published two studies that described same case (excluded one of studies from review) |
Ovarian neoplasms in children and adolescents in Papua New Guinea |
Sengupta SK, Everett VJ |
Review articles/book chapters |
Diagnostic dilemma: non-gestational or gestational choriocarcinoma of the ovary |
Bhatia K, Vaid AK |
Hormonally Active Organ Tumors in Children and Adolescents |
Hicks ML, Danzey TJ |
Gestational Choriocarcinoma |
Hui P |
Recent advances in the pathology and classification of ovarian germ cell tumors |
Roth LM, Talerman A |
Clinical syndromes associated with ovarian neoplasms: a comprehensive review |
Shanbhogue AK, Shanbhogue DK, Prasad SR, Surabhi VR, Fasih N, Menias CO
|
Germ Cell Tumors of the Ovary |
Talerman A, Vang R
|
Pathology of Germ Cell Tumors |
Zaloudek CJ
|
Classification
Since 1980, a total of 22 possible cases of pure nongestational ovarian choriocarcinoma have been published in the English language. Of these, nine occurred in premenarchal females [12-18], 11 occurred in postmenarchal females but were confirmed by DNA analysis [18,19-25], and eight were considered possible cases of pure NGOC according to the strict criteria described above in the methods section [18,26-32] (Tables 1-3).
Age
In the premenarchal group, the average age at diagnosis was 13.6 years. One study did not include the age of the patient at presentation [20]. Another case occurred in a 39 year-old female with a history of gonadal dysgenesis and primary amenorrhea [19].
In the DNA confirmed group, the average age at diagnosis was 24.5 years. One study that reported on three of these cases did not include the age at diagnosis [21].
In the possible cases group, the average age at diagnosis was 14.0 years.
FIGO Stage
In the premenarchal group, the FIGO stage of eight of the cases did not state the FIGO stage. For the one case that did, the FIGO stage was reported as IC [16].
For the DNA confirmed group, the FIGO stage of five cases was not reported. The remaining cases had FIGO stages of IA [26], IIIC [23], and IV [25].
For the possible cases group, the FIGO stage of five cases was not reported. The remaining cases had FIGO stages of IA [31], I [32], and III [35].
Surgery performed
In the premenarchal group (n=9), surgical therapy was reported for five of the cases but not stated for four cases. Of the five cases which reported surgical therapy, two had a unilateral oophorectomy performed [15], one had a unilateral salpingo-oopherectomy performed [17], one had a unilateral salpingo-oopherectomy and partial omentectomy performed [16], and one had a bilateral salpingo-oopherectomy, hysterectomy, omentectomy, and thoracoscopy and wedge resection for pleural lesions [19].
For the DNA confirmed group (n=11), surgical therapy was reported for all but five of the cases. Of the 11 cases which reported surgical therapy, one had a total abdominal hysterectomy, bilateral salpingo-oopherecotmy, and omentectomy performed two months after completion of chemotherapy [22]; one had an initial removal of the ovarian mass and two rounds of chemotherapy which were followed by followed by a total abdominal hysterectomy, bilateral salpingo-oopherecotmy, omental resection, pelvic lymph node dissection, and appendectomy [23]; one had a total abdominal hysterectomy, bilateral salpingo-oopherecotmy, omentectomy, and pelvic lymph node dissection performed [24]; two had a left salpingo-oopherectomy and partial omentectomy performed [25,26]; and one had a right salpingo-oopherectomy performed [27].
For the possible cases group (n=8), surgicial treatment was not reported for one case. Surgery was not performed in one case [3]. Unilateral salpingo-oopherectomy was performed in two cases [30,34]. The remaining cases were treated surgically with a left salpingo-oopherectomy, total omentectomy, and inversion appendectomy [29]; a left salpingo-oopherectomy and partial omentectomy [31]; a left salpingo-oopherectomy and right ovarian cystectomy [32]; and a right salpingo-oopherectomy, left ovarian cystectomy and omentectomy [35].
Chemotherapy
In the premenarchal group, one case did not state whether chemotherapy was used [21]. Two cases were treated with surgical therapy alone [15]. Two cases were treated with PVB (cisplatin, bleomycin, and vinblastine) therapy [16, 18]. One case was treated with vincristine, methotrexate, leukovorin, bleomycin, Adriamycin, and cyclophosphamide [14]. One cases was treated with multiple rounds of various chemotherapeutic agents, see Table 1 [19]. One case was treated with “radiotherapy and chemotherapy,” but the study did not describe any further details [20]. One case was treated with “three drug chemotherapy,” but the study did not expound upon what these three drugs were [20].
For the DNA group, five cases did not include information of chemotherapy treatment. The BEP chemotherapy regimen was used in two cases for four and five cycles, respectively [22] and [23]. One patient was treated with the MAC regimen for four cycles [16]. One patient was treated with the EMA regimen for four cycles [27]; and one patient was treated with one course of EMA followed by 7 cycles of just etoposide and actinomycin due to methotrexate-toxicity [26].
For the possible cases group, chemotherapy treatment was not reported in two cases. Chemotherapy was not given in one case [33] The BEP regiment was used in two cases for three and six cycles, respectively [30,31]. The BEP regimen was also used on another patient for four cycles, followed by high-dose CTx with carboplatin, etoposide, ifosphamide with subsequent bone marrow transplantation [35]. Finally, two patients were treated methotrexate, actinomycin, and an alkylating agent (MAC) regimen for 4 cycles [32] and 5 cycles [34].
Survival
In the premenarchal group, survival outcomes were not reported for three cases [16,17,21]. One patient was reported to be alive and well at ten-year follow-up [15], one patient had no evidence of disease at six-month follow-up [18], one had no evidence of disease at 17-month follow-up [19], and one had no evidence of disease at one year follow-up [20]. One patient died from immediate postoperative complications [15], and one patient was reported dead of disease [20].
For the DNA-confirmed group, outcome was not reported in six cases. Four patients had no evidence of disease at varying follow-up intervals: one month [22], 12 months [26], 18 months [24], and 30 months [23]. One patient was reported dead of disease at 4 months [27].
For the possible cases group, outcome was not reported in two cases. Five patients had no evidence of disease at varying follow-up intervals: 5 months [32], 9 months [34] 14 months [31], 36 months [35], and 62 months [30]. In one case, the patient arrested during initial imaging and all resuscitation efforts were unsuccessful [33] (Tables 1-3).
This study highlights several features of pure nongestational ovarian choriocarcinoma. First, this study emphasizes the rarity of pure nongestational ovarian choriocarcinoma. Nine cases of premenarchal pure nongestational ovarian choriocarcinoma and eight cases of possible pure nongestational choriocarcinoma have been reported since 1980. Just twelve cases of pure ovarian nongestational ovarian choriocarcinoma have been confirmed by DNA polymorphism analysis. Because of the rarity of this disease process, stricter diagnostic criteria should be used in order to correctly categorize nongestational origin from gestational origin, as unless confirmed by DNA analysis or the disease occurs in a patient who is premenarchal, one cannot with absolute certainty classify an ovarian choriocarcinoma. Recently, DNA analysis has been used to successfully determine nongestational versus gestational origin of ovarian choriocarcinoma. This technology will allow for appropriate classification of this disease process which will ultimately lead to improved therapeutic strategies as more information is learned about pure nongestational ovarian choriocarcinoma.
Secondary to the rarity of this disease process, no standard therapy has been established. Treatment is often extrapolated from treatment strategies for gestational choriocarcinoma and germ cell tumors, thereby leading to significant heterogeneity in treatment strategies for pure nongestional ovarian choriocarcinoma. In reviewing the clinical outcomes of those with pure ovarian suspected choriocarcinoma (confirmed and suspected), it is difficult to make definitive treatment recommendations secondary to heterogeneity in, and inconsistent reported of, relevant clinical factors, including disease classification, patient age, stage, surgery, adjuvant therapy and outcomes, combined with the rarity of this particular entity. For those reported cases in which outcomes were reported (n=16), at a follow-up ranging from one month to ten years, 12 were reported as NED, a majority (n=11) were treated with adjuvant chemotherapy (Tables 1-3). Commonly used adjuvant combinational treatment regimens include BEP and EMA.
Table 1. Pure NGOC cases in premenarchal females since 1980
Case |
Age (years) |
Menarchal Status |
hCG |
Surgery |
FIGO Stage |
Chemotherapy |
Outcome |
[15] |
6 |
Premenarchal |
NS* |
RO |
NS |
None |
NED at 10 years |
[15] |
11 |
Premenarchal |
NS |
RO |
NS |
None |
DOD—“attributed to immediate postoperative complications” |
[16] |
10 |
Premenarchal |
7957 mIU/L at 7 days postop |
LSO, partial omentectomy |
IC |
PVB x 5 cycles |
NS |
[17] |
11 |
Premenarchal |
Not performed prior to surgery; negative postop |
RSO |
NS |
Vincristin, methotrexate, leukovorin, bleomycin, adriamycin, cyclophosphamide |
NS |
[18] |
9 |
Premenarchal |
NS |
NS |
NS |
PVB x 3 courses |
NED at 6 months |
[19] |
39 |
History of gonadal dysgenesis and primary amenorrhea
45XO/46XY karyotype |
26392 mIU/ml at postop referral |
Hysterectomy, BSO, omentectomy
Thoracoscopy for pleural lesion resection and wedge resection of lung nodules |
NS |
Cisplatin and etoposide x 4 courses
BEP x 1 course
Cisplatin, etoposide, ifofsamide x 2 course
Oral etoposide x 7 days
Carboplatin, vinblastine, Adriamycin x 1 course
High-dose CTx + autologous BMT |
NED at 17 months |
[20] |
NS |
Presented with precocious puberty |
“precocious puberty 2/2 tumor production of hCG” |
NS |
NS |
“Radiotheray and chemotherapy” |
DOD |
[20] |
11 |
Premenarchal |
“increased” |
NS |
NS |
“3 drug CTx” |
NED at 1 year |
[21] |
12 |
Premenarchal |
Elevated |
NS |
NS |
NS |
NS |
* Abbreviations: NS: Not Stated, AAW: Alive and Well, DOD: Dead of Disease, NED: No Evidence of Disease, TAH: Total Abdominal Hysterectomy, R: Right, L: Left, B: Bilateral, S: Salpingectomy, O: Oophorectomy, MAC: Methotrexate, Actinomycin, Alkylating Agent, BEP: Bleomycin, Etoposide, Cisplatin, PVB: Cisplatin, Bleomycin, Vinblastine, EMA: Etoposide, Methotrexate, Actinomycin, EMA/CO: Etoposide, Methotrexate, Actinomycin, Cyclophosphamide, Oncovin
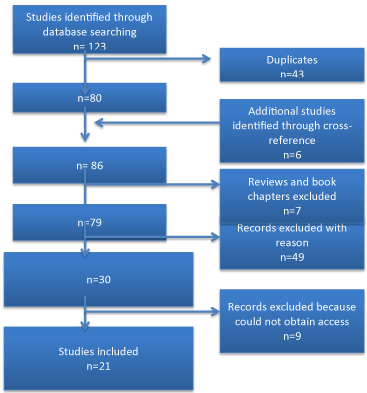
Figure 1. Flow diagram of included studies.
Table 2. Pure NGOC cases confirmed by DNA analysis
Case |
Age (years) |
Reproductive Status |
hCG |
Surgery |
FIGO Stage |
Chemotherapy |
Outcome |
DNA analysis |
[22] |
24 |
G1P0A1 |
675,713 mIU/mL |
TAH, BSO, omentectomy 2 months after completion of CTx |
NS |
BEP x 4 cycles |
NED at 1 month |
DNA polymorphism analysis at 8 loci |
[23] |
23 |
G3P1 |
26,516 mIU/mL |
Initially, removal of mass and omental biopsy
TAH, BSO, omental resection, pelvic lymph node dissection, appendectomy following 2 courses of BEP CTx |
IIIC |
BEP x 5 cycles |
NED at 30 months |
Histo: ovarian CC and some immature seminiferous tubulues
DNA polymorphism analysis with 5 STR loci
Karyotype analysis of peripheral blood 46XX
FISH confirmed absence of SRY hybridization signal in tumor; all nuclei of ovary and tumor hybridized with two XX signals
In testicular tissue both two XX signals (75%) and one X signal (25%) |
[24] |
33 |
G0 |
185,000 mIU/mL |
TAH, BSO, omentectomy, pelvic lymph node dissection |
NS |
MAC x 4 courses |
NED at 18 months |
DNA polymorphism analysis using eight microsatellite markers |
[25] |
19 |
G0, virgin |
|
LSO, partial omentectomy, R ovarian biopsy |
IV |
EMA/CO x “multiple courses” |
NS |
DNA polymorphism analysis of two loci |
[26] |
19 |
G0, virgin |
206,949.7 mIU/mL |
LSO, partial omentectomy |
IA |
EMA x 1 course
Etoposide, actinomycin x 7 courses |
NED at 12 months |
DNA polymorphism analysis at 15 loci |
[21] |
NS |
G0, virgin |
|
|
|
|
|
DNA polymorphism analysis at 12 loci |
[21] |
NS |
“married” |
|
|
|
|
|
DNA polymorphism analysis at 12 loci |
[21] |
NS |
“married” |
|
|
|
|
|
DNA polymorphism analysis at 12 loci |
[27] |
26 |
G0 |
64,000 IU/L |
RSO |
NS |
EMA x 4 courses |
DOD at 4 months |
DNA polymorphism analysis at 9 loci |
[28] |
25 |
G0 |
NS |
NS |
NS |
NS |
NS |
DNA polymorphism analysis at 11 loci |
[28] |
27 |
G0 |
NS |
NS |
NS |
NS |
NS |
DNA polymorphism analysis at 11 loci |
*See Table 1 for explanations of abbreviations.
Table 3. Possible pure NGOC cases since 1980
Case |
Age (years) |
Reproductive Status |
hCG |
Surgery |
FIGO Stage |
Chemotherapy |
Outcome |
[29] |
15 |
G0, virgin |
Urine positive x 2
Blood negative x 3 |
LSO, total omentectomy, inversion appendectomy |
NS |
NS |
NS |
[30] |
10 |
Menarche 6 months prior to presentation |
6,600 ng/mL |
RSO |
NS |
BEP x 3 courses |
NED at 62 months |
[31] |
12 |
G0, virgin |
20,257 mIU/mL |
LSO, partial omentectomy, multiple peritoneal biopsies |
IA |
BEP x 6 courses |
NED at 14 months |
[32] |
18 |
G0, virgin |
Urine positive |
LSO, R ovarian cystectomy, omental and peritoneal biopsies |
I |
MAC x 4 courses |
NED at 5 months |
[33] |
16 |
G0, virgin |
NS |
Not performed |
NS |
Not given |
Cardiac arrest during imaging, all resuscitating measures unsuccessful |
[34]
|
13 |
G0, virgin |
Urine positive |
RSO |
NS |
MAC x 5 courses |
NED at 9 months |
[21] |
16 |
G0, virgin |
NS |
NS |
NS |
NS |
NS |
[35] |
12 |
G0 |
1,100,000 IU/L after initial surgery |
RSO, L ovarian cystectomy, omentectomy |
III |
BEP x 4 courses
High-dose CTx with carboplatin, etoposide, ifosphamide followed by BMT |
NED at 3 years |
*See Table 1 for explanation of abbreviations.
Herein, in this scoping review, we have detailed the classification of, and clinical aspects of, pure ovarian choriocarcinoma. Secondary to its rarity and variability in reporting, conclusive recommendations regarding ideal therapy is lacking. Going forward, definitive categorization, via DNA polymorphism analysis, and creation of an international tumor registry is warranted for rare disease entities such as pure ovarian choriocarcinoma, in order to facilitate better comprehension of its etiology and standardization of therapy with optimization of outcomes.
2021 Copyright OAT. All rights reserv
The authors would like to thank Kathy Shrawder for her expertise in the preparation and formatting of this manuscript.
- Nucci MR, Olivia E (2009) Gynecologic Pathology. Edinburgh: Elsevier Churchill Livingstone; c2009. 592p. Chapter 13, Germ cell tumors of the ovary: 501-538.
- Di Saia PJ, Creasman WT (2012) Clinical Gynecologic Oncology. (8th edn). Philadelphia: Saunders; c2012. Chapter 12, Germ cell, stromal, and other ovarian tumors: 329-356.
- Brinton LA, Bracken MB, Connelly RR (1986) Choriocarcinoma incidence in the United States. Am J Epidemiol 123: 1094-1100. [Crossref]
- Smith HO, Berwick M, Verschraegen CF, Wiggins C, Lansing L, et al. (2006) Incidence and survival rates for female malignant germ cell tumors. Obstet Gynecol 107: 1075-1085. [Crossref]
- Fisher RA, Savage PMMacDermott C, Hook J, Sebire NJ, et al. (2007) The impact of molecular genetic diagnosis on the management of women with hCG-producing malignancies. Gynecol Oncol 3: 413-419. [Crossref]
- Jacobs AJ, Newland JR, Green RK (1982) Pure choriocarcinoma of the ovary. Obstet Gynecol Surv 37: 603-609. [Crossref]
- Buza N, Rutherford T, Hui P (2014) Genotyping Diagnosis of Nongestational Choriocarcinoma Involving Fallopian Tube and Broad Ligament: A Case Study. Int J Gynecol Pathol 33: 58-63. [Crossref]
- Goonewardene M, Amaratunga K (1995) Pure choriocarcinoma of ovary, probably non-gestational in origin. Ceylon Med J 40: 49-50. [Crossref]
- Liu Y, Yang J, Ren T, Zhao J, Feng F, et al. (2014) The encouraging prognosis of nongestational ovarian choriocarcinoma with lung metastases. J Reprod Med 59: 221-226. [Crossref]
- Ozturk E, Ugur MG, Cebesoy FB, Aydin A, Sever T, et al. (2010) Good prognosis for primary ovarian pure nongestational choriocarcinoma using the EMA/CO regimen. Eur J Gynaecol Oncol 31: 123-125. [Crossref]
- Radotra BD (2001) Pure non-gestational choriocarcinoma of ovary a case report with autopsy findings. Indian J Pathol Microbiol 44: 503-505. [Crossref]
- Goonewardene M, Amaratunga K (1995) Pure choriocarcinoma of ovary, probably non-gestational in origin. Ceylon Med J 40: 49-50. [Crossref]
- Liu Y, Yang J, Ren T, Zhao J, Feng F, et al. (2014) The encouraging prognosis of ovarian choriocarcinoma with lung metastases. J Reprod Med 59: 221-226. [Crossref]
- Shigematsu T, Kamura T, Arima T, Wake N, Nakano H (2000) DNA polymorphism analysis of a pure non-gestational choriocarcinoma of the ovary: case report. Eur J Gynaecol Oncol 21: 153-154. [Crossref]
- Axe SR, Klein VR, Woodruff JD (1985) Choriocarcinoma of the ovary. Obstet Gynecol 66: 111-114. [Crossref]
- Kong B, Tian YJ, Zhu WW, Qin YJ (2009) A pure nongestational ovarian choriocarcinoma in a 10-year-old girl: case report and literature review. J Obstet Gynaecol Res 35: 574-578. [Crossref]
- Sengupta SK, Hamilton DR (1981) Primary non-gestational choriocarcinoma of the ovary. Australian & New Zealand Journal of Obstetrics & Gynecology 21: 252-253.
- Vance RP, Geisinger KR (1985) Pure nongestational choriocarcinoma of the ovary. Report of a case. Cancer 56: 2321-2325. [Crossref]
- Chou HH, Lai CH, Wang PN, Tsai KT, Liu HP, et al. (1997) Combination of high-dose chemotherapy, autologous bone marrow/peripheral blood stem cell transplantation, and thoracoscopic surgery in refractory nongestational choriocarcinoma of a 45XO/46XY female: A case report. Gynecol Oncol 64: 521-525. [Crossref]
- Gribbon M, Ein SH, Mancer K (1992) Pediatric malignant ovarian tumors: a 43-year review. J Pediatr Surg 27: 480-484. [Crossref]
- Jiao LZ, Xiang Y, Feng FZ, Wan XR, Zhao J, et al. (2010) Clinical analysis of 21 cases of nongestational ovarian choriocarcinoma. Int J Gynecol Cancer 20: 299-302. [Crossref]
- Exman P, Takahashi TK, Gattas GF, Cantagalli VD, Anton C, et al. (2013) Primary ovary choriocarcinoma: individual DNA polymorphic analysis as a strategy to confirm diagnosis and treatment. Rare tumors 5: 89-92. [Crossref]
- Hu T, Yang M, Zhu H, Shi G, Wang H (2011) Pure non-gestational ovarian choriocarcinoma in a 45,XO/46,XX SRY-negative true hermaphrodite. J Obstet Gynaecol Res 37: 1900-1905. [Crossref]
- Koo HL, Choi J, Kim KR, Kim JH (2006) Pure non-gestational choriocarcinoma of the ovary diagnosed by DNA polymorphism analysis. Pathol Int 56: 613-616. [Crossref]
- Tsujioka H, Hamada H, Miyakawa T, Hachisuga T, Kawarabayashi T (2003) A pure nongestational choriocarcinoma of the ovary diagnosed with DNA polymorphism analysis. Gynecol Oncol 89: 540-542. [Crossref]
- Yamamoto E, Ino K, Yamamoto T, Sumigama S, Nawa A, et al. (2007) A pure nongestational choriocarcinoma of the ovary diagnosed with short tandem repeat analysis: case report and review of the literature. Int J Gynecol Cancer 17: 254-258. [Crossref]
- Arima T, Imamura T, Amada S, Tsuneyoshi M, Wake N (1994) Genetic origin of malignant trophoblastic neoplasms. Cancer Genet Cytogenet 73: 95-102. [Crossref]
- Arima T, Imamura T, Sakuragi N, Higashi M, Kamura T, et al. (1995) Malignant trophoblastic neoplasms with different modes of origin. Cancer Genet Cytogenet 85: 5-15. [Crossref]
- Aggarwal A, Ocon AJ, Nibhanipudi K (2012) A 15-year-old with amenorrhea, abdominal distention, and elevated human chorionic gonadotropin pregnancy, right? Not so fast. Pediatr Emerg Care 28: 1057-1059. [Crossref]
- Hayashi S, Abe Y, Tomita S, Nakanishi Y, Miwa S, et al. (2015) Primary non-gestational pure choriocarcinoma arising in the ovary: A case report and literature review. Oncol Lett 9: 2109-2111. [Crossref]
- Heo EJ, Choi CH, Park JM, Lee JW, Bae DS, et al. (2014) Primary ovarian choriocarcinoma mimicking ectopic pregnancy. Obstet Gynecol Sci 57: 330-333. [Crossref]
- Goswami D, Sharma K, Zutshi V, Tempe A, Nigam S (2001) Nongestational pure ovarian choriocarcinoma with contralateral teratoma. Gynecol Oncol 80: 262-266. [Crossref]
- Raju GC, Woo J, Marchack D, Naraynsingh V (1985) Primary nongestational choriocarcinoma of the ovary. Postgrad Med J 61: 757-758. [Crossref]
- Ozdemir I, Demirci F, Yucel O, Demirci E, Alper M (2004) Pure ovarian choriocarcinoma: a difficult diagnosis of an unusual tumor presenting with acute abdomen in a 13-year-old girl. Acta Obstet Gynecol Scand 83: 504-505. [Crossref]
- Inaba H, Kawasaki H, Hamazaki M, Okugawa T, Honzumi M, et al. (2000) A case of metastatic ovarian non-gestational choriocarcinoma: successful treatment with conservative type surgery and myeloablative chemotherapy. Pediatr Int 42: 383-385. [Crossref]

