Hepatic hemangiomas (HHs) are defines as “giant” when are larger than 4 cm. The following case is reviewed due its unusual evolution. A case of 64-year-old woman was found at CT scan to have a “giant” HH (>20 cm in diameter). In 2015 (8 years after initial diagnosis), in the absence of treatments, abdominal CT scan highlighted the initial spontaneous regression of the HH, that progressed over time. Management of giant hemangiomas remains debated. Surgery should be restricted to specific situations, depending on growth pattern, symptom persistence, risk of complications and patient anxiety. Usually HHs remain stable in size over time and only a prolonged clinical and radiological follow-up is advised. The commonly known natural history of HHs in non-cirrhotics do not include decrease in size or regression. Clearly documented cases of spontaneous regression of giant HHs in non-cirrhotic adult patients have not yet been reported.
hepatic hemangioma, giant hemangioma, benign liver neoplasm, conservative treatment, computed tomography (CT) and adult no-cirrhotic patient.
Hepatic hemangiomas (HHs) are the most common benign neoplasms of the liver with an estimated prevalence of 20%. They can be found at any age, but 60–80% of cases are diagnosed in 30- to 50-year-old patients and have an incidence rate of 0.4%-20% in the general population [1]. Usually, they are asymptomatic and in most cases are incidentally diagnosed during imaging studies for the follow up of other diseases. HHs may range from 1 mm up to 50 cm and are defined as “giant” when they are larger than 4 cm [2]. Previous studies reported that HHs can enlarge during pregnancy or estrogen drug therapy. On the other hand, in cirrhotic patients, HHs were previously described to decrease in size from radiological imaging [3]. These types of neoplasms consist of groups of cavities filled with blood with an endothelial cell lining, all feeding from the hepatic artery. Their etiology is not well understood, however some do consider them vascular malformations that enlarge through dilation rather than hypertrophy or hyperplasia. It is suggested that hormones could play a key role in the enlargement of these masses. Patients exposed to estrogen and progesterone during pregnancy or those undergoing hormone replacement therapy have shown regression after withdrawal. Nevertheless, these estrogen receptors are not present in all masses and growth has been made evident without hormone replacement, as well as in post-menopausal women [4]. To date, a giant liver hemangioma in non-cirrhotic patients which spontaneously shrunk without treatment, has not yet been reported. The following case has been reviewed due its unusual evolution.
In 2002, a 64-year-old woman was found to have a heterogeneous mass diagnosed as “giant” HH (20.7 cm in diameter) at CT scan, which occupied the entire right lobe of the liver, associated with two other smaller hepatic lesions evoking hemangiomas in segments II and V with compensatory hypertrophy of the left liver (Figure 1). Her performance status was zero. No HH- related comorbidities and/or signs/symptoms were reported. Lab test results were always within the normal range. Hepatitis B- related markers and hepatitis C antibodies were negative. No history of alcohol consumption or estrogen therapy. The patient was referred to undergo a surgical evaluation. There was no radiological argument in favor of eventual posttraumatic rupture or hemorrhage and, for this reason, an annual clinical/radiological observation was carried out. We examined clinical symptoms/signs and distinguished any changes in size, number or form of the hemangioma every year. As expected, until 2014 the HH remained stable in size from imaging studies. No therapeutic procedure for the HH had been performed during the previous year. However, in 2015 (13 years after first diagnosis) an abdominal CT scan revealed an initial spontaneous regression of the HH, which progressed over time with a 40% reduction in size (Figure 2). In 2021, the patient still displays no symptoms or signs from the clinical outpatient medical evaluation and the hemangioma continues to be stable in size (12.5 cm in diameter) from the last imaging studies performed.
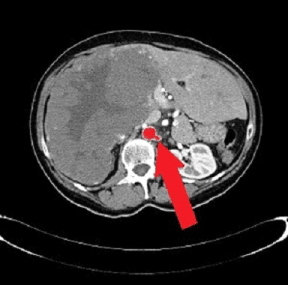
Figure 1: CT scan taken at hemangioma first diagnosis in 2002 (white arrows show the stump of the left renal artery)
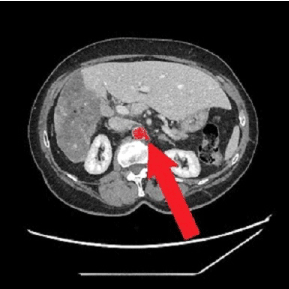
Figure 2: Most recent CT taken in 2019, with a progressive shrinking of the hemangioma (white arrows show the stump of the left renal artery)
HHs are the most common benign vascular neoplasms of the liver, with a 20% incidence in autopsy series. They are more frequent in women (80%) and are often diagnosed incidentally during imaging studies, which are carried out for unrelated pathologies, since most patients remain asymptomatic throughout their life. Since the natural history of HH is benign and an increase in size progression arises in less than 40%, most patients can be reassured and need only observation. A lesion of more than 4 cm in diameter is called a giant HH, which could cause abdominal symptoms, such as pain, nausea, anorexia, and a feeling of abdominal distention [3].
HHs, if associated with symptoms, should require intervention. The right lobe is the most common site. The knowledge about etiopathogeny of HHs remains unclear, with several mechanisms reported including genetic connection, mesenchymal origin or congenital hematoma. Although the mechanism is still under debate, the size of hepatic hemangioma in adults may seldom change [1].
High VEGF expression, which plays an important role in tumor progression, leads to increased angiogenic formation in hemangioma endothelial cells [5]. HHs do not cause symptoms in most cases. Rarely do HHs present life-threatening conditions such as an acute traumatic rupture or coagulation disorders. When symptoms are expressed, they are nonspecific, similar to other diseases, as digestive origin and related to the size [3].
HHs on a dynamic CT scan show an initial intense peripheral nodular enhancement with a gradual central fill-in. Because of this typical radiologic appearance, HHs can be differentiated from other tumors. Management of giant HHs remains under debate. Surgery should be restricted to specific cases, depending on their growth pattern, symptom persistence, risk of complications and patient anxiety. Size alone should not be the sole reason for surgery. Absolute surgical indication for HH is hemoperitoneum, due to those rare spontaneous or traumatic ruptures, intratumoral bleeding and consumptive coagulophathy in infants (Kassabach-Merrit syndrome). Usually, HHs remain stable in size over time and only prolonged clinical and radiological follow-ups are advised. If enlargement is documented, surgical removal should be considered. Based on the high VEGF expression theory, some studies reported that anti-VEGF treatment has been shown to have a direct and rapid effect in tumors due to the effect of chemotherapy drugs which play a role in decreasing liver hemangiomas [6].
In literature, it has been reported that shrunken HHs are derived by the activity of bevacizumab or sorafenib [6,7]. Bevacizumab is an anti-VEGF monoclonal antibody and is reported to have antitumor activity against HCC, ovarian cancer, renal cell cancer, pancreatic cancer, and soft tissue sarcoma when it is administered alone or in combination with chemotherapy [6]. Sorafenib has been reported to have an antitumor effect on various solid tumors. It is a multikinase inhibitor that plays a part in inhibiting epidermal growth factor receptor and it is active against hepatocellular carcinoma and soft tissue sarcomas [7].
Kefeng J. et al. reported a case of a spontaneous disappeared giant HH in patient with a diagnosis of hepatitis B, liver cirrhosis and hepatocellular carcinoma. They supported that the process of a HH spontaneous disappearance may be due to VEGF decreasing from low to high levels, and then decreasing in cirrhotic patients [8].
Also Guillonnet A et al. showed that the level of VEGF in the cirrhotic group was significantly lower than that in the non-cirrhosis group confirming what, in literature, is already reported regarding VEGF theory [9].
Usually, they reduce in size only when the VEGF levels change or after selective hepatic artery embolization, while the commonly known natural history of HHs in non-cirrhotics do not include a decrease in size or regression. In 2001, Okano H et al., reported a spontaneous regression of a small (30 mm) HH without therapy in a young patient (28 years old male) who had a history of poorly controlled diabetes mellitus. The authors enabled that an impaired of his coagulation balance, due to his insulin-dependent diabetes mellitus, could have led to HH disappearance [10].
In our case, the patient with spontaneous regression of HH had no cirrhosis, no hepatitis B- or C-related issues, no history of comorbidities and no liver lesion related symptoms. In summary, we have documented and reported the first case of a non-cirrhotic adult woman with a giant HH which had regressed without the use of drugs or procedures. To date, documented cases of spontaneous regression of giant HHs in non-cirrhotic adult patients have not yet been reported in literature. This outcome suggests that prolonged clinical and imaging follow-up of HHs without related symptoms should be required.
The authors of this manuscript have no conflicts of interest to disclose as described by Integrative Cancer Science and Therapeutics.
Informed consent was obtained from the patient for the publication of their information and imaging.
- Bioulac-Sage P, Laumonier H, Laurent C, Blanc JF, Balabaud C (2008) Benign and malignant vascular tumors of the liver in adults. Semin Liver Dis 28: 302-314. [Crossref]
- Yang Z, Tan H, Liu X, Sun Y (2017) Extremely Giant Liver Hemangioma (50 cm) with Kasabach-Merritt Syndrome. J Gastrointest Surg 21: 1748-1749. [Crossref]
- Brancatelli G, Federle MP, Blachar A, Grazioli L (2001) Hemangioma in the cirrhotic liver: diagnosis and natural history. Radiology 219: 69-74. [Crossref]
- Conter RL, Longmire WP Jr (1988) Recurrent hepatic hemangiomas. Possible association with estrogen therapy. Ann Surg 207: 115-119. [Crossref]
- Mahajan D, Miller C, Hirose K, McCullough A, Yerian L (2008) Incidental reduction in the size of liver hemangioma following use of VEGF inhibitor bevacizumab. J Hepatol 49: 867-870. [Crossref]
- Homsi J, Daudd AI (2007) Spectrum of activity and mechanism of action of VEGF/PDGF inhibitors. Cancer Control 14: 285-294. [Crossref]
- Llovet JM, Ricci S, Mazzaferro V, Hilgard P, Gane E, et al. (2008) Sorafenib in advanced hepatocellular carcinoma. N Engl J Med 359: 378-390. [Crossref]
- Kefeng J, Changlu Y, Cheng S, Yujuan H (2018) A case of a spontaneous disappeared giant liver hemangioma and its literature review. Medical Case Reports 4: 3-76. [Crossref]
- Guillonnet A, Sibileau E, Benadjaoud S, Tavano A, Rousseau C, et al. (2013) Spontaneous and simultaneous regression of multiple hepatic haemangiomas: First case reported. Diagn Interv Imaging 94: 897-900. [Crossref]
- Okano H, Shiraki K, Inoue H, Ito T, Yamanaka T, et al. (2001) Natural course of cavernous hepatic hemangioma. Oncol Rep 8: 411-414. [Crossref]


